Abstract
A series of three- and four-coordinate iron(II) complexes with nitrogen, chlorine, oxygen, and sulfur ligands is presented. The electronic variation is explored by measuring the association constant of the neutral ligands, and the reduction potential of the iron(II) complexes. Varying the neutral ligand gives large changes in Keq, which decrease in the order CNtBu > pyridine > 2-picoline > DMF > MeCN > THF > PPh3. These differences can be attributed to a mixture of steric effects and electronic effects (both σ and π). The binding constants and the reduction potentials are surprisingly insensitive to changes in an anionic spectator ligand. This suggests that three-coordinate iron(II) complexes may have similar binding trends as proposed three-coordinate iron(II) intermediates in the FeMoco of nitrogenase, even though the anionic spectator ligands in the synthetic complexes differ from the sulfides in the FeMoco.
Introduction
Nitrogenases are fascinating iron metalloenzymes that have the ability to convert dinitrogen to ammonia at ambient temperature and pressure.1,2 They also reduce a variety of organic substrates such as alkynes, nitriles, cyanide, isocyanide, CO2, azide, nitrous oxide and cyclopropene.3 So far, scientists have not unambiguously determined the binding location of any substrate in a nitrogenase enzyme.
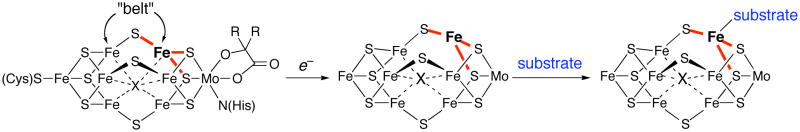
The current “best guesses” for substrate binding sites are based on a 1.16 Å resolution structure of the resting state of the iron-molybdenum nitrogenase from A. vinelandii.4 The active site of the iron-molybdenum enzyme is a cluster called the FeMo cofactor (FeMoco), identified as MoFe7S9X(homocitrate), where X represents C4−, N3−, or O2− (Figure 1). Other nitrogenases lack molybdenum but have a similarly shaped cluster, based on EXAFS evidence.5
Figure 1.
The FeMoco of iron-molybdenum nitrogenase in the resting state.4 X is a light atom (C, N, or O) that lies 2.0 Å from the six “belt” iron atoms. The iron atoms along the “belt” are coordinated to three bridging sulfides. We hypothesize that reduction of the cofactor transiently gives a three-coordinate iron site that can bind substrate. 6
One reasonable hypothesis for the location of substrate binding on the FeMoco is the iron atoms in the “belt” of the enzyme, and this idea has been supported by the substrate dependence of nitrogenase mutants.7,8 Even though the belt iron atoms are four-coordinate in the crystallographically characterized resting state, they could become three-coordinate and coordinatively unsaturated in the reduced form that binds substrates.9 This idea has motivated the study of isolable three-coordinate iron(II) complexes that mimic the “activated” three-coordinate sites on the FeMoco.6,10,11,12
Though it might be most desirable to study iron complexes with three sulfur donors, the available S-coordinated three-coordinate Fe complexes have a highly congested iron environment, and are prone to ligand dissociation.13,14 We have focused instead on three-coordinate complexes in which two of the donors come from a bulky β-diketiminate ligand (LtBu,iPr2, Scheme 1), because these complexes are easy to prepare, highly crystalline, and suitable for detailed spectroscopic and mechanistic analysis.15 One potential point of concern is that the identity of the donor atoms to iron is different in the β-diketiminate complexes (N donors) than in the FeMoco (S donors). It is reasonable to suspect that this difference in spectator ligands might influence the binding constants and redox potentials, invalidating the β-diketiminate complexes as nitrogenase mimics. Therefore, it is of interest to learn the influence of spectator ligands in binding to three-coordinate iron complexes. In this paper, we vary one of the three donors in three-coordinate iron complexes, and measure the binding constant (Kassoc) of a number of neutral ligands in order to gain systematic knowledge about the trends in these complexes.
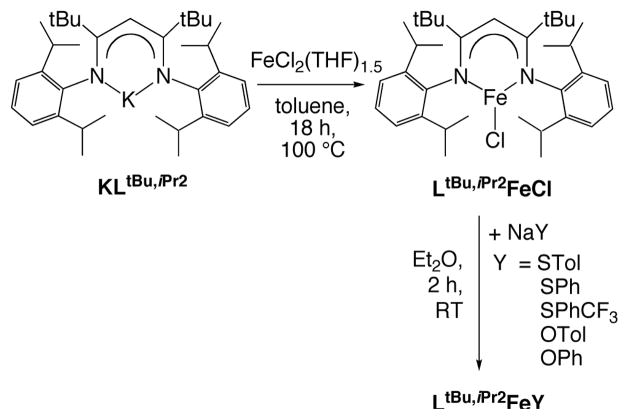
Scheme 1.
There are more general implications as well. First, the binding preferences of trigonal-planar iron complexes are not well understood.16,17 Also, the influence of spectator ligands on binding constants of neutral ligands is of general interest in coordination chemistry and catalysis, and has rarely been studied quantitatively for a range of different types of ligands. Binding of neutral ligands to porphyrin-iron complexes has been studied in some detail, but can be complicated by spin state changes upon binding.18,19,20 In contrast, the iron(II) complexes studied here are always high-spin as three- or four-coordinate complexes.
Results
Synthesis of Three-coordinate FeII Complexes
We have shown that LtBu,iPr2FeCl21 is a versatile starting material for the synthesis of a variety of three-coordinate iron(II) compounds.15 In previous studies, LtBu,iPr2FeCl was synthesized from reaction of FeCl2(THF)1.5 and the lithium salt of the diketiminate ligand, producing LiCl as a byproduct. Unfortunately, the solubilities of LiCl and LtBu,iPr2FeCl are not different enough to enable easy separation of the compounds without Soxhlet extraction, which is inconvenient on a large scale. Here, this difficulty is avoided by using the potassium salt of the bulky β-diketiminate ligand (KLtBu,iPr2). This potassium salt was synthesized by treating the diimine LtBu,iPr2H with benzylpotassium22 in diethyl ether.23 Reaction of KLtBu,iPr2 with FeCl2(THF)1.5 in toluene reproducibly gave a clean product from which KCl could be removed by filtration. LtBu,iPr2FeCl was isolated as red crystals in very high yield (97%).
Double-metathesis reactions of LtBu,iPr2FeCl led to three-coordinate iron-thiolate (LtBu,iPr2FeSPh, LtBu,iPr2FeSPhCF3, LtBu,iPr2FeSTol) and phenoxide (LtBu,iPr2FeOPh, LtBu,iPr2FeOTol)24 complexes. In some of these compounds, para-substituents on the aryl rings were used to enable isolation of single crystals, or to introduce an electronic effect (see below). Reactions of LtBu,iPr2FeCl and 1 equiv of the appropriate sodium salt were carried out in diethyl ether at room temperature. The complexes were isolated as red-orange crystals by cooling pentane solutions, in yields of 70–85%. The purity of these compounds was typically ascertained using UV-vis spectroscopy, which showed a characteristic visible band in each complex between 500 – 600 nm, and using 1H NMR spectroscopy.
As with other three-coordinate iron(II) diketiminate complexes,15 the 1H NMR spectra had peaks over a range from roughly +120 to −120 ppm. The signals could be integrated to give tentative assignments. In cases where this left ambiguity, resonances were assigned based on proximity to the paramagnetic iron(II) center (protons closer to the metal center are shifted further from 0 ppm).15 The number of resonances in the spectra are as expected for averaged C2v symmetry, indicating rapid rotation around the Fe-S, Fe-O, S-C, and O-C bonds on the NMR time scale. The Evans method25,26 was used to calculate the solution magnetic moments, which were 5.5 ± 0.4 Bohr magnetons for all compounds. This indicates a ground state of S=2, consistent with the expectations for high-spin Fe(II) with substantial orbital angular momentum, as studied in detail for LtBu,iPr2FeCl and LtBu,iPr2FeCH3.27
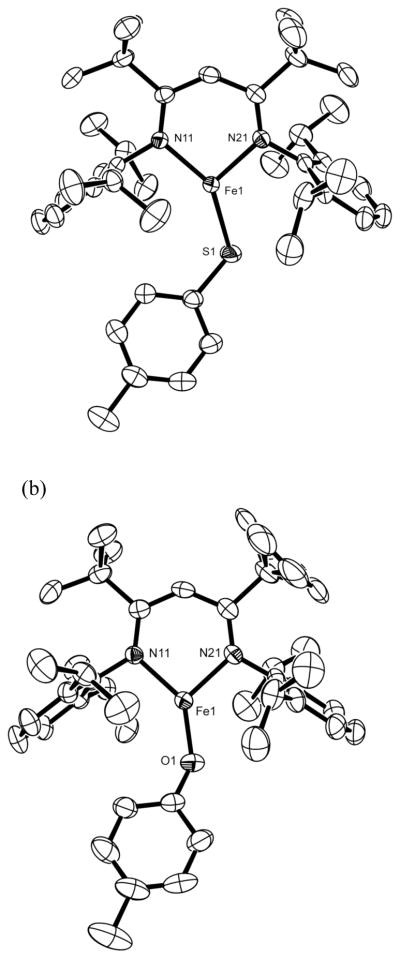
The molecular structures of LtBu,iPr2FeSPh, LtBu,iPr2FeSTol, LtBu,iPr2FeSPhCF3, and LtBu,iPr2FeOTol were determined by X-ray crystallography. Thermal-ellipsoid plots of the crystallographic models are shown in Figure 2, and relevant bond lengths and angles are given in Table 1. The iron centers are planar, and the sum of the three angles is greater than 359°.21,24,28 Interestingly, the sulfur atoms lie off the C2 axis of the diketiminate-iron group, giving N-Fe-S angles that differ by 25°, 28° and 30° in the three thiolate complexes. The cause for this “T” shape may be that the sulfur atom prefers a relatively small angle. The Fe-S-C angles in our complexes range from 110–115°, which fall close or within a standard deviation of the average Fe-S-C angle, 110(4)°, in the Cambridge Structural Database (CSD). To accommodate a thiolate ligand with this Fe-S-C angle between the bulky aryl rings of the diketiminate, the iron coordination must distort. In the aryloxide complex LtBu,iPr2FeOTol, the angle at oxygen is somewhat larger at 138.8(2)°, also within a standard deviation of the average Fe-O-C angle in the CSD, 133(8)°. Because the larger angle at oxygen causes less steric pressure on the iron coordination geometry the N-Fe-O angles in the aryloxide complex are more similar (Δ ~ 15°) than in the more distorted thiolates (Δ ~ 25–30°).
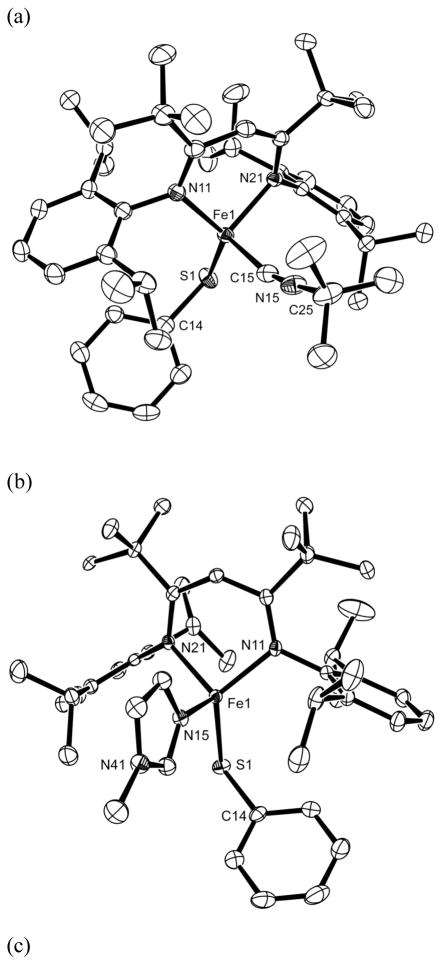
Figure 2.
ORTEP drawings of the molecular structures of (a) LtBu,iPr2FeSTol and (b) LtBu,iPr2Fe(OTol). Analogous drawings of LtBu,iPr2Fe(SPh) and LtBu,iPr2Fe(SPhCF3) are given in the Supporting Information. Thermal ellipsoids are shown at 50% probability. Hydrogen atoms have been omitted for clarity.
Table 1.
Relevant bond lengths and angles. E represents S or O.
| Bond/Angle | LtBu,iPr2Fe(SPh) | LtBu,iPr2Fe(STol) | LtBu,iPr2Fe(SPhCF3) | LtBu,iPr2Fe(OTol) |
|---|---|---|---|---|
| Fe-N (Å) | 1.959(2), 1.969(2) | 1.969(1), 1.971(1) | 1.960(1), 1.968(1) | 1.965(2), 1.972(2) |
| Fe-E (Å) | 2.2523(6) | 2.2584(5) | 2.2642(5) | 1.833(2) |
| N-Fe-N(°) | 95.20(6) | 94.49(5) | 94.94(5) | 94.76(8) |
| N-Fe-E (°) | 144.69(5), 119.98(5) | 146.28(4), 118.56(4) | 146.97(4), 117.13(4) | 140.20(8), 125.01(9) |
| Fe-E-C (°) | 110.06(7) | 114.37(5) | 114.70(5) | 138.8(2) |
Cyclic Voltammetry Studies of Iron(II) Complexes
The redox chemistry of the three-coordinate complexes was examined using cyclic voltammetry in diethyl ether (Et2O) with 0.1 M NBu4BArF as electrolyte (BArF = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate). The use of this unusual solvent/electrolyte combination is necessary because more polar solvents like THF and acetonitrile (MeCN) coordinate to the iron atom, as shown below.
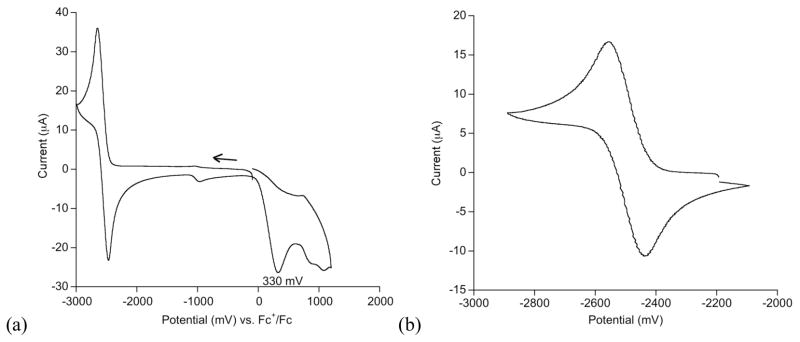
The complexes show reversible reductions at low potential that we ascribe to an iron(II)/iron(I) couple (Figure 3). Peak separation (Epa-Epc) values for the iron(II)/iron(I) couple are between 100 mV and 200 mV, which is typical for solvents of relatively low polarity.29 Additionally, an irreversible oxidation (Epa) occurs between +0.3 to +0.5 V (for example, see Figure 3a). We have not explored the oxidation products.
Figure 3.
Voltammograms of LtBu,iPr2FeCl in Et2O with 0.1 M NBu4BArF electrolyte. (a) Full scan at 500 mV/s. (b) The Fe2+/1+ wave at 100 mV/s. Other voltammograms are given in the Supporting Information.
The cyclic voltammetry of the complexes was also measured in acetonitrile (MeCN) with 0.1 M NBu4PF6 electrolyte. As discussed below, MeCN binds to these three-coordinate complexes to give a four-coordinate solvent adduct at high concentrations of MeCN. Therefore, results obtained in MeCN reflect the redox behavior of the four-coordinate complex LtBuFe(Y)(NCMe). These four-coordinate complexes showed a quasireversible reduction at low potential and an irreversible oxidation (Epa) at 0 to +0.3 V. The only difference between the different complexes is that LtBu,iPr2FeOPh possesses an additional quasireversible response corresponding to an oxidation at E1/2 = −93 mV.
The E1/2 values for the iron(II)/iron(I) couple in both solvents are shown in Table 2. These values are averages from scans with sweep rates between 100 – 1000 mV/s, and are referenced to the ferrocene couple (+0.64 V vs. NHE).30 A precipitate formed during the measurement of LtBu,iPr2Fe(OPh)(NCMe), which caused the peak to shift in the negative direction. This change is reflected in the larger error (±60 mV) associated with the LtBu,iPr2Fe(OPh) complex. Although these reduction potentials do not strictly represent thermodynamic potentials, they can be used for comparison of the complexes as described below in the Discussion section.
Table 2.
E1/2 values for reduction of complexes LtBu,iPr2FeY in Et2O/NBu4BArF and LtBu,iPr2FeY(NCMe) in MeCN/NBu4PF6. Potentials are given in volts relative to the ferrocenium/ferrocene couple at 0 V. 31
| Y | Et2O | MeCN |
|---|---|---|
| Cl | −2.46(1) | −2.32(2) |
| OPh | −2.56(3) | −2.27(6) |
| SPh | −2.41(2) | −2.30(6) |
| SPhCF3 | −2.40(2) | −2.33(1) |
Characterization of Four-coordinate Fe(II) Complexes
Each of the three-coordinate iron(II) complexes was capable of binding an additional neutral ligand. The amount of added ligand necessary to ensure binding was different depending on the ligand (see below), but was always accompanied by a change from red to an orange color.
The four-coordinate iron(II) complexes have broader 1H NMR spectra than the three-coordinate iron(II) complexes, and fall in a narrower chemical shift range (see Supporting Information). This was observed for every added ligand, suggesting that the broader peaks arise not from ligand exchange, but rather from slower electronic relaxation in the four-coordinate complexes. Consistent with this idea, 1H NMR spectra like these have been observed previously for four-coordinate iron(II) diketiminate complexes.32
In order to verify the geometry of the adducts, we determined the solid-state structures of LtBu,iPr2Fe(SPh)(L) (L = tert-butyl isocyanide, CNtBu; 1-methylimidazole, MeIm; N,N-dimethyl-formamide, DMF) using X-ray crystallography. Thermal-ellipsoid plots are shown in Figure 4, and important bond lengths and angles are listed in Table 3. The fourth donor binds from the direction normal to the plane of the three-coordinate complexes: the angles between the N2S plane and the Fe-L bond were 77°, 86°, and 83° in the three structures. This is consistent with the trigonal-pyramidal geometry observed previously in some ligand adducts of LtBu,iPr2FeCl.24,28
Figure 4.
ORTEP drawings of the molecular structures of (a) LtBu,iPr2Fe(SPh)(CNtBu), (b) LtBu,iPr2Fe(SPh)(MeIm) and, (c) LtBu,iPr2Fe(SPh)(DMF). Thermal ellipsoids are shown at 50% probability. Co-crystallized solvent in the LtBu,iPr2Fe(SPh)(MeIm) structure, and all hydrogen atoms, are omitted for clarity.
Table 3.
Important bond distances and angles for the four-coordinate Fe(II) complexes. L represents the fourth donor atom (C, N, or O).
| Bond/Angle | CNtBu | MeIm | DMF |
|---|---|---|---|
| Fe-N (Å) | 2.000(2), 2.013(2) | 2.021(2), 2.0262(19) | 2.0181(11), 2.0212(11) |
| Fe-S (Å) | 2.2776(15) | 2.2955(11) | 2.2877(4) |
| Fe-L (Å) | 2.089(2) | 2.094(2) | 2.0832(10) |
| N-Fe-N (°) | 96.19(5) | 96.85(8) | 95.44(4) |
| S-Fe-L (°) | 118.04(7) | 125.58(6) | 110.31(3) |
| Fe-S-L (°) | 104.36(6) | 107.05(8) | 112.94(5) |
The greater coordination number in the ligand adducts increased the lengths of the bonds to the spectator ligands. The Fe-S bond length on average increased 0.035(2) Å while the Fe-N bonds increased 0.0526(4) Å from the three-coordinate thiolate complex LtBu,iPr2Fe(SPh). The difference between the two N-Fe-S angles within the same complex ranged from 12° – 18°, as in the three-coordinate thiolate complexes. The M-S-C angles are typical of those found in the CSD (109 ± 4°).
Binding Constants of Neutral Donors to LtBu,iPr2FeCl, LtBu,iPr2Fe(SPh), LtBu,iPr2Fe(SPhCF3), and LtBu,iPr2Fe(OPh)
We determined the trends in the binding constants for added neutral ligands, as a function of the added ligand (L) and of the anionic spectator ligand (Y). To carry out the binding studies we monitored the visible absorbance peak of the three-coordinate iron complexes using UV-vis spectrophotometry.
For these experiments, 10 mM stock solutions of the three-coordinate complexes in toluene were sequentially diluted to give samples that were 1.0 mM in LtBu,iPr2FeY. The donor ligand, L, was present in amounts varying from 0.25 mM to 1.0 M. A color change from red to orange is observed depending on the particular ligand and amount added. This corresponds to the disappearance of the absorbance peak in the 500 – 600 nm range. An isosbestic point is observed near 500 nm because of the growth of a shoulder in the four-coordinate complex around 400 nm (see Supporting Information). Equilibrium is immediately established between the three-coordinate complex (M) and the four-coordinate complex (ML) in solution (eq 1) upon addition of L. The equilibrium constant (Keq) can be represented as shown in Equations 1 and 2.
| (1) |
| (2) |
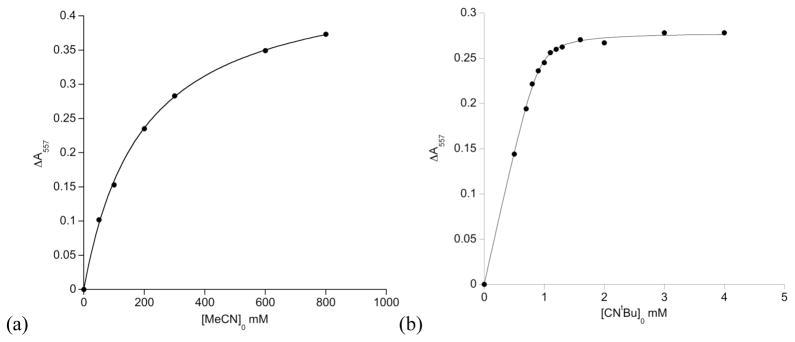
While no clear absorbance band is associated with the four-coordinate complex, its concentration can be calculated from the loss of absorbance (ΔAλmax) of the distinct peak of the three-coordinate complex near 550 nm with addition of L. The value of ΔAλmax is plotted against the initial concentration of ligand added, [L]0, to produce a binding curve (Fig 5). Data were only used if an isosbestic point was observed.
Figure 5.
Binding curves for (a) LtBu,iPr2FeSPh to acetonitrile, an example of weak binding, and (b) LtBu,iPr2FeSPh to CNtBu, an example of strong binding.
The equilibrium constant (Keq) is determined by fitting the binding curves to one of two equations depending on whether the ligands bind weakly or strongly to LtBu,iPr2FeY. A ligand that binds most of the iron at one equivalent of L is defined as being “strong binding.” Ligands that show less than one-half binding of iron at one equivalent are considered “weak binding.”
In the weak binding scenario, the concentration of ML at equilibrium can be represented by taking the difference of initial and final concentration of M ([ML] = [M]0 − [M]f). This can be incorporated into an equation to fit a binding curve (equation 3).
| (3) |
In equation 3, [L]0 is substituted for [L], because in the weak binding situation the concentration of free ligand at equilibrium [L] is virtually identical to the initial concentration of ligand [L]0. However, this assumption is not valid under strong binding conditions. Therefore, eq 4 was formulated taking into account this difference.
| (4) |
For these experiments, we were interested in learning about the dependence of the binding constant on (a) the type of neutral donor ligand L, and (b) the nature of the spectator ligand Y. To address point (a), a number of representative ligands L were chosen with different donor atoms, steric size, and electronic properties. The values of Keq were determined for binding to both LtBu,iPr2FeCl and to LtBu,iPr2Fe(SPh), to ensure that any trends seen were not peculiar to a single choice of Y. To address point (b), two of the ligands (MeCN and DMF) were evaluated across a broader range of supporting ligands Y, including the phenolate complex and the CF3-substituted thiophenolate complex.
Irrespective of Y, added ligands PPh3, THF, MeCN, DMF and 2-picoline were appropriate for use of the “weak binding” equation (Eq. 3) and exhibited Keq values between 0.15 and 660. Pyridine and CNtBu were fitted with the “strong binding” equation (Eq. 4) and were associated with Keq values above 104. These data are summarized in Table 4.
Table 4.
Calculated binding constants for neutral ligands to LtBu,iPr2FeCl, LtBu,iPr2Fe(SPh), LtBu,iPr2Fe(SPhCF3), and LtBu,iPr2Fe(OPh).
| Ligand | LtBu,iPr2FeCl Keq | LtBu,iPr2Fe(SPh) Keq | LtBu,iPr2Fe(SPhCF3) Keq | LtBu,iPr2Fe(OPh) Keq | Donor Atom | ||
|---|---|---|---|---|---|---|---|
| PPh3 | 0.70 ± 0.08 | 1.0 ± 0.5 | 0.7 ± 0.5 | P | |||
| THF | 0.80 ± 0.01 | 0.15 ± 0.01 | 5.3 ± 0.1 | O | |||
| MeCN | 9.8 ± 0.5 | 5.2 ± 0.2 | 1.9 ± 0.1 | 11.7 ± 0.9 | 4.7 ± 0.4 | N | |
| DMF | 390 ± 20 | 160 ± 5 | 2.4 ± 0.1 | 230 ± 10 | 160 ± 10 | O | |
| 2-picoline | 660 ± 50 | 300 ± 30 | 2.2 ± 0.1 | N | |||
| Pyridine | 41000 ± 6000 | 11000 ± 2000 | 3.7 ± 0.3 | N | |||
| CNtBu | 73000 ± 27000 | 41000 ± 7000 | 1.8 ± 0.7 | C |
The values of Keq are highly dependent on the neutral ligand L. However, the Keq values are similar with the same added ligand L and different anionic ligands Y. Equivalently, the trends of L binding are similar no matter which Y is used. The similarity in the trends is most evident when comparing Keq values of LtBu,iPr2FeCl and LtBu,iPr2Fe(SPh), where the Keq values for LtBu,iPr2FeCl are approximately twice that of LtBu,iPr2Fe(SPh).
Discussion
Three-coordinate Iron(II) Thiolate Complexes
The three LtBu,iPr2FeSAr complexes described here add to the few examples of three-coordinate iron thiolate complexes. The most common S-coordinated iron complexes with a coordination number of three are Fe2(μ-SR)2 dimers.33,34,35,36,37,38 Previous three-coordinate iron thiolates have extremely bulky ligands that enable them to resist the tendency of thiolates to bridge between metals.13,28,36,39,40 The SR group in most literature compounds is a substituted thiophenolate with bulky tert-butyl groups in the ortho- and para-positions. Here, on the other hand, the use of a bulky β-diketiminate ligand enables the use of smaller thiolates.
Binding of Neutral Ligands to Low-Coordinate Iron
In this paper we report the binding constants for a range of ligands to low-coordinate Fe(II) complexes, determined using UV-vis spectroscopy. Binding of the added donor ligand is instantaneous as observed by the immediate color change of the solution. Crystal structures show that the ligand binds to LtBu,iPr2FeY in a 1:1 stoichiometry. The 1:1 binding is further supported by the observation of an isosbestic point when comparing samples at different concentrations of added ligand (see Figures S3 – S6 for examples).
The measured values of Keq varied greatly with variation of the neutral ligand, but were independent of the donor atom and hybridization of the donor atom in the neutral ligand. In an effort to discern what factors were responsible for the observed trend, we explored both steric and electronic explanations in a systematic fashion.
Steric Effect of Ligands
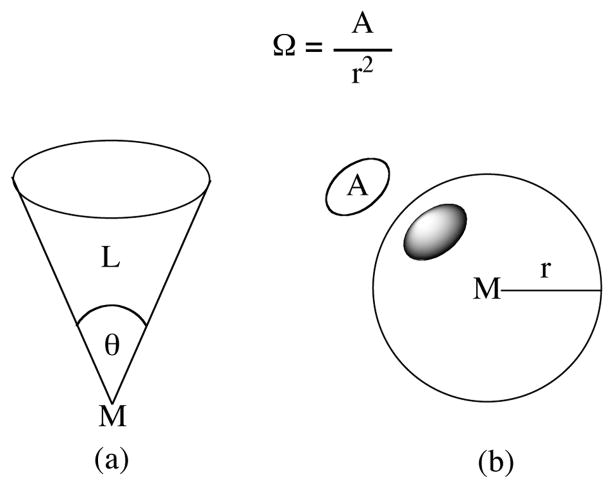
Steric contributions were surveyed by calculating the cone and solid angles of the fourth donor ligands. The more familiar cone angle (θ), as described by Tolman,41 describes the amount of space a ligand occupies by measuring the angle created at the apex of a cone that completely contains the ligand (Figure 6a). The concept of a solid angle (Ω) is less familiar, but is perhaps a more accurate way to represent the steric congestion of a ligand by taking the ligand’s shape into account. It is measured in steradians and represented by Eq. 5 where A is the area of a circle created on a sphere if a light is shone on the ligand from a metal located at the center of the sphere (Figure 6b).
Figure 6.
Graphical representations of a cone angle (a) and solid angle (b).
| (5) |
The cone angles and solid angles for ligands of interest were calculated using the program SolidG, provided by Ilia Guzei.42 For each ligand, we chose ten representative structures (listed in the Supporting Information) containing the ligand of interest in the CSD. The values for each ligand were calculated and averaged to give the values shown in Table 5.
Table 5.
Calculated solid and cone angles of ligands used in this study.
| Ligand | Solid Ang (Ω) | Cone Ang (θ) | Keq(Cl) |
|---|---|---|---|
| PPh3 | 3.32(6) | 124(1) | 0.70 |
| THF | 1.81(6) | 89(2) | 0.80 |
| MeCN | 1.60(0) | 83.69(3) | 9.80 |
| DMF | 1.63(4) | 85(1) | 390 |
| 2-Picoline | 2.36(3) | 102.8(6) | 660 |
| Pyridine | 1.90(2) | 91.6(5) | 41000 |
| CNtBu | 1.65(1) | 85.0(3) | 90000 |
Despite a variety of spectator ligands in the reference complexes (see Supporting Information for full lists of reference complexes), there was little variation in the derived cone angle and solid angle values for the same target ligand. A further non-systematic survey confirmed that the coordination number and metal type do not significantly affect the cone and solid angle calculated for a given ligand. In addition, the cone angle and solid angle correlate quite well with one another. So, these values are likely to be accurate representations of the relative amount of steric hindrance caused by each of these ligands in the four-coordinate complexes LtBu,iPr2Fe(Y)(L).
The size of most ligands was similar: aside from 2-picoline and triphenylphosphine, the solid angles varied in the narrow range 1.60 to 1.90. Given the wide variation in Keq between the ligands of similar size, it is clear that steric effects are not dominant in this system. However, the two largest ligands are insightful. 2-Picoline was chosen as a ligand that is electronically similar to pyridine, but is substantially larger (solid angle of 2.36 vs. 1.90). The added size reduces the binding constant by a factor of 30–60. In addition, the bulkiest ligand, PPh3, was the most weakly binding. Overall, steric effects have an influence on Keq for 2-picoline and PPh3, but do not explain the trends with the other neutral ligands.
Electronic Properties of Ligands: σ-Effects
In order to rationalize the σ-bonding capacity of different ligands, we compared the binding constants to the gas-phase proton affinities of ligands as well as the pKa of their conjugate acids. We were not able to find a literature value for the pKa of protonated t-butyl isocyanide in MeCN, and so it was calculated using a recently reported semiempirical DFT method. 43 The relevant values are shown in Table 6.
Table 6.
H+ affinity values and pKa values in MeCN for the ligands studied.
| Ligand | H+ affinitya (kJ/mol) | pKa (MeCN) | Keq(Cl) |
|---|---|---|---|
| PPh3 | 972.8 | 7.61b | 0.7 |
| THF | 822.1 | 1.1c | 0.8 |
| MeCN | 779.2 | 0 | 9.8 |
| DMF | 887.5 | 6.1c | 390 |
| 2-picoline | 949.1 | 13.32c | 660 |
| Pyridine | 930 | 12.53b | 41000 |
| CNtBu | 870.7 | 2.71d | 90000 |
Hunter, E. P.; J. Phys. Chem. 1998, 27, 3991–3999.
Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. J. Org. Chem. 2005, 70, 1019–1028.
Izutsu, K. Acid-Base Dissociation Constants in Dipolar Aprotic Solvents; Blackwell Scientific: Oxford, 1990, pp 17–35.
Calculated: see ref 43 and Experimental Section.
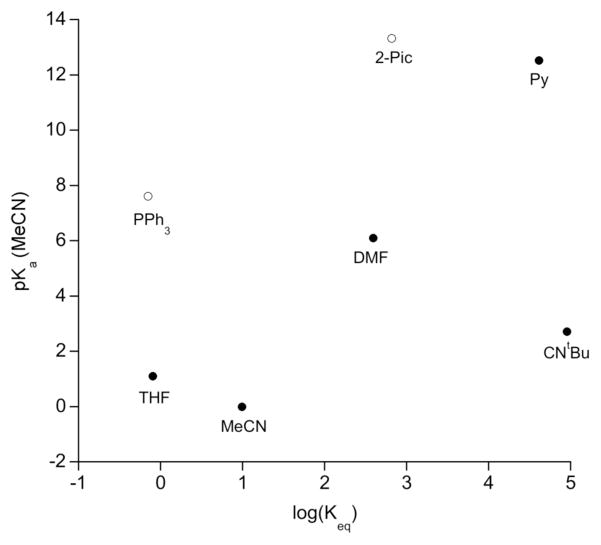
No correlation was found between Keq and the proton affinities. There was a weak correlation of Keq with the pKa values if CNtBu and the bulky ligands are left out (Figure 7). Therefore, the ligands that bind most tightly to a proton also bind most tightly to the iron atom. However, the large deviation of the isocyanide indicates that σ-effects are not the sole explanation for the electronic effects on Keq.
Figure 7.
Plot of log(Keq) vs. pKa (MeCN) for different ligands L. As discussed above, PPh3 and 2-picoline fall from the trend due to steric congestion in PPh3 and 2-picoline (Table 5). The deviation of CNtBu may be attributable to π-backbonding (see below).
Electronic Properties of Ligands: π-Effects
t-Butyl isocyanide is the strongest π-acceptor ligand studied here and has the largest equilibrium constant, suggesting that π-backbonding might account for the remaining deviation from the trends discussed above. Previous binding studies of low-coordinate iron(I) to alkynes and alkenes showed that π-backbonding was the dominant factor determining the affinity of different ligands.44 A very recent computational study has found that the binding constants in iridium systems can be related to backbonding. 45
Out of the ligands studied, the ability to accept electron density is greatest for CNtBu.46 Low-lying LUMOs are also present in pyridine and PPh3, but in the last case the great steric hindrance of the phenyl groups on the phosphine prevents this ligand from binding strongly. In attempts to quantitatively compare the π-backbonding ability of these ligands, the carbonyl stretch values of tungsten pentacarbonyl complexes (W(CO)5L) containing the ligand of interest were collected (see Table 7). Unfortunately, there was no evident correlation between this measure of backbonding ability and the equilibrium constant for binding to three-coordinate iron.
Table 7.
Collected CO stretch values (cm−1) of W(CO)5L.
| W(CO)5L | A1 | A1 | E | Keq(Cl) |
|---|---|---|---|---|
| PPh3a | 2072 | 1942 | 1939 | 0.7 |
| THFb | 2074 | 1912 | 1933 | 0.8 |
| MeCNc | 2083 | 1931 | 1948 | 9.8 |
| DMFc | 2067 | 1847 | 1917 | 390 |
| Pyridined | 2076 | 1980 | - | 41000 |
| CNtBue | 2065 | 1922 | 1952 | 90000 |
Bancroft, G. M.; Dignard-Bailey, L.; Puddephatt, R. J. Inorg. Chem. 1986, 25, 3675–3680.
We substituted the value known for dihydrofuran: Paur-Afshari, R.; Lin, J.; Schultz, R. H. Organometallics 2000, 19, 1682–1691.
Stolz, I. W.; Dobson, G. R.; Sheline, R. K. Inorg. Chem. 1963, 2, 323–326.
Kraihanzel, C. S.; Cotton, F. A. Inorg. Chem. 1963, 2, 533–540.
King, R. B.; Saran, M. S. Inorg. Chem. 1974, 13, 74–78.
In order to further test the importance of π-backbonding, LtBu,iPr2FeCl and LtBu,iPr2FeSPh were each treated with 1 atm of CO under a range of conditions. However, these reactions yielded starting material, decomposition products or diamagnetic products as observed by 1H NMR. Infrared spectroscopy of these reactions showed no absorbances from 1700 – 2600 cm−1 where C-O stretches of β-diketiminate iron(I) and iron(II) carbonyl compounds have been observed.10,47,48 Therefore, if CO binds at all to the three-coordinate iron complexes, it is a very weak interaction. Note that related three-coordinate iron-alkyl complexes react with CO to give a low-spin iron-acyl product from CO insertion, so weak CO interactions must be possible in some three-coordinate iron(II) compounds.47
Therefore, despite a likely influence of π-backbonding in isocyanide complexes, it is not the dominant factor either. Overall, the trends in the binding strength of neutral ligands are determined by a combination of steric and electronic effects, none of which dominates.
Steric and Electronic Effects from the Spectator Ligand Y
As noted above, there was a small increase in binding constants of neutral ligands to LtBu,iPr2FeCl as compared to the binding constants to LtBu,iPr2FeSPh. One could reasonably attribute this difference to electronic effects (the greater electronegativity of chlorine vs. sulfur), or to steric effects (the smaller size of chlorine vs. sulfur). Even though the effect was small, we took this opportunity to compare the binding constants with two additional complexes: one with an electron-withdrawing substituent on the arylthiolate, LtBu,iPr2Fe(SC6H4CF3), and one with oxygen in place of sulfur, LtBu,iPr2Fe(OPh). Both of these ligands are nearly isosteric with the arylthiolate ligand in LtBu,iPr2FeSPh.
Because the binding constants are most precise with weak-binding ligands, we used MeCN and DMF as added neutral donors across the range of spectator ligands Y. The binding curves using LtBu,iPr2Fe(OPh) and LtBu,iPr2Fe(SPhCF3) yielded Keq values that were similar to the chloride and thiophenolate complexes (see Table 4 above). No clear trends are discernable, casting doubt on an electronic explanation for the small difference between binding to thiolate and to chloride complexes.
We also attempted to evaluate the electronic effect of Y on the complex using cyclic voltammetry (CV) to determine the reduction potential (the oxidations were irreversible). These studies were performed in both Et2O, to evaluate the three-coordinate species, and MeCN, to evaluate the four-coordinate species. The Fe2+/1+ waves were more reversible in Et2O than MeCN. This may indicate greater stability of the three-coordinate iron(I) species than the four-coordinate iron(I) species. Only small variations in potential are observed upon changing the anionic ligand Y. In the two solvents, these small shifts follow opposite trends. For example, LtBu,iPr2zFe(OPh) has the most negative reduction potential in Et2O, while LtBu,iPr2Fe(OPh)(NCMe) has the least negative reduction potential value in MeCN. The reduction potentials appear to be roughly 150 mV less negative in MeCN than in Et2O. However, because the waves in MeCN are quasireversible, it would be dangerous to draw any conclusion from this trend. Overall, the similarity of the potentials with different Y suggests that the electronegativity of the third donor does not significantly influence the electronic properties of the metal center.
Implications for Modeling Nitrogenase
As described in the Introduction, our group has used diketiminate complexes as mimics of one- and two-iron “belt” sites on nitrogenase. 6 This has naturally raised questions about the use of nitrogen donors to mimic a sulfur-coordinated iron site. In this study, the diketiminate was not varied, but one of the ligands was systematically changed over a range from “hard” oxygen and chlorine ligands to “soft” thiolate. This enabled us to experimentally gauge the influence of changing one spectator ligand on the binding ability and other properties of a three-coordinate iron complex.
In this context, it is interesting that the binding constants for a variety of neutral ligands are similar with oxygen, sulfur, or chloride spectator ligands in one position. This similarity was evident with neutral ligands that are π-donors (e.g. THF) or π-acceptors (e.g. CNtBu). The reduction potentials of the iron(II) complexes were also similar over the range of spectator ligands. This insensitivity to the spectator ligand suggests that changes in donor, such as from diketiminates to thiolates or sulfides, may give only a small influence on binding energies and redox potentials. Of course, in the broader field of coordination chemistry, the identity of spectator ligands often influences binding energies and mechanisms:49,50 however, in the three-coordinate systems studied here, the effects of changing one ligand are surprisingly small.
In recent publications, Holland15 and Peters51 have explored geometric influences on the unusual electronic structures and reactivity of high-spin, late-metal complexes with three or four ligands, respectively. These works show that the geometry of the metal has a pronounced effect on its electronic structure, and it is likely that the geometric influences are much more important than the precise electronics of the spectator ligand in determining the properties of the resulting complex. Further systematic studies are needed in order to evaluate the influence of geometry on measurable chemical parameters like binding constants.
Experimental
Synthetic Methods
All manipulations were performed under a nitrogen atmosphere by standard Schlenk techniques or in an M. Braun glovebox maintained at or below 1 ppm of O2 and H2O. Glassware was dried at 150 °C overnight. 1H NMR data were recorded on a Bruker Avance 500 spectrometer (500 MHz) at 22 °C and referenced internally to residual protiated solvent (C6HD5 at δ 7.16 ppm). Peaks of these paramagnetic complexes are all singlets. Relative integrations of peaks and assignments are given except for the four-coordinate complexes, where overlap of peaks is too great to integrate accurately. Solution magnetic susceptibilities were determined at 294 K using Evans’ method.25,26 Electronic spectra were recorded between 400 nm and 800 nm on a Cary 50 UV-visible spectrophotometer, using screw-cap quartz cuvettes of 1 cm optical path length. Elemental analyses were performed by the Microanalysis Laboratory at the University of Illinois (Urbana, Illinois) or Columbia Analytical Services (Tucson, AZ). Infrared spectra (600–4000 cm−1) were recorded on KBr pellets in a Shimadzu FTIR spectrophotometer (FTIR-8400S).
Acetonitrile, pentane, diethyl ether, tetrahydrofuran (THF), and toluene were purified by passage through activated alumina and “deoxygenizer” columns obtained from Glass Contour Co. DMF was dried over 3Å molecular sieves. Deuterated benzene was dried over activated alumina in a bomb flask, and the alumina was filtered off into a storage container prior to use. Before use, an aliquot of each solvent (except acetonitrile) was tested with a drop of sodium benzophenone ketyl in THF solution. Celite and alumina were dried overnight at 200 °C under vacuum. FeCl2(THF)1.5 was synthesized by the method of Kern. 52 KLtBu,iPr2 was synthesized by adding benzylpotassium22 to HLtBu,iPr2 in diethyl ether.23
CV measurements were obtained using a Cypress Systems 3100 potentiostat. The working electrode was glassy carbon with a 1-mm diameter working area, and Ag wires were used as auxiliary and reference electrodes. All measurements were referenced with an internal ferrocene standard, and reported relative to the Cp2Fe+/Cp2Fe couple. Acetonitrile used for CV experiments was vacuum transferred after alumina treatment.
X-ray Crystallography
Single crystals were mounted on a glass capillary tube or fiber and mounted on a Siemens SMART CCD or Bruker SMART APEX II CCD Platform diffractometer for data collection under a cold N2 stream at 193(2) K or 100.0(1) K. Full data collection was carried out using MoKα radiation (graphite monocromator) with appropriate frame times ranging from 20–90 s and typical detector distance around 5.09 cm. Total data collection time was generally between 12 and 24 h. Structures were refined using SIR97 or SHELXS-97 and refined using SHELXL-97. Space groups were determined based on systematic absences and intensity statistics. A direct-methods solution was calculated which provided most non-hydrogen atoms from the E-map. Full-matrix least squares/difference Fourier cycles were performed which located the remaining non-hydrogen atoms. All non-hydrogen atoms were refined with anisotropic displacement parameters. All hydrogen atoms were placed in ideal positions and refined as riding atoms with relative isotropic displacement parameters.
Modified Procedure for LtBu,iPr2FeCl.21
Under an atmosphere of N2, KLtBu,iPr2 (0.50 g, 0.92 mmol), FeCl2(THF)1.5 (0.202 g, 0.919 mmol), and toluene (25 mL) were added to a 150-mL resealable tube. The mixture was heated in an oil bath at 100 °C for 18 h and turned very dark red in color. The solution was cooled, filtered through Celite, and concentrated to 7 mL. The solution was warmed to dissolve a small amount of solid formed during concentration, and cooled to −40 °C overnight to afford dark red crystals. The supernatant was decanted and the red solid was washed with 1 mL of pentane to remove trace LtBu,iPr2H, leaving LtBu,iPr2FeCl (0.531 g, 97%). 1H NMR (C6D6, 500 MHz): δ 104 (s, 1H, α-H), 42 (s, 18H, tBu), 2.6 (s, 4H, m-aryl), −27 (s, 12H, iPr methyl), −108 (s, 4H, iPr methine), −111 (s, 12H, iPr methyl), −115 (s, 2H, p-aryl). Occasionally, trace water gives up to 5% of LtBu,iPrH, which is observed as a white solid, or as peaks at 1–2 ppm of the 1H NMR spectrum. The most reliable way to gauge purity is UV-visible spectroscopy in toluene (λmax = 559 nm; ε= 63(3) M−1 cm−1).
LtBu,iPr2Fe(Y) (Y = SPh, STol, SPhCF3, OPh, OTol)
The appropriate thiophenol or phenol was treated with a slight excess of NaH (1.05 equiv), and stirred in THF for 2 h. The solution was filtered through Celite, and solvent was removed under vacuum.
To a stirring solution of LtBu,iPr2FeCl (0.0593 g, 0.100 mmol) in Et2O (5–10 mL), 1.0 equiv of the appropriate sodium salt was added. The resulting orange colored solution and suspension was stirred for 2 h. The solvent was evaporated under vacuum, and the residue was extracted with pentane (10 mL) and filtered through Celite to give a clear red-orange solution. This solution was concentrated to 5 mL, warmed briefly to achieve a homogeneous solution, cooled and placed in a freezer (−35 °C) to afford red-orange crystals. Specific yields are given below.
LtBu,iPr2Fe(SPh)
Yield 80%. 1H NMR (C6D6, 500 MHz): δ 87 (1H, α-H), 56 (2H, o-SPh), 50 (2H, m-SPh), 40 (18H, tBu), 6.1 (4H, m-Ar), −7.6 (1H, p-SPh), −24 (12H, iPr methyl), −99 (4H, iPr methine), −112 (12H, iPr methyl), −114 (2H, p-Ar) ppm. μeff (Evans, C6D6): 5.1(5) μB. UV-vis (Tol) λmax: 550 nm (51(3) M−1 cm−1). IR (KBr): 2960 (s), 2870 (m), 1624(w), 1577 (w), 1502 (m), 1473 (w), 1459 (w), 1430 (s), 1375 (m), 1354 (s), 1317 (m), 1217 (w), 1096 (w), 1024 (w), 780 (w), 736 (m), 697 (w) cm−1 Anal. Calcd for C41H58N2SFe: C, 73.84; H, 8.76; N, 4.20%. Found: C, 74.20; H, 9.01; N, 4.27%.
LtBu,iPr2Fe(STol)
Yield 75%. 1H NMR (C6D6, 500 MHz): δ 87 (1H, α-H), 54 (2H, o-STol), 50 (2H, m-STol), 45 (3H, CH3-STol), 40 (18H, tBu), 5.9 (4H, m-Ar), −24 (12H, iPr methyl), −99 (4H, iPr methine), −111 (12H, iPr methyl), −114 (2H, p-Ar) ppm. UV-vis (Tol.) λmax: 553 nm (45(2) M−1 cm−1). IR (KBr, cm−1): 2963 (s), 2867 (m), 1624 (m), 1535 (w), 1503 (m), 1485 (m), 1458 (m), 1430 (m), 1375 (m), 1355 (s), 1316 (m), 1218 (w), 1108 (w), 1087 (m), 932 (w), 805 (m), 762 (m). Anal. Calcd for C42H60N2SFe: C, 74.09; H, 8.88; N, 4.11%. Found: C, 73.78; H, 8.84; N, 4.04%.
LtBu,iPr2Fe(SPhCF3)
Yield 85%. 1H NMR (C6D6, 500 MHz): δ 84 (1H, α-H), 54 (2H, o-SPh), 49 (2H, m-SPh), 40 (18H, tBu), 7.9 (4H, m-Ar), −24 (12H, iPr methyl), −98 (4H, iPr methine), −111 (12H, iPr methyl), −118 (2H, p-Ar) ppm. μeff (Evans, C6D6): 4.9(5) μB. UV-vis (Tol) λmax: 563 nm (42(2) M−1 cm−1). IR (KBr cm−1): 2964 (s), 2873 (w), 1600 (m), 1503 (m), 1353 (s), 1323 (vs), 1158 (m), 1116 (m), 1091 (m), 1062 (w), 1014 (w), 827 (w), 800 (w), 781 (w). Anal. Calcd for C42H57N2SFe: C, 68.64; H, 7.82; N, 3.81%. Found: C, 68.37; H, 8.09; N, 3.80%.
LtBu,iPr2Fe(OPh)
Yield 78%. 1H NMR (C6D6, 500 MHz): δ 119 (1H, α-H), 94 (2H, o-OPh), 60 (2H, m-OPh), 42 (18H, tBu), −4.4 (4H, m-Ar), −7.2 (1H, p-OPh), −30 (12H, iPr methyl), −103 (2H, p-Ar), −118 (4H, iPr methine, 12H, iPr methyl) ppm. μeff (Evans, C6D6): 5.6(5) μB. UV-vis (Tol) λmax: 533 nm (56(3) M−1 cm−1). IR (KBr cm−1): 2959 (s), 2867 (w), 1588 (m), 1490 (s), 1430 (w), 1376 (m), 1359 (s), 1318 (m), 1294 (m), 1217 (w), 1180 (w), 1096 (w), 871 (w), 802 (w), 755 (m), 691 (w). Anal. Calcd for C41H58N2OFe: C, 75.67; H, 8.98; N, 4.30%. Found: C, 75.29; H, 8.90; N, 4.08%.
LtBu,iPr2Fe(OTol)
Yield 75%. 1H NMR (C6D6, 400 MHz): δ 117 (1H, α-H), 94 (2H, o-OTol), 83 (3H, CH3-OTol), 55 (2H, m-OTol), 41 (18H, tBu), −4.1 (4H, m-Ar), −29 (12H, iPr methyl), −101 (2H, p-Ar), −115 (4H, iPr methine, 12H, iPr methyl) ppm. UV-vis (Et2O) λmax: 531 nm (79(4) M−1 cm−1). IR (KBr cm−1): 2963 (s), 2870 (w), 1618 (w), 1502 (s), 1459 (w), 1430 (w), 1360 (s), 1319 (m), 1280 (m), 1105 (w), 934 (w), 873 (w), 821 (m), 779 (w), 762 (m). Anal. Calcd for C42H60N2OFe: C, 75.88; H, 9.09; N, 4.21%. Found: C, 74.83; H, 9.13; N, 4.22%. This compound is somewhat unstable in solution, depositing a solid over time. Therefore, its purity is suspect, consistent with the elemental analysis data.
LtBu,iPr2Fe(SPh)(CNtBu)
In a 20 mL scintillation vial, CNtBu (70 μL, 0.619 mmol) was added via syringe to a red-orange solution of LtBu,iPr2Fe(SPh) (81.0 mg, 0.122 mmol) in Et2O (8 mL). The solution was cooled to −35 °C overnight to give orange crystals (67.8 mg, 75%). 1H NMR (C6D6, 500 MHz): δ 33, 27, 25, 20, 13, 8.1, 4.0, 2.1, 1.2, 0.29, −4.4, −4.9, −17, −50 ppm. IR (KBr): 3057 (w), 2962 (vs), 2868 (m), 2168 (s), 2127 (m), 1618 (m), 1491 (m), 1473 (m), 1465 (w), 1431 (m), 1352 (s), 1315 (s), 1254 (w), 1215 (m), 1153 (w), 1105 (w), 1024 (w), 933 (w), 887 (w), 795 (w), 762 (w), 739 (w), 692 (w) cm−1. Anal. Calcd for C46H67N3SFe: C, 73.67; H, 9.00; N, 5.60%. Found: C, 72.87; H, 9.01; N, 5.58%.
LtBu,iPr2Fe(SPh)(DMF)
In a 20 mL scintillation vial, DMF (94 μL, 1.21 mmol) was added via syringe to a red-orange solution of LtBu,iPr2Fe(SPh) (81.8 mg, 0.123 mmol) in Et2O (10 mL). Upon swirling the solution orange crystals began to form. Cooling to −35 °C gave orange crystals in two crops (84.5 mg, 93%). Anal. Calcd for C45H67N3OSFe: C, 71.42; H, 8.85; N, 5.68%. Found: C, 70.66; H, 8.71; N, 5.46%. IR (KBr): 3063(w), 2968 (vs), 2868 (m), 1651 (vs), 1578 (m), 1527 (w), 1487 (m), 1460 (s), 1431 (s), 1383 (s), 1360 (s), 1315 (m), 1251 (w), 1217 (w), 1184 (w), 1153 (w), 1109 (m), 1063 (w), 1024 (w), 933 (w), 885 (w), 794 (w), 764 (m), 739 (m) 692 (m) cm−1. 1H NMR (C6D6, 500 MHz): δ 33, 18.0, 17.6, 5.8, 1.4, 1.2, −3.9, −20, −34, −67 ppm.
LtBu,iPr2Fe(SPh)(MeIm)
In a 20 mL scintillation vial, N-methylimidazole (20 μL, 0.251 mmol) was added dropwise via syringe to a red-orange solution of LtBu,iPr2Fe(SPh) (80.8 mg, 0.121 mmol) in Et2O (10 mL). Upon swirling the solution, an orange solid precipitated immediately. Placing in the freezer (−35°C) overnight gave a fluffy orange solid (81.7 mg, 90%). This complex can be crystallized from benzene. 1H NMR (C6D6, 500 MHz): δ 49, 27, 23, 22, 12, 1.2, 2.0, 5.6, 20, 28, 48, 89 ppm. IR (KBr): 3110 (w), 2966 (s), 2866 (m), 1578 (m), 1533 (m), 1489 (s), 1466 (m), 1433 (m), 1379 (s), 1356 (vs), 1315 (s), 1284 (w), 1252 (w), 1217 (m), 1186 (w), 1153 (w), 1099 (m), 1022 (w), 939 (w), 779 (w), 742 (m) cm−1. Anal. Calcd for C45H64N4SFe: C, 72.17; H, 8.61; N, 7.46%. Found: C, 72.82; H, 8.71; N, 6.93%.
UV-vis Equilibrium Experiments
Three-coordinate iron complexes were dissolved in toluene and filtered through a plug of Celite before adding any reagent(s). Fitting used KaleidaGraph 3.6.2 (Synergy Software). For weak binding, the plot of [L]0 vs. ΔAλmax was fit to equation 3 with refinement of the binding constant Keq and a scaling factor. For strong binding, the plot of [L]0 vs. ΔAλmax was fit to equation 4 with refinement of [M]0, Keq, and a scaling factor. It was necessary to refine [M]0 because the quality of the fit was drastically affected by small variations in [M]0, which are unavoidable due to small amounts of decomposition. The refined value of [M]0 was always between 0.8 mM and 1.1 mM, consistent with the intended 1.0 mM concentration of iron complex.
Computations
The SolidG program42 was used to calculate cone and solid angles for the neutral ligand of interest. The program utilizes the crystallographic information file (CIF) of a crystal structure to determine spatial orientation of a ligand. The values in Table 5 reflect the average solid and cone angles determined from ten structures. We used our crystallographic coordinates for DMF and CNtBu complexes (Figure 4). To supplement these data, we used the Cambridge Structural Database (CSD) to find other iron complexes containing these and other ligands of interest1. Priority was given to four-coordinate iron(II) complexes. When necessary, complexes with an increased coordination number and/or strong-field ligands were also used. Due to the scarcity of iron complexes with 2-picoline as a ligand, cobalt and nickel complexes were used to determine the cone and solid angles of this ligand. A full listing of complexes used is in the Supporting Information.
The pKa of CNtBu was calculated through means of the acid-base reactions
| (6) |
where B is a reference base whose pKa is already known experimentally. This approach 43 bypasses the treatment of the solvated proton, whose chemical structure is often unknown and difficult to model theoretically.
The pKa of tBuNCH+ can be determined by calculating the total free energy change of eq 6:
| (7) |
The total free energy change (ΔG) can be evaluated as a combination of gas-phase free energies and solvation free energies:
| (8) |
where ΔGg(tBuNCH+) represents the gas-phase free energy change of the deprotonation reaction, tBuNCH+ → tBuNC + H+.
The gas-phase geometry optimization and free energy calculation were performed at the B3LYP/6-311++G(d,p) level of theory. The solvation free energies in MeCN were obtained using the Polarizable Continuum Model (PCM) at the B3LYP/6-31+G(d) level. The gas-phase optimized geometries were used for all of the solution-phase calculations.
From the gas-phase free energies and solvation free energies, we can obtain the pKa of tBuNCH+ using eq 7. In our calculations, we chose several bases as our reference: PhNH2 (experimental pKa = 10.6); Et3N (experimental pKa = 18.8); pyridine (experimental pKa = 12.5); and, PhNMe2 (experimental pKa = 11.4).53 Using these reference bases gave pKa values for tBuNCH+ of 2.70, 2.82, 3.11, and 2.23, respectively. We take the average value of 2.71 as the pKa of tBuNCH+ in MeCN.
Supplementary Material
Table 8.
X-ray structure parameters for the three- and four-coordinate complexes discussed in this work.
| LtBu,iPr2Fe(SPh) | LtBu,iPr2Fe(SPhCF3) | LtBu,iPr2Fe(STol) | LtBu,iPr2Fe(OTol) | LtBu,iPr2Fe(SPh)(CNtBu) | LtBu,iPr2Fe(SPh)(MeIm)·C6H6 | LtBu,iPr2Fe(SPh)(DMF) | ||
|---|---|---|---|---|---|---|---|---|
| empirical formula | C41H58N2SFe | C42H57N2F3SFe | C42H60N2SFe | C42H60N2OFe | empirical formula | C46H67N3SSFe | C51H70N4SFe | C44H65N3OSFe |
| fw | 666.8 | 734.81 | 680.83 | 664.77 | fw | 749.94 | 827.02 | 739.9 |
| temperature (K) | 193(2) | 193(2) | 193(2) | 193(2) | temperature (K) | 100.0(1) | 100.0(1) | 100.0(1) |
| cryst system | monoclinic | monoclinic | monoclinic | monoclinic | cryst system | monoclinic | triclinic | triclinic |
| space group | P21/n | P21/c | P21/c | P21/c | space group | P21/n | P-1 | P-1 |
| a (Å) | 10.8405(15) | 22.1226(10) | 22.1137(11) | 22.0892(14) | a (Å) | 10.693(9) | 9.575(5) | 10.3921(12) |
| b (Å) | 16.368(2) | 9.6586(4) | 9.6346(5) | 9.6154(6) | b (Å) | 19.719(16) | 12.487(5) | 12.4143(14) |
| c (Å) | 21.579(3) | 20.9138(9) | 20.4372(10) | 20.5038(13) | c (Å) | 21.014(17) | 20.562(5) | 17.392(2) |
| β (°) | 91.007(2) | 114.980(1) | 114.858(1) | 115.819(1) | α (°) | 90 | 79.982(5) | 102.907(2) |
| V (Å3) | 3828.3(9) | 4050.7(3) | 3950.9(3) | 3920.2(4) | β (°) | 96.683(12) | 77.463(5) | 99.220(2) |
| Z | 4 | 4 | 4 | 4 | γ (°) | 90 | 88.120(5) | 94.130(2) |
| ρ(g/cm3) | 1.157 | 1.205 | 1.145 | 1.126 | V (Å3) | 4401(6) | 2363.2(17) | 2145.2(4) |
| μ (mm−1) | 0.477 | 0.468 | 0.464 | 0.147 | Z | 4 | 2 | 2 |
| R1, wR2 [I >2σ(I)] | 0.0420, 0.1012 | 0.0344, 0.0946 | 0.0377, 0.0897 | 0.0534, 0.1114 | ρ(g/cm3) | 1.132 | 1.162 | 1.145 |
| GOF | 1.017 | 1.087 | 1.039 | 1.058 | μ (mm−1) | 0.423 | 0.400 | 0.434 |
| R1, wR2 [I>2σ(I)] | 0.0489, 0.1113 | 0.0562, 0.1016 | 0.0431, 0.0949 | |||||
| GOF | 1.018 | 0.975 | 1.010 |
Acknowledgments
The authors thank William Jones and Joseph Dinnocenzo for valuable input and discussions, and thank Eleni Skrombolas for initial experiments. This work was funded by the National Institutes of Health (Grant GM065313 to P.L.H.) and the Department of Education (GAANN fellowship to K.P.C.).
Footnotes
Supporting Information available: Details of NMR spectra, equilibrium measurements, cyclic voltammetry, and solid angle calculation (PDF) and crystallography (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Karen P. Chiang, Department of Chemistry, University of Rochester, Rochester, New York 14627
Pamela M. Barrett, Department of Chemistry, University of Rochester, Rochester, New York 14627
Feizhi Ding, Department of Chemistry and Biochemistry, New Mexico State University, Las Cruces, New Mexico 88003.
Jeremy M. Smith, Department of Chemistry and Biochemistry, New Mexico State University, Las Cruces, New Mexico 88003
Savariraj Kingsley, Department of Chemistry, University of Rochester, Rochester, New York 14627.
William W. Brennessel, Department of Chemistry, University of Rochester, Rochester, New York 14627
Meghan M. Clark, Department of Chemistry, University of Rochester, Rochester, New York 14627
Rene J. Lachicotte, Department of Chemistry, University of Rochester, Rochester, New York 14627
Patrick L. Holland, Department of Chemistry, University of Rochester, Rochester, New York 14627
References
- 1.Holland PL. In: Comprehensive Coordination Chemistry II. McCleverty J, Meyer TJ, editors. Vol. 8. Elsevier; Oxford: 2004. pp. 569–599. [Google Scholar]
- 2.Barney BM, Lee H-I, Dos Santos PC, Hoffman BM, Dean DR, Seefeldt LC. Dalton Transactions. 2006:2277–2284. doi: 10.1039/b517633f. [DOI] [PubMed] [Google Scholar]
- 3.Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983–3011. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 4.Einsle O, Tezcan FA, Andrade SLA, Schmid B, Yoshida M, Howard JB, Rees DC. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 5.Krahn E, Weiss BJR, Kröckel M, Groppe J, Henkel G, Cramer SP, Trautwein AX, Schneider K, Müller A. J Biol Inorg Chem. 2002;7:37–45. doi: 10.1007/s007750100263. [DOI] [PubMed] [Google Scholar]
- 6.Holland PL. Can J Chem. 2005;83:296–301. [Google Scholar]
- 7.Seefeldt LC, Dance IG, Dean DR. Biochemistry. 2004;43:1401–1409. doi: 10.1021/bi036038g. [DOI] [PubMed] [Google Scholar]
- 8.Dos Santos PC, Igarashi RY, Lee H-I, Hoffman BM, Seefeldt LC, Dean DR. Acc Chem Res. 2005;38:208–214. doi: 10.1021/ar040050z. [DOI] [PubMed] [Google Scholar]
- 9.Schimpl J, Petrilli HM, Blöchl PE. J Am Chem Soc. 2003;125:15772–15778. doi: 10.1021/ja0367997. [DOI] [PubMed] [Google Scholar]
- 10.Smith JM, Sadique AR, Cundari TR, Rodgers KR, Lukat-Rodgers G, Lachicotte RJ, Flaschenriem CJ, Vela J, Holland PL. J Am Chem Soc. 2006;128:756–769. doi: 10.1021/ja052707x. [DOI] [PubMed] [Google Scholar]
- 11.Lees NS, McNaughton RL, Gregory WV, Holland PL, Hoffman BM. J Am Chem Soc. 2008;130:546–555. doi: 10.1021/ja073934x. [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Sadique AR, Smith JM, Dugan TR, Cowley RE, Brennessel WW, Flaschenriem CJ, Bill E, Cundari TR, Holland PL. J Am Chem Soc. 2008;130:6624–6638. doi: 10.1021/ja710669w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonnell FM, Ruhlandt-Senge K, Ellison JJ, Holm RH, Power PP. Inorg Chem. 1995;34:1815–22. [Google Scholar]
- 14.Lee SC, Holm RH. Proc Natl Acad Sci USA. 2003;100:3595–3600. doi: 10.1073/pnas.0630028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland PL. Acc Chem Res. 2008;41:905–914. doi: 10.1021/ar700267b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummins CC. Prog Inorg Chem. 1998;47:685–836. [Google Scholar]
- 17.Alvarez S. Coord Chem Rev. 1999;193–195:13–41. [Google Scholar]
- 18.Sanders JKM, Bampos N, Clyde-Watson Z, Darling SL, Hawley JC, Kim HJ, Mak CC, Webb SJ. Porphyrin Handb. 2000;3:1–48. [Google Scholar]
- 19.Ellis PE, Jones RD, Jr, Basolo F. J Chem Soc, Chem Commun. 1980:54–55. [Google Scholar]
- 20.Ellis PE, Linard JE, Szymanski T, Jones RD, Budge JR, Basolo F. J Am Chem Soc. 1980;102:1889–1896. [Google Scholar]
- 21.Smith JM, Lachicotte RJ, Holland PL. Chem Commun. 2001:1542–1543. doi: 10.1039/b103635c. [DOI] [PubMed] [Google Scholar]
- 22.Bailey PJC, RA, Dick CM, Fabre S, Henderson LC, Herber C, Liddle ST, Lorono-Gonzalez D, Parkin A, Parsons S. Chem Eur J. 2003;9:4820–4828. doi: 10.1002/chem.200305053. [DOI] [PubMed] [Google Scholar]
- 23.Adhikari D, Tran BL, Zuno-Cruz FJ, Sanchez Cabrera G, Mindiola DJ, Chiang KP, Cowley RE, Dugan TR, Holland PL. Inorg Synth. in press. [Google Scholar]
- 24.Eckert NA, Smith JM, Lachicotte RJ, Holland PL. Inorg Chem. 2004;43:3306–3321. doi: 10.1021/ic035483x. [DOI] [PubMed] [Google Scholar]
- 25.Schubert EM. J Chem Educ. 1992;69:62. [Google Scholar]
- 26.Evans DF. J Chem Soc. 1959:2003–2005. [Google Scholar]
- 27.Andres H, Bominaar E, Smith JM, Eckert NA, Holland PL, Münck E. J Am Chem Soc. 2002;124:3012–3025. doi: 10.1021/ja012327l. [DOI] [PubMed] [Google Scholar]
- 28.Vela J, Smith JM, Yu Y, Ketterer NA, Flaschenriem CJ, Lachicotte RJ, Holland PL. J Am Chem Soc. 2005;127:7857–7870. doi: 10.1021/ja042672l. [DOI] [PubMed] [Google Scholar]
- 29.Barriere F, LeSuer RJ, Geiger WE. Trends Mol Electrochem. 2004:413–444. [Google Scholar]
- 30.Connelly NG, Geiger WE. Chem Rev. 1996;96:877–910. doi: 10.1021/cr940053x. [DOI] [PubMed] [Google Scholar]
- 31.Connelly NG, Geiger WE. Chem Rev. 1996;96:877–910. doi: 10.1021/cr940053x. The potential of ferrocene in MeCN/NBu4PF6 is +0.40 V vs. SCE, and SCE is at +0.24 V vs. NHE. [DOI] [PubMed] [Google Scholar]
- 32.Vela J, Cirera J, Smith JM, Lachicotte RJ, Flaschenriem CJ, Alvarez S, Holland PL. Inorg Chem. 2007;46:60–71. doi: 10.1021/ic0609148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohki Y, Ikagawa Y, Tatsumi K. J Am Chem Soc. 2007;129:10457–10465. doi: 10.1021/ja072256b. [DOI] [PubMed] [Google Scholar]
- 34.Ohta S, Ohki Y, Ikagawa Y, Suizu R, Tatsumi K. J Organomet Chem. 2007;692:4792–799. [Google Scholar]
- 35.Power PP, Shoner SC. Angew Chem. 1991;103:308–9.(See also Angew Chem, Int Ed Engl. 1991;30(3):330–2.).
- 36.Hauptmann R, Kliß R, Schneider J, Henkel G. Z Anorg Allg Chem. 1998;624:1927–36. [Google Scholar]
- 37.Ruhlandt-Senge K, Power PP. Bull Soc Chim Fr. 1992;129:594. [Google Scholar]
- 38.Sydora OL, Henry TP, Wolczanski PT, Lobkovsky EB, Rumberger E, Hendrickson NH. Inorg Chem. 2006;45:609–26. doi: 10.1021/ic051289u. [DOI] [PubMed] [Google Scholar]
- 39.Lee HK, Luo BS, Mak TCW, Leung WP. J Organomet Chem. 1995;489:C71–C73. [Google Scholar]
- 40.Groysman S, Wang JJ, Tagore R, Lee SC, Holm RH. J Am Chem Soc. 2008;130:12794–12807. doi: 10.1021/ja804000k. [DOI] [PubMed] [Google Scholar]
- 41.Tolman CA. Chem Rev. 1977;77:313–348. [Google Scholar]
- 42.Guzei IA, Wendt M. Dalton Trans. 2006:3991–3999. doi: 10.1039/b605102b. [DOI] [PubMed] [Google Scholar]
- 43.Ding F, Smith JM, Wang HJ. J Org Chem. 2009;74:2679–2691. doi: 10.1021/jo802641r. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, Smith JM, Flaschenriem CJ, Holland PL. Inorg Chem. 2006;45:5742–5751. doi: 10.1021/ic052136+. [DOI] [PubMed] [Google Scholar]
- 45.Gusev DG. Organometallics. 2009;28:763–770. [Google Scholar]
- 46.Barbeau C, Turcotte J. Can J Chem. 1976;54:1603–1611. [Google Scholar]
- 47.Smith JM, Lachicotte RJ, Holland PL. Organometallics. 2002;21:4808–4814. [Google Scholar]
- 48.Sadique AR, Brennessel WW, Holland PL. Inorg Chem. 2008;47:784–786. doi: 10.1021/ic701914m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bryndza HE, Domaille PJ, Paciello RA, Bercaw JE. Organometallics. 1989;8:379–85. [Google Scholar]
- 50.Henderson RA. Chem Rev. 2005;105:2365–2437. doi: 10.1021/cr030706m. In [4Fe-4S] clusters, changing from terminal chloride to thiolate ligands has been reported to change the substitution mechanism from associative to dissociative. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins DM, Peters JC. J Am Chem Soc. 2005;127:7148–7165. doi: 10.1021/ja045310m. [DOI] [PubMed] [Google Scholar]
- 52.Kern RJ. J Inorg Nucl Chem. 1962;24:1105–9. [Google Scholar]
- 53.Kaljurand I, Kutt A, Soovali L, Rodima T, Maemets V, Leito I, Koppel IA. J Org Chem. 2005;70:1019–1028. doi: 10.1021/jo048252w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.