Abstract
During the last few years new methods for the measurement of hepcidin concentration have been developed. In this perspective article, Drs. Bergamaschi and Villani examine the potential clinical usefulness of serum hepcidin determination. See related paper on page 1748.
Given its potential toxicity and low bioavailability, iron metabolism in humans is a tightly regulated process. Two main levels of regulation have been described: a cellular regulation that, through the binding of iron-responsive proteins (IRPs) to iron-responsive elements in mRNA from genes encoding proteins of iron metabolism, controls the synthesis of transferrin receptor (necessary for cellular iron procurement) and ferritin (the iron storage protein), and a systemic regulation that modulates intestinal iron absorption and iron mobilization from macrophages and tissue stores. The peptide hormone hepcidin, produced by hepatocytes and acting on target cells situated both outside and within the liver, plays a key role in the systemic regulation of iron metabolism.1
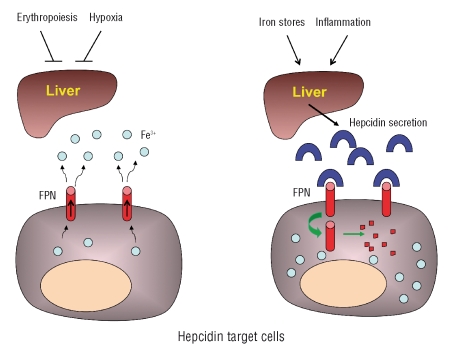
Hepcidin was initially identified as a cysteine-rich urinary antimicrobial peptide.2,3 The subsequent observations that, in mice, hepcidin was over-expressed by hepatocytes during iron overload4 and that knockout animals accumulated excess iron5 showed that hepcidin has a role in iron homeostasis. Several studies demonstrated that hepcidin modulates the release of iron from different cell sources, including enterocytes, macrophages and hepatocytes, to plasma. Through these effects, hepcidin controls iron absorption, the recycling of iron derived from senescent and damaged erythrocytes, and the release of iron from tissue stores. These different tasks are accomplished through a unique biochemical mechanism: the interaction of hepcidin with ferroportin, a transmembrane protein that represents the sole known cellular iron exporter in vertebrates.6 By triggering ferroportin internalization, ubiquitination and degradation, hepcidin blocks the release of iron from cells (Figure 1). Since ferroportin is expressed at the highest levels by iron exporting cells such as the enterocytes and macrophages, hepcidin represents a negative regulator of iron absorption and macrophage iron release.
Figure 1.
Model of the systemic regulation of iron exchange. Ferroportin (FPN) is the only known cellular iron exporter in vertebrates and it is mainly expressed by enterocytes and macrophages (exemplified by the cells shown here). Increased erythropoiesis and hypoxia suppress hepcidin production by the liver, and iron absorption or iron release proceed unopposed (cell on the left). Increased iron stores and inflammation induce hepcidin production by the liver; this causes the internalization and degradation of ferroportin by target cells, thus blocking enterocyte iron absorption and iron recycling by macrophages (cell on the right).
Iron homeostasis is controlled by a regulatory network involving four main components: bone marrow erythropoiesis, tissue oxygen delivery, iron stores and inflammation.7 The interest in hepcidin increased dramatically following the demonstration that the peptide represents the final common pathway on which the components of the regulatory network converge to control tissue iron exchange and iron absorption. In fact, increased erythropoietic activity and reduced tissue oxygen delivery suppress hepcidin production, thereby stimulating iron absorption/mobilization, whereas increased iron stores and inflammation act in the opposite way (Figure 1). Consequently, the abnormal iron status and iron homeostasis that occur in several conditions, such as in most forms of hereditary hemochromatosis,8–10 iron-loading anemias11 and anemia of inflammation,12 are mediated by a dysregulation of hepcidin production. One possible exception to this rule is type 4 hemochromatosis in which the gene encoding ferroportin, the downstream target of hepcidin, is primarily affected. However, the number of cases of ferroportin disease examined for hepcidin level is too low to allow firm conclusions to be drawn about the behavior of hepcidin in these conditions.13,14
Hepcidin production is regulated at the transcriptional level, and the concentration of the peptide in biological fluids is believed to reflect its gene expression. Since investigation of mRNA in liver biopsy samples is not routinely feasible, assays of hepcidin in biological fluids have been proposed as biomarkers useful for the diagnosis of disorders of iron homeostasis. For example, low or inappropriately low levels of hepcidin, relative to serum ferritin concentration, suggest inadequate responses to iron loading and this pattern, associated with an increased transferrin saturation, is typical of hemochromatoses types 1, 2 and 3.8–10,15
Until recently there was only one laboratory where hepcidin concentration could be determined, and the test was essentially used for research applications in disorders of iron metabolism. Several technical reasons were responsible for this limitation, including the existence of different isoforms of the peptide (of 20, 22 and 25 amino acids, respectively) whose specific role in iron metabolism remains uncertain,16 and the difficulty in raising antibodies to hepcidin. The latter problem is probably related to the small size of the molecule, to the presence in the native peptide of four disulfide bonds determining a hairpin structure which may hide antigenic epitopes, and to the high degree of conservation between different animal species.17 During the last few years, however, new methods for the determination of hepcidin concentration have been developed, most of them based on mass spectrometry (MS), but it is unknown to what extent results obtained with different methods are comparable.18,19
In the present issue of Haematologica a collaborative study, involving 8 laboratories that used different immunochemical (IC) or MS-based assays for hepcidin determination, reports the results of the first international round robin for the quantification of urinary and plasma hepcidin assays.20 This is an essential step towards the standardization of hepcidin determination and eventual introduction of hepcidin assays in the clinical practice. As often the case in biological research, the results of the study are double-faced. The bad news is that wide differences between methods were observed in the absolute hepcidin concentrations. Differences between methods may be due to several reasons, the most important being the heterogeneity of the hepcidin standards used by different laboratories, and the variable capacity of different methods to detect the hepcidin-20 and hepcidin-22 isoforms in addition to the bioactive hepcidin-25. The good news is that the between-sample variation and the analytical variation of the methods are similar, indicating that all methods are suitable to distinguish hepcidin levels of different samples. These results, however, represent only an initial step and more work is needed for a better standardization of the assays and to define clinical applications. Assay standardization requires that the role of different hepcidin isoforms (do different isoforms influence test results and what is their role in the pathophysiology of disorders of iron metabolism?), as well as the influence of circadian variations in serum concentration and urinary excretion of the peptide are more clearly defined. In addition, we need to know which, among serum concentration and urinary excretion, is a more accurate biomarker of iron metabolism.21 To date, most studies on hepcidin determination have been performed through the evaluation of hepcidin urinary excretion in small series of patients. Clinical applications require that, following assay standardization, results are confirmed by studies in larger series of patients.
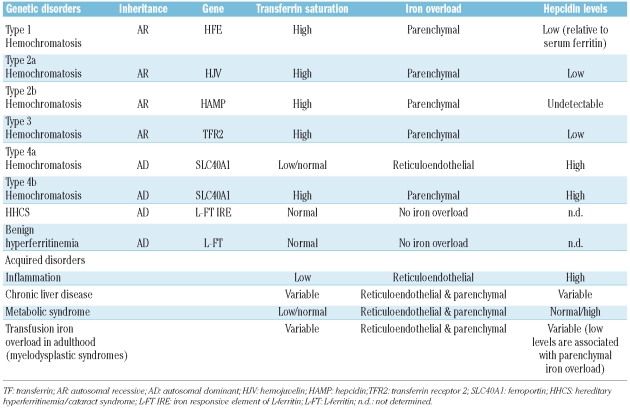
Hopefully, the above studies will provide relevant information on the pathophysiology of several disorders of iron metabolism, including often overlooked conditions such as liver disease, alcohol abuse, obesity and the metabolic syndrome in which it has been known for a long time that patients may have high serum ferritin levels and some degree of iron overload, but the underlying mechanisms responsible for this alteration of iron homeostasis remain elusive. In addition, hepcidin assays could be useful in the differential diagnosis of primary forms of iron overload and of genetic and acquired disorders associated with hyperferritinemia (Table 1),22 in the detection of iron deficiency in patients with inflammation, a condition in which traditional parameters of iron status such as serum ferritin and transferrin saturation are not reliable, and for the prediction of response to treatment with erythropoiesis stimulating agents (ESA). In patients undergoing treatment with ESA, in fact, the increase in red cell production and hemoglobin level is preceded by an expansion of erythropoiesis that, in turn, stimulates iron absorption/mobilization through the suppression of hepcidin production. Finally, in adult patients with transfusion iron overload, e.g. those with myelodysplastic syndromes receiving blood transfusions,23 inappropriately low hepcidin levels might indicate a high risk of parenchymal iron loading and the need for iron chelation therapy.
Table 1.
Expected behavior of hepcidin in genetic and acquired disorders characterized by hyperferritinemia without anemia (adapted from Camaschella and Poggiali, 2009).22
Footnotes
The work was supported by a grant from Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Dr. Bergamaschi is a Consultant Physician at the Department of Medicine, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. Dr. Villani is a Research Fellow at the same institution.
No potential conflict of interest relevant to this article was reported.
References
- 1.Zhang A-S, Enns CA. Iron Homeostasis: recently identified proteins provide insight into novel control mechanisms. J Biol Chem. 2009;284:711–5. doi: 10.1074/jbc.R800017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–50. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 3.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–10. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 4.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–9. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 5.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98:8780–5. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Constante M, Layoun A, Santos MM. Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood. 2009;113:3593–9. doi: 10.1182/blood-2008-08-173641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105:1803–6. doi: 10.1182/blood-2004-08-3042. [DOI] [PubMed] [Google Scholar]
- 9.Piperno A, Girelli D, Nemeth E, Trombini P, Bozzini C, Poggiali E, et al. Blunted hepcidin response to oral iron challenge in HFE-related hemochromatosis. Blood. 2007;110:4096–100. doi: 10.1182/blood-2007-06-096503. [DOI] [PubMed] [Google Scholar]
- 10.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 11.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–5. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, et al. Hepcidin in iron overload disorders. Blood. 2005;105:4103–5. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoller H, McFarlane I, Theurl I, Stadlmann S, Nemeth E, Oxley D, et al. Primary iron overload with inappropriate hepcidin expression in V162del ferroportin disease. Hepatology. 2005;42:466–72. doi: 10.1002/hep.20775. [DOI] [PubMed] [Google Scholar]
- 15.van Dijk BA, Laarakkers CM, Klaver SM, Jacobs EM, van Tits LJ, Janssen MC, Swinkels DW. Serum hepcidin levels are innately low in HFE-related haemochromatosis but differ between C282Y-homozygotes with elevated and normal ferritin levels. Br J Haematol. 2008;142:979–85. doi: 10.1111/j.1365-2141.2008.07273.x. [DOI] [PubMed] [Google Scholar]
- 16.Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T. The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study. Blood. 2006;107:328–33. doi: 10.1182/blood-2005-05-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006;290:G199–203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kemna EHJM, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93:90–7. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- 19.Piperno A, Mariani R, Trombini P, Girelli D. Hepcidin modulation in human diseases: From research to clinic. World J Gastroenterol. 2009;15:538–51. doi: 10.3748/wjg.15.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroot JJC, Kemna EHJM, Bansal SS, Busbridge M, Campostrini N, Girelli D, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94:1748–52. doi: 10.3324/haematol.2009.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;3:620–8. doi: 10.1373/clinchem.2006.079186. [DOI] [PubMed] [Google Scholar]
- 22.Camaschella C, Poggiali E. Towards explaining “unexplained hyperferritinemia”. Haematologica. 2009;94:307–9. doi: 10.3324/haematol.2008.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cazzola M, Della Porta MG, Malcovati L. Clinical relevance of anemia and transfusion iron overload in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2008;2008:166–75. doi: 10.1182/asheducation-2008.1.166. [DOI] [PubMed] [Google Scholar]