Abstract
Mesenchymal stem cells (MSCs) are an attractive cell source for cartilage tissue engineering and regenerative medicine. However, the use of these cells has been limited by their reduced ability to form functional tissue compared to chondrocytes when placed in three-dimensional culture systems. To optimize MSC functional chondrogenesis, we examined the effects of increasing seeding density and transient application of transforming growth factor beta 3 (TGF-β3), two factors previously shown to improve growth of chondrocyte-based constructs. Chondrocytes seeded in agarose at 20 million cells/mL and MSCs seeded at 20 or 60 million cells/mL agarose were cultured for 7 weeks under continuous or transient application of TGF-β3. In the transient group, cell-laden constructs were exposed to TGF-β3 for the initial 3 weeks, followed by 4 weeks of culture in medium without TGF-β3. Compressive properties, biochemical content, and gene expression were assessed at 3, 5, and 7 weeks. Matrix distribution and collagen type was determined using histology and immunohistochemistry, and chondrogenic and osteogenic markers were assessed using real-time polymerase chain reaction. When maintained continuously with TGF-β3, chondrocyte-seeded constructs achieved a higher equilibrium compressive modulus than MSCs similarly maintained. Although properties of both groups increased with respect to starting values, there was no difference in bulk mechanical or biochemical properties with higher seeding density when MSCs were cultured with constant TGF-β3. Findings also showed that while transient application of TGF-β3 elicited robust growth from chondrocyte-laden gels, MSCs seeded at the same density failed to respond, although constructs maintained their previously accrued properties and continued to express cartilaginous genes after TGF-β3 removal. Conversely, MSCs seeded at 60 million cells/mL exhibited a strong anabolic response with transient TGF-β3 exposure, achieving an equilibrium modulus of approximately 200 kPa. Although this represents the highest modulus we have been able to achieve with MSC-seeded constructs using our culture system, further work remains to optimize MSC chondrogenesis for cartilage tissue engineering, particularly in terms of collagen content and dynamic mechanical properties.
Introduction
Articular cartilage lines the surfaces of joints and functions to distribute the forces arising from joint movement. This load-bearing role is enabled by a dense extracellular matrix (ECM) composed of proteoglycans (∼3–10% wet weight) and type II collagen (∼10–30% wet weight).1–3 These matrix constituents are responsible for the unique mechanical properties of cartilage, including a high equilibrium compressive modulus (0.5–1 MPa) and an even higher dynamic compressive modulus (16–40 MPa).4–6 Under normal physiological conditions, the metabolic activity of cartilage consists of a fine balance of anabolic and catabolic events that resident chondrocytes that make up 10% of the tissue volume regulate.7 With traumatic injury or joint disease, degenerative changes may permanently compromise mechanical function due to the limited capacity of articular cartilage for self-renewal.8 To date, few strategies exist for restoring damaged articular surfaces; therefore, cartilage tissue engineering has emerged as a means of generating replacement tissues.9 To optimize growth and maturation of tissue-engineered constructs, various methodologies have been employed, including three-dimensional (3D) culture in a wide range of biomaterials10–14 coupled with mechanical stimulation15–18 and growth factor supplementation.19,20 In addition to these strategies, variations in cell-seeding density and medium formulations have also been shown to have pronounced effects on the final properties accrued by engineered cartilage.21–23
While significant progress has been made with these chondrocyte-based approaches, the use of chondrocytes for cartilage tissue engineering may prove impractical because of limitations in cell availability and donor site morbidity. Therefore, recent efforts have used mesenchymal stem cells (MSCs), which readily undergo chondrogenesis when cultured in chemically defined media with transforming growth factor beta (TGF-β) family members.24,25 In the presence of these biofactors, MSCs deposit cartilaginous matrix when seeded in hydrogels26–28 or fibrous scaffolds.29 However, although MSCs can generate cartilage-like ECM, it has also been shown that MSCs do not reach functional parity with donor-matched chondrocytes cultured under the same conditions. Our previous work suggests that MSCs, starting at the same seeding density, reach at best 50% of the compressive equilibrium properties of chondrocyte-seeded agarose constructs.30 A number of studies have examined the effects of varying cell seeding density on MSC chondrogenesis, although in most studies, the seeding densities employed were lower (<10 million cells/mL) than those used in cartilage tissue engineering with chondrocytes, and functional properties were not assayed.31–36 Cell–cell contact and communication is a recognized factor in the initiation of chondrogenesis in pellet cultures,37 although the effect of variation in this parameter has yet to be investigated in the context of emerging mechanical properties of MSC-seeded 3D constructs.
In addition to cell-density effects, recent studies have also shown that transient application of TGF-β3 in a serum-free, chemically defined medium enhances the compressive properties and glycosaminoglycan (GAG) content of chondrocyte-laden hydrogels to near-native tissue levels.38,39 In those studies, after removal of the growth factor, constructs achieved equilibrium compressive moduli of approximately 0.8 MPa and proteoglycan levels of 6% to 7% wet weight in less than 2 months of culture. It is not yet clear whether similar transient application of growth factor can accelerate the maturation of MSC-based constructs. Two recent studies using MSC-laden hydrogels indicate that this phenomenon may be operative for MSCs in 3D culture.40,41 In one study by Mehlhorn et al., human MSCs in alginate beads synthesized lower amounts of aggrecan after transient application of TGF-β3 than constructs cultured continuously with TGF-β3 over 2 weeks. However, the level of aggrecan accrued was still greater than that of control constructs, and chondrogenic genes remained expressed, suggesting maintenance of the chondrocytic phenotype.41 Indeed, differentiated MSCs were resistant to subversion of the chondrogenic phenotype when challenged with osteogenic media. However, this study did not examine mechanical properties of the formed constructs. Caterson et al. also observed a continued chondrogenic response by MSCs in alginate treated with a single pulse of TGF-β1 (50 ng/mL) for 3 days.40 At 21 days of culture, treated constructs stained for proteoglycans and type II collagen and continued to express aggrecan and type II and type IX collagens. Constructs also briefly expressed osteocalcin at 14 days of culture.
Taken together, these studies indicate that brief exposure to TGF-β may be sufficient to initiate and maintain chondrogenesis, although the effect of this treatment on mechanical function was not investigated. It is also unknown whether transient application of TGF-β enhances MSC chondrogenesis and improves the development of functional properties of MSC-based constructs. While standard practice relies on continuous treatment with TGF-β, previous studies using chondrocyte-based constructs suggest that temporal exposure to this morphogen is more effective in generating tissue replacements with near-native properties. The effects of transient TGF-β treatment has not been investigated in terms of mechanical properties of MSC-laden constructs. In addition, because TGF-β is known to suppress chondrocyte hypertrophy42 and can retard or abrogate osteogenic progression of MSCs in monolayer culture,43,44 it was also not clear whether removal of the growth factor would initiate mineralization or other hypertrophic changes in our stem cell populations.
To specifically address these questions, this study evaluated mechanical and biochemical properties in chondrocyte- and MSC-laden hydrogels with transient exposure of TGF-β3 in a chemically defined medium. In addition, we explored the effects of varying seeding density in MSC-laden constructs on resultant functional properties. We hypothesized that increasing seeding density would improve mechanical properties and biochemical content. We also hypothesized that transient application of TGF-β3 would improve functional properties of MSC-laden constructs and that differences in cartilaginous gene expression would mark these changes.
Materials and Methods
MSC and chondrocyte isolation and culture
MSCs were isolated from the carpal bone marrow of three 3- to 6-month-old calves (Research 87, Bolyston, MA), as previously described.32 MSCs were maintained in medium containing 10% fetal bovine serum and 1% penicillin/streptomycin/Fungizone (PSF), with cultures up to passage 3 used for these studies. Chondrocytes were harvested from carpometacarpal articular cartilage,32 digested with pronase and collagenase,21,41 and encapsulated immediately upon isolation.
Construct fabrication and long-term 3D culture
Primary chondrocytes or MSCs were suspended in a chemically defined medium (CM) and combined 1:1 with sterile type VII agarose (49°C, 4% w/v, Sigma, St Louis, MO) in phosphate buffered saline (PBS) at room temperature. CM consisted of high-glucose Dulbecco's modified Eagle medium supplemented with 1× PSF; 0.1 μM dexamethasone; 50 mg/mL ascorbate 2-phosphate; 40 mg/mL L-proline; 100mg/mL sodium pyruvate; and 1× 6.25 μg/mL insulin, 6.25μg/mL transferrin, 6.25 ng/mL selenious acid, 1.25 mg/mL bovine serum albumin (BSA), and 5.35 μg/mL linoleic acid. Chondrocytes were seeded at a density of 20 million cells/mL, and MSCs were seeded at 20 or 60 million cells/mL. The resultant cell–agarose mixture was cast between parallel plates to create a 2.25-mm sheet and allowed to gel for 20 min at room temperature. After gelation, disks (Ø4 mm) were cored and cultured with continuous or transient TGF-β3 treatment in 1 mL of CM per construct. In the continuous treatment group, disks were cultured in CM supplemented with 10 ng/mL TGF-β3 (CM+, R&D Systems, Minneapolis, MN) for 7 weeks. In the transient TGF-β3 treatment group, disks were maintained in CM+ for the first 3 weeks and then cultured in CM without TGF-β3 for an additional 4 weeks. At 3, 5, and 7 weeks, constructs were evaluated for mechanical properties, biochemical content, and gene expression. Constructs were measured with digital calipers at each time point to monitor dimensional stability. Each experiment was repeated at least once to confirm results with data from all samples pooled.
MSC pellet formation and long-term culture
Pellets containing 250,000 MSCs per pellet were formed by centrifugation (5 min, 300× g) in 96-well polypropylene conical plates (Nalge Nunc International, Rochester, NY). Pellets were cultured with continuous or transient application of TGF-β3, as described above. At 3, 5, and 7 weeks, pellets were evaluated for sulfated GAG and DNA content. Histological analysis was performed at 7 weeks.
Mechanical testing of engineered constructs
A custom mechanical testing device was used to evaluate compressive properties of engineered constructs.30 Disks were tested in unconfined compression between two impermeable platens. First, samples were equilibrated in creep under a static load of 2 g for 5 min. After creep testing, samples were subjected to 10% strain (calculated from post-creep thickness values) applied at 0.05%/s followed by relaxation for 1000 s until equilibrium. Dynamic testing was performed by applying 1% sinusoidal deformation at 1.0 Hz. The equilibrium modulus was determined from the equilibrium stress (minus tare stress) normalized to the applied strain. The dynamic modulus was determined from the slope of dynamic stress–strain response. After mechanical testing, constructs were frozen at −20°C for biochemical evaluation.
Biochemical analysis
To assess biochemical content, samples were digested for 16 h in papain (0.56 U/mL in 0.1 M sodium acetate, 10 M cysteine hydrochloric acid, 0.05M ethylenediaminetetraacetic acid, pH 6.0) at 60°C. Agarose disks were digested in 1 mL/construct of papain, and cell pellets were digested in 300 μL/sample of papain with three pellets combined per sample. After digestion, the 1,9-dimethylmethylene blue dye-binding assay was used to determine GAG content in digests against a standard curve of chondroitin-6-sulphate. Digested samples were also evaluated for collagen content after acid hydrolysis using the orthohydroxyproline (OHP) assay,45 with a 1:7.14 OHP:collagen ratio used, as described previously.46 DNA content was determined from papain digests using the PicoGreen dsDNA assay (Molecular Probes, Eugene, OR). GAG and collagen values are reported as percentages of construct wet weight, and DNA content is reported as quantity per disk.
Real-time polymerase chain reaction
Total RNA was extracted by two sequential isolations in TRIZOL-chloroform and quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Reverse transcription was performed using the First Strand complementary DNA (cDNA) Synthesis kit (Invitrogen Life Technologies, Carlsbad, CA), and cDNA amplification was performed using an Applied Biosystems 7300 real-time polymerase chain reaction (PCR) system with intron-spanning primers and SYBR Green Reaction Mix (Applied Biosystems, Carlsbad, CA). Expression levels of seven cartilage-specific markers (chondroitin-4-sulfotransferase-1 (C4ST-1), chondroitin-4-sulfotransferase-2 (C4ST-2), xylosyltransferase (XT-1), GalNAc4,6S-disulfotransferase (Galnac), aggrecan, collagen II, and link protein) and two bone-related markers (collagen type I and osteocalcin) were determined and normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase.
Histology
Samples for histology were fixed in 4% paraformaldehyde, dehydrated in a graded series of ethanol, and embedded in paraffin (Paraplast, Lab Storage, St. Peters, MO). Samples were sectioned at 8-μm thickness and stained with hematoxylin and eosin (H&E, Sigma), Alcian Blue (pH 1.0), and Picrosirius Red (0.1% w/v in saturated picric acid) for cell distribution, sulfated proteoglycans, and collagens, respectively. Mineralization in engineered constructs was assessed using Von Kossa staining (American Mastertech, Lodi, CA), with a positive calcium control slide stained simultaneously for comparison. Color images were captured at 5×, 10×, or 20× magnification using a microscope equipped with a color charge coupled device digital camera and the QCapturePro (QImaging, Surrey, BC, Canada) acquisition software. Images captured at 5× magnification (showing the majority of the construct expanse) can be found in Supplementary Figures 1–3. (Supplementary figures available online at www.liebertonline.com/ten.)
Immunohistochemistry
For immunohistochemical analysis, 8-μm sections were deparaffinized and rehydrated in a graded series of ethanol to water. Antigen retrieval was performed by incubating sections in citrate buffer (10 mM citric acid with 0.05% Tween 20 at pH 6.0) heated to 99°C for 25 min. Citrate buffer and samples were then transferred to room temperature and allowed to cool for an additional 20 min. After antigen retrieval, samples were incubated for 1 h at room temperature in 300 μg/mL hyaluronidase (Type IV, Sigma) in PBS. Primary antibodies to collagen type I (MAB3391, Millipore, Billerica, MA), collagen type II (11-116B3, Developmental Studies Hybridoma Bank, Iowa City, IA), and collagen type X (X-AC9, Developmental Studies Hybridoma Bank) were used for immunolabeling, as described previously.32 Briefly, samples were treated with 3% hydrogen peroxide followed by treatment with a blocking reagent (3,3′ diaminobenzidine chromogen reagent (DAB150 IHC Select), Millipore) and incubated with primary antibodies in 3% BSA with non-immune controls. After incubation with primary antibodies, samples were treated with biotinylated goat anti-rabbit immunoglobulin (Ig)G secondary antibodies and streptavidin horse radish peroxidase and then reacted with DAB150 IHC Select. Color images were captured at 10× magnification as above.
Statistical analysis
Statistical analysis was performed using SYSTAT software (v10.2, SYSTAT Software Inc., San Jose, CA). Mechanical and biochemical data for cell-seeded constructs was analyzed using two-way analysis of variance (ANOVA), with significance set at p < 0.05. Biochemical content of cell pellets was assessed using one-way ANOVA, with significance set at p < 0.05. Where significance was indicated by ANOVA analysis, Tukey's post hoc testing was performed to enable comparisons between groups. All values are reported as means ± standard deviations.
Results
Compressive properties of cell-seeded agarose
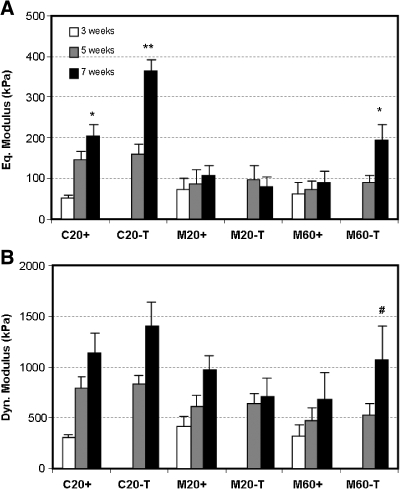
To optimize functional properties of MSC-laden constructs, MSCs were seeded at 20 (M20) or 60 (M60) million cells/mL of agarose and cultured under continuous (+) or transient (T) exposure to TGF-β3 for 7 weeks in chemically defined, serum-free medium. Chondrocytes were seeded at 20 million cells/mL (C20) and maintained under these same conditions. Over time in culture, chondrocyte and MSC-seeded constructs became increasingly opaque and, consistent with previous findings, increased in volume relative to starting values. Construct thickness did not change with transient exposure to TGF-β3, although at every time point assayed, MSC-seeded gels were thicker than chondrocyte-seeded gels, regardless of seeding density. There were no observable differences in MSC-seeded construct diameters until day 49. As with thickness measurements, diameter size was comparable between M20 and M60 at every time point, and both were larger than C20 by day 49 (Table 1).
Table 1.
Changes in Construct Dimensions with Time
| |
Thickness (mm) |
Diameter (mm) |
||||
|---|---|---|---|---|---|---|
| Day 21 | Day 35 | Day 49 | Day 21 | Day 35 | Day 49 | |
| C20+ | 2.24 ± 0.02 | 2.42 ± 0.06* | 2.48 ± 0.07* | 4.01 ± 0.01 | 4.06 ± 0.07 | 4.18 ± 0.17 |
| C20-T | NA | 2.41 ± 0.04* | 2.45 ± 0.06* | NA | 4.02 ± 0.03 | 4.04 ± 0.05 |
| M20+ | 2.55 ± 0.10# | 2.63 ± 0.09# | 2.71 ± 0.16# | 4.08 ± 0.11 | 4.13 ± 0.09 | 4.40 ± 0.15* |
| M20-T | NA | 2.58 ± 0.13# | 2.67 ± 0.12# | NA | 4.13 ± 0.08 | 4.33 ± 0.23*# |
| M60+ | 2.58 ± 0.08# | 2.67 ± 0.08# | 2.81 ± 0.10# | 4.22 ± 0.25 | 4.28 ± 0.14 | 4.53 ± 0.12*# |
| M60-T | NA | 2.69 ± 0.11# | 2.76 ± 0.09# | NA | 4.35 ± 0.08# | 4.41 ± 0.20# |
*Significant difference from day 21 within cell type and seeding density (p < 0.05). #Significant difference from C20 within each time point and medium group (p < 0.05).
For all groups, the equilibrium and dynamic compressive modulus improved with time in culture (p < 0.05). Consistent with our previous findings,30 the equilibrium modulus of C20+ disks increased through week 7, reaching 203 ± 27 kPa (p = 0.008), whereas M20+ constructs plateaued by 3 weeks at a lower level of 107 ± 23 kPa (p = 1.0, Fig. 1A). There was no difference in equilibrium modulus between M20+ and M60 + at any time point (p > 0.9), although the moduli of both groups were much greater than starting agarose values (typically 5–10 kPa). Also consistent with our previous findings,39 the equilibrium modulus of the C20-T group increased dramatically, with an 8-fold increase 4 weeks after removal of TGF-β3 (p < 0.001), compared to only a 4-fold increase over the same time period with continuous exposure (p < 0.001). Although the equilibrium properties of M20 constructs were not different in transient and continuous medium conditions (p = 0.9), a 4-fold increase to 193 ± 40 kPa was observed in M60 constructs by week 7 when cultured in transient compared to continuous media exposure (p < 0.001). These M60-T samples reached modulus values similar to that of week 7 C20+ constructs (p = 1.0, Fig. 1A). Despite the difference in the equilibrium modulus between C20+ and M20+, the dynamic moduli of these constructs were not significantly different by week 7 (p = 0.9). At 7 weeks, the dynamic modulus of M20+ was also similar to that of M60+ (p = 0.1). Under transient medium conditions, the dynamic moduli of C20 and M20 constructs were not greater than that of control constructs in + conditions (p > 0.2), but the dynamic modulus of M60-T constructs was greater than that of M60+ (p = 0.003, Fig. 1B).
FIG. 1.
Time-dependent compressive properties of engineered constructs with variation in cell type, seeding density, and medium formulation. (A) Equilibrium and (B) dynamic modulus of cell-seeded constructs. *Greater than chondrocytes seeded at 20 million cells/mL of agarose under continuous exposure to transforming growth factor beta 3 (TGF-β3) (C20+) at week 5 (p < 0.05); **greater than C20+ at week 7 (p < 0.05); #greater than mesenchymal stem cell (MSC) seeded at 60 million cells/mL of agarose and cultured under continuous exposure to TGF-β3 (M60+) at 7 weeks. Data represent the mean and standard deviation of seven to eight samples per group per time point.
Biochemical content of cell-seeded agarose
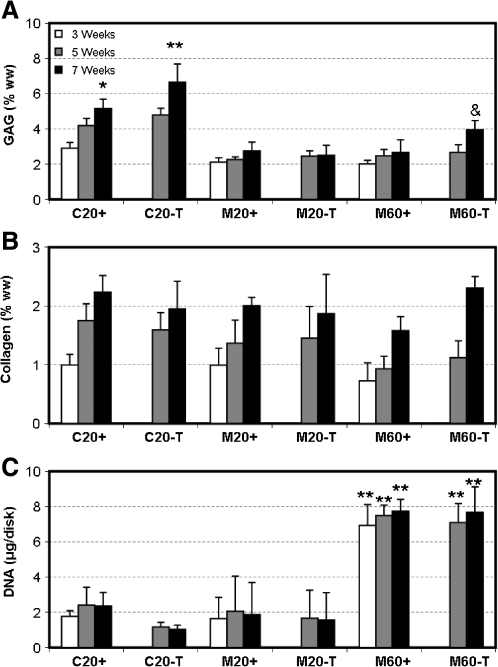
Biochemical content for C20+ and M20+ disks increased with time, with more GAG deposited in C20+ than M20+ by week 7 (p < 0.001, Fig. 2A). As with the equilibrium modulus, the GAG content of C20+ continued to improve through the final time point (p < 0.001), whereas M20+ disks plateaued by week 3 (p > 0.4, Fig. 2A). Under transient conditions, a marked increase in GAG content was observed in the C20-T and M60-T groups (p < 0.001, Figure 2A) compared to their continuous-exposure controls. Although the total GAG content of week 7 M60-T constructs was markedly higher than that of all other MSC groups, it remained below that of week 7 chondrocyte-laden constructs. Collagen content was not significantly different between groups by week 7, except in the M60+ group, which was lower than C20+ and M60-T (p < 0.05, Fig. 2B). Assessment of DNA content showed that higher cell density was maintained in the 60M-seeded MSC groups and that transient application of TGF-β3 had no effect on cell proliferation, regardless of cell type (p = 1.0, Fig. 2C). When normalized to DNA content, GAG content in M60 constructs cultured under either medium condition was dramatically lower than that of M20 constructs similarly maintained, suggesting that GAG synthesis per cell was impaired with increasing cell number (not shown).
FIG. 2.
Biochemical composition of engineered constructs with variation in time in culture, cell type, seeding density, and medium formulation. (A) Glycosaminoglycan (GAG), (B) collagen, and (C) DNA content of chondrocyte- and MSC-laden gels. *Greater than C20+ at week 5 (p < 0.05); **greater than C20+ at week 7, &No difference from C20+ at week 5 (p > 0.05). Data represent the mean and standard deviation of seven to eight samples per group per time point.
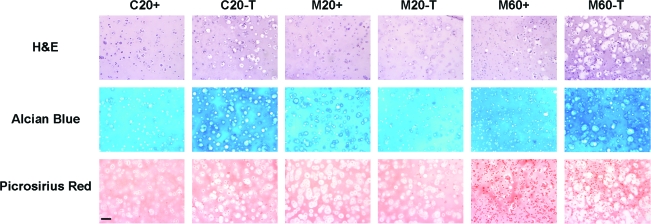
Histological analysis of cell-seeded agarose
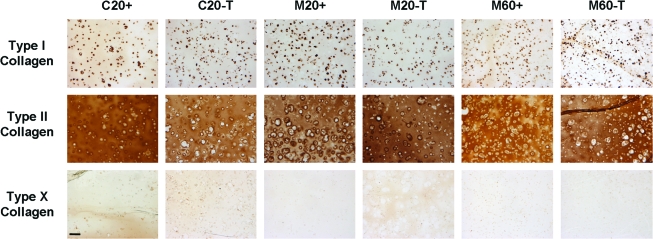
Histological staining for cellularity, GAG, and collagen deposition largely confirmed biochemical measures. Consistent with biochemical analyses of DNA content, H&E staining for cell distribution at week 7 showed greater cell density for gels seeded at 60 million cells/mL than those seeded at 20 million cells/mL (Fig. 3). From H&E staining, it appeared that transient application of TGF-β3 resulted in larger cell lacunae in the C20 and M60 groups. These C20-T and M60-T groups also showed greater staining for proteoglycan deposition than controls with continuous medium conditions (Fig. 3). Constructs showed little discernible difference in collagen staining with variation in cell type or seeding density. Collagen deposition was further assessed using immunohistochemistry to distinguish between type I, type II, and type X collagens. At week 7, all groups showed weak pericellular staining for type I collagen and intense inter-territorial staining for type II collagen (Fig. 4). There was no increase in type I collagen accumulation with removal of TGF-β3 in any group, nor was type X collagen staining evident under any condition. Von Kossa staining for calcium was also performed to assess mineralization in cell-seeded gels; no evidence of calcium deposition was observed in any group (Fig. 5).
FIG. 3.
Histological appearance of engineered constructs after 7 weeks of culture. Hematoxylin and eosin, Alcian Blue, and Picrosirius Red staining of C20, M20, and M60 constructs cultured in continuous (+) and transient (T) media. C20-T and M60-T showed greater proteoglycan deposition and changes in cell morphology consistent with hypertrophic events. Images were acquired at 10× magnification. Scale bar: 100 μm. Color images available online at www.liebertonline.com/ten.
FIG. 4.
Distribution of collagen types I, II, and X in engineered constructs cultured in + or T medium for 7 weeks. Chondrocyte- and MSC-laden constructs showed weak pericellular staining for type I collagen and intense staining for type II collagen regardless of initial seeding density or medium formulation. Type X collagen was not apparent in any group. Scale bar: 100 μm. Color images available online at www.liebertonline.com/ten.
FIG. 5.
Von Kossa staining of engineered constructs cultured in T medium for 7 weeks. Mineralization (black staining) was not observed in chondrocyte- or MSC-laden gels. Images were acquired at 10× magnification. Scale bar: 100 μm. Color images available online at www.liebertonline.com/ten.
Biochemical and histological analysis of MSC pellets
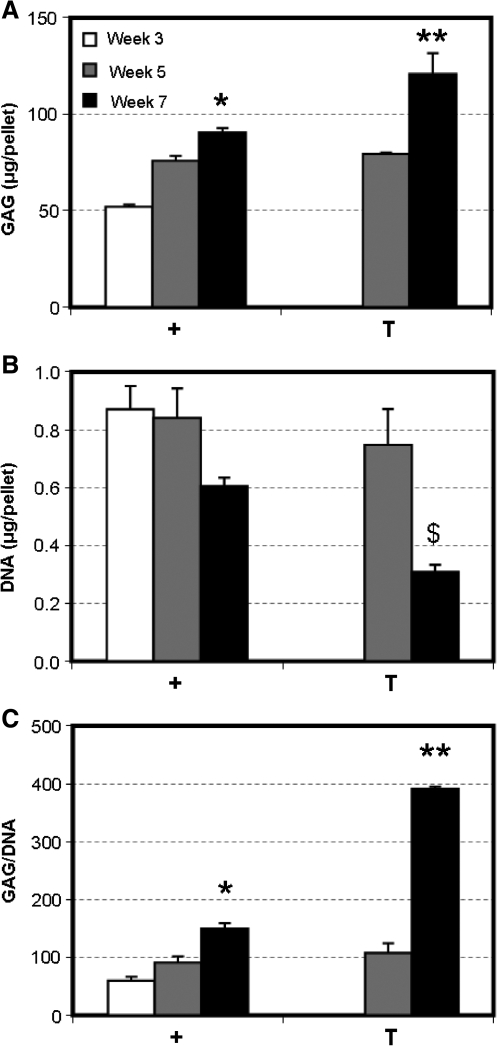
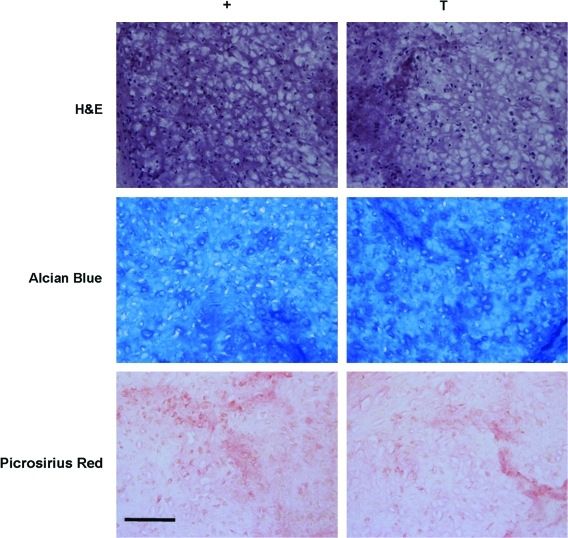
Because MSC response to transiently applied TGF-β3 appeared to depend on initial seeding density, a cell-pellet model of chondrogenesis was used to determine whether results similar to that achieved with gels seeded at 60 million cells/mL could be generated when cultured under transient conditions. Cells are tightly packed in pellet culture (i.e., no ECM at the initiation of culture), and they may be considered ‘infinite’ with respect to the other seeding densities used in this study (20 or 60 million cells/mL). MSC pellets accrued increasing amounts of GAG with time through week 7 when cultured continuously with TGF-β3. With transient exposure to TGF-β3, the GAG content of pellets was significantly greater by week 7 than the GAG content of pellets cultured continuously with TGF-β3 (p < 0.001, Fig. 6A). Contrary to our findings in 3D hydrogel culture, DNA content of pellets was lower for both groups at week 7, with greater loss in pellets cultured under transient medium conditions than with continuous exposure (Fig. 6B). When normalized to cell number, the difference in GAG content at week 7 between pellets cultured under transient and continuous conditions was even more pronounced, with a 2.6 times difference in GAG deposition (Fig. 6C). No differences in collagen content were observed with transient application of TGF-β3 (not shown). By week 7, there was a noticeable difference in cell number between pellets maintained continuously or transiently in TGF-β3, as shown in the H&E stains. As with cell-seeded constructs, Alcian Blue staining for proteoglycans demonstrated conspicuously darker regions of staining in pellets cultured under transient than under continuous conditions, consistent with biochemical measures of GAG content. Picrosirius red staining for collagen was of equal intensity between the two groups (Fig. 7).
FIG. 6.
Biochemical composition of MSC pellets with variation in time in culture and medium formulation. (A) GAG (μg/pellet), (B) DNA (μg/pellet), and (C) GAG/DNA content of MSC pellets. Transient application of TGF-β3 resulted in greater GAG deposition than in pellets cultured continuously with TGF-β3. *Greater than + pellets at week 5 (p < 0.05), **greater than + pellets at week 7 (p < 0.001), $lower than + pellets at week 7 and T pellets at week 5 (p < 0.01). Data represent the mean and standard deviation of three samples per group per time point.
FIG. 7.
Histologic appearance of MSC pellets after 7 weeks of culture. Hematoxylin and eosin, Alcian Blue, and Picrosirius Red staining of MSC pellets cultured in + and T media. Images were taken at 20× magnification. Scale bar: 100 μm. Color images available online at www.liebertonline.com/ten.
Gene expression
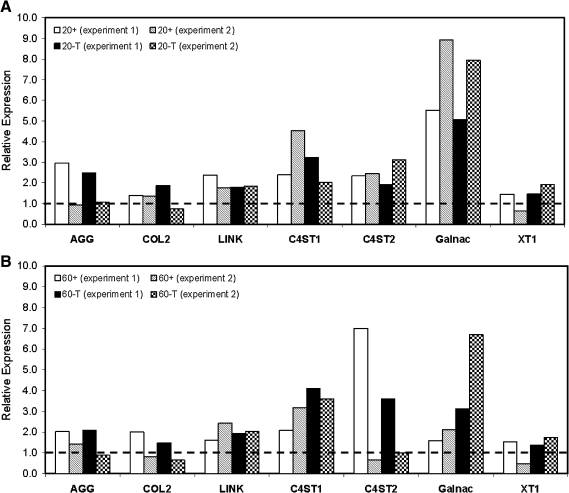
All groups cultured continuously with TGF-β3 expressed chondrogenic markers. Removal of TGF-β3 did not adversely affect the chondrocytic phenotype of MSCs seeded at 20 or 60 million cells/mL. Expression levels of C4ST-1 and Galnac continued to increase for M20-T and M60-T after 3 weeks, whereas aggrecan and collagen type II expression remained relatively stable (Fig. 8). C20-T groups followed a similar pattern of expression, with large increases in Galnac from 3 weeks to 7 weeks. For all MSC and chondrocyte groups exposed transiently to TGF-β3, Galnac expression at 7 weeks was markedly higher than in control gels maintained continuously with TGF-β3. These data are from two biological replicates (separate studies), with similar findings in each. Although all groups expressed collagen type I, osteocalcin expression was undetectable (not shown).
FIG. 8.
Expression of cartilage markers in MSC-laden constructs with variation of initial seeding density. Relative gene expression normalized to 3-week control constructs (dashed line) of (A) M20 and (B) M60 constructs at 7 weeks in + and T media. The chondrocyte-like phenotype is maintained in constructs cultured in T medium 4 weeks after TGF-β3 withdrawal. Data from two experiments are presented.
Discussion
Bone marrow-derived MSCs are an attractive cell source for regenerative medicine, given their easy isolation and expansion capacity and their ability to differentiate toward a number of musculoskeletal lineages. For cartilage tissue engineering, MSCs readily undergo chondrogenesis in 3D culture in the presence of specific biofactors and deposit a cartilage-like matrix. This matrix formation improves mechanical properties critical to the function of the native tissue, including compressive and tensile properties.30,47 Despite their maturation potential, engineered cartilage formed from MSCs is limited by the reduced equilibrium compressive properties generated, compared to fully differentiated chondrocytes. Consistent with previous findings,30 this study showed that the mechanical properties of MSC-laden constructs were lower than those of chondrocyte-laden constructs similarly maintained. To further optimize chondrogenesis, we examined the effects of two parameters, initial seeding density and transient application of TGF-β3, on the mechanical properties and biochemical content of MSC-laden gels. In chondrocyte-seeded hydrogels, both factors have been previously shown to improve equilibrium compressive properties.21,39
Contrary to our original hypothesis, MSCs seeded in agarose at 20 and 60 million cells/mL and maintained continuously with TGF-β3 showed no differences in functional properties or biochemical content after 7 weeks in free-swelling culture. Although several studies have reported enhanced chondrogenesis with greater seeding density,31,33,35,36 the range of cell densities assayed were generally less than 10 million cells/mL,33,35 and outcome parameters were limited to biochemical measures or gene expression. In one study comparing seeding densities greater than 10 million cells/mL, total GAG deposition was indistinguishable between constructs seeded at 12 to 48 million cells/mL, consistent with the current findings.31 In another study comparing human MSCs seeded at different densities in alginate, an initial density of 25 million cells/mL resulted in significantly greater GAG synthesis per cell after 14 days of culture than in constructs seeded at 50 million cells/mL.36 In keeping with this observation, we found markedly less GAG deposition per cell (∼75% reduction) in constructs seeded at 60 million MSCs/mL than in constructs seeded at 20 million MSCs/mL.
During chondrogenesis in the limb bud and in in vitro micromass models using these cells, greater cellularity promotes cell–cell contact and enhances differentiation.48,49 Our results and those of others suggest that there exists an optimal MSC density that maximizes GAG production per cell, even when cells are not in direct contact in 3D hydrogel systems. At seeding densities higher than this optimal value, chondrogenesis may be adversely affected. Studies using chondrocyte-based constructs have also shown that, although matrix biosynthesis is initially high during early culture periods, when the pericellular environment is being established de novo, a decline in synthesis rate is observed with gradual accumulation of ECM.50 At earlier time points than those assayed in our studies, a higher seeding density may promote matrix biosynthesis until a certain threshold value of bulk GAG content is attained. To assess whether such negative feedback mechanisms regulate matrix accumulation rates in MSC-based constructs, future work will assess GAG synthesis at earlier time points. Alternatively, a deficit in nutrient supply may reduce the extent of differentiation or matrix production observed at the higher seeding density. For example, we have shown more robust production of ECM in chondrocyte-seeded gels in medium containing 20% FBS than in 10% FBS medium.21 Additional studies will be required to address this important question.
To further enhance MSC chondrogenesis, we also examined the effect of transient application of TGF-β3 in the context of changing seeding densities. Although chondrocytes responded favorably to transient exposure to TGF-β3 by dramatically increasing the equilibrium modulus, MSCs seeded at the same density (20 million/mL) showed no change with removal of the growth factor. Although the “release” phenomenon was not observed in MSCs seeded at this density, removal of TGF-β3 did not abrogate accrued properties, indicating that MSCs were able to maintain their differentiated phenotype and functional properties. Conversely, when MSCs were seeded at a higher density (60 million cells/mL), transient application of TGF-β3 had a pronounced effect on the equilibrium modulus and GAG accumulation. This finding suggests the potential for quorum sensing in triggering the “release” phenomenon in MSCs, whereby paracrine signaling between cells may potentiate the “release” response after TGF-β3 withdrawal. Alternatively, at a higher seeding density (60 million cells/mL), a smaller fraction of MSCs may undergo chondrogenesis, or the extent of chondrogenesis might be limited in induced cells, potentially through negative feedback mechanisms or nutrient limitations and growth factor supply. Enhanced recruitment of undifferentiated cells or enhanced GAG production by committed MSCs may cause the increase in total GAG content and equilibrium compressive properties in these constructs with removal of TGF-β3. Whether chondrogenically committed or uncommitted cells initiate the triggering response is as yet unknown.
Despite improved GAG production in these MSC-laden constructs with the removal of TGF-β3, the GAG content on a per-cell basis remained markedly lower than with MSC constructs seeded at 20 million cells/mL (∼65% lower). This suggests that simply adding MSCs does not overcome their inherent matrix-forming limitations compared with fully differentiated chondrocytes. Furthermore, although MSCs seeded at 20 million cells/mL did not respond to “release” in general, in two instances (out of six), increases in equilibrium modulus were observed on par with that of cells seeded at 60 million cells/mL. This observation implies that, if this response depends on the initial seeding density, 20 million cells/mL may be close to the threshold density. To test whether seeding density was a factor in this “release” response, we also cultured MSC pellets under transient conditions and assessed GAG content and histological features. Consistent with findings for MSC constructs seeded at 60 million cells/mL, transient exposure to TGF-β3 induced significant increases in GAG accumulation in MSC pellets.
The ability of MSCs to generate functional matrix is merely one aspect of engineering viable replacement tissue. For clinical application, the phenotype of these cells must be maintained once removed from the controlled, prochondrogenic, chemically defined culture environment to the much less defined, and often inflammatory, in vivo setting. As other in vitro studies have demonstrated, we have shown here that a chondrocyte-like phenotype is maintained in constructs after initiation of chondrogenesis with transient exposure to TGF-β3. Furthermore, we have shown continuous expression of genes encoding cartilage matrix elements, as well as four genes encoding enzymes involved in proteoglycan synthesis. Although most of these genes were insensitive to TGF-β3 withdrawal, the expression levels of Galnac increased rapidly after removal of the growth factor. This was consistent across all “release” groups, including MSCs seeded at 20 million cells/mL (which did not improve in properties), and the levels achieved by 7 weeks exceeded that of the continuous exposure control groups. Although “release” may modulate the expression of Galnac to some extent, the complete molecular mechanism underlying the dramatic increases in mechanical properties and GAG content associated with the “release” response is still unclear. Larger-scale screening methods (e.g., microarray analysis) may be required to fully understand this phenomenon. Alternatively, factors not examined in this study, such as post-translational modifications, may play a crucial role and warrant further study.
Although transient application and removal of TGF-β3 from the culture medium did not adversely affect chondrogenic gene expression, histological analysis of our constructs revealed enlarged cell lacunae. This morphological feature is prominent in cells with high metabolic activities (producing large amounts of highly charged proteoglycan)51 but is also consistent with the hypertrophic changes associated with endochondral ossification. This morphology was not detected in MSCs seeded at 20 million cells/mL or in constructs cultured continuously in TGF-β3 (groups not undergoing a “release” response). Despite the presence of these enlarged cells, there was no additional evidence indicative of hypertrophic transitions taking place. There was no evidence of matrix mineralization or a shift to type I collagen production 4 weeks after removal of TGF-β3, and type X collagen deposition was also not observed. One study, which showed that TGF-β withdrawal alone was not sufficient to induce hypertrophy or mineralization in MSC pellets, supports this finding.52
Although we did not observe phenotypic changes in our MSC constructs in this study, the long-term stability of the cartilage phenotype of differentiated MSCs in the absence of prochondrogenic factors remains to be determined. Recent work assessing the chondrogenic commitment of MSCs demonstrated pronounced instability of phenotype when cultured under in vivo conditions.53,54 Implantation of chondrogenically differentiated MSCs resulted in extensive mineralization, coupled with the persistence of cartilage-like regions. Remarkably, articular chondrocytes showed no signs of ossification or hypertrophy when implanted in vivo, suggesting that intrinsic differences remain between these two cell types. In this study, enlarged lacunae were observed in chondrocyte- and MSC-seeded constructs undergoing release. Although TGF-β removal may not be sufficient to induce phenotypic changes, its absence may create a permissive environment for other factors present in vivo to drive osteogenesis in cells that are less complete in their commitment to the chondrocyte phenotype. In one in vitro study, Mueller et al. induced mineralization of chondrogenically differentiated MSC pellets by adding triiodothyronine and β-glycerophosphate after withdrawing TGF-β.52 Additional work, using chondrocyte- and MSC-seeded constructs, will be required to demonstrate the stability of the cartilage phenotype in our “released” constructs if they are to be viable candidates for regenerative therapies.
The work described herein demonstrates that initial seeding density and transient application of TGF-β3 regulate functional MSC chondrogenesis. The equilibrium modulus reported here (∼200 kPa) for constructs seeded at 60 million cells/mL and cultured with transient TGF-β3 exposure is the highest value we have achieved for MSCs seeded in agarose hydrogels. This modulus is approximately 50% of that of native bovine tissue5,6 and suggests the potential of these cells for the production of functional engineered cartilage constructs. Although the parameters used in this study generated MSC constructs with the highest equilibrium modulus we have ever achieved using these cells, the properties attained still fall below chondrocyte construct values when cultured under similar conditions. This finding further underscores the inherent differences that remain between these two cells types, even after MSCs commit to the chondrocyte phenotype. Although GAG content and compressive equilibrium properties approached native levels, collagen content and dynamic properties remained low for all cell-seeded constructs. Continuing optimization strategies should focus on these critical aspects of engineered cartilage constructs. We have recently developed high-throughput screening methods to identify and optimize new small-molecule mediators of chondrogenesis. We and others have also deployed novel materials55,56 and constructed mechanical loading systems57,58 that may better foster the chondrogenic differentiation process. These advances have the potential to generate functional cartilage replacements based on MSCs, especially if combined with the defined media regimes and seeding density requirements indicated from our results. Recent work using chondrocyte-based constructs demonstrate that mechanical loading applied in concert with transient exposure to TGF-β3 can generate neotissues with mechanical properties exceeding that of either treatment alone.38 Despite this potential, expectations must be tempered regarding the capacity of a newly differentiated cell type relative to fully differentiated cells and must take heed of the continuing necessity of promoting and maintaining the differentiated phenotype and functional properties after in vivo implantation.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (RO3 AR053668) and a Graduate Research Fellowship from the National Science Foundation (AHH). Additional support was provided by the Penn Center for Musculoskeletal Disorders. The type II collagen and type X collagen antibodies developed by Thomas F. Linsenmayer were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biology, University of Iowa, Iowa City, Iowa.
Disclosure Statement
The authors have no competing financial interests.
References
- 1.Williamson A.K. Chen A.C. Sah R.L. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19:1113. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 2.Ateshian G.A. Hung C.T. Functional properties of native articular cartilage. In: Guilak F., editor; Butler D.L., editor; Goldstein S.A., editor; Mooney D.J., editor. Functional Tissue Engineering. New York: Springer-Verlag; 2003. pp. 46–68. [Google Scholar]
- 3.Muir H. The chemistry of the ground substance of joint cartilage. In: Sokoloff L., editor. The Joints and Synovial Fluid. New York: Academic Press; 1980. pp. 27–94. [Google Scholar]
- 4.Park S. Hung C.T. Ateshian G.A. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage. 2004;12:65. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Athanasiou K.A. Rosenwasser M.P. Buckwalter J.A. Malinin T.I. Mow V.C. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9:330. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 6.Ateshian G.A. Warden W.H. Kim J.J. Grelsamer R.P. Mow V.C. Finite deformation biphasic material properties of bovine articular cartilage from confined compression experiments. J Biomech. 1997;30:1157. doi: 10.1016/s0021-9290(97)85606-0. [DOI] [PubMed] [Google Scholar]
- 7.Mankin H.J. Mow V.C. Buckwalter J.A. Iannotti J.P. Ratcliffe A. Form and function of articular cartilage. In: Simon S.R., editor. Orthopaedic Basic Science. Rosemont, IL: American Academy of Orthopedic Surgeons; 1994. pp. 2–43. [Google Scholar]
- 8.Buckwalter J.A. Martin J. Mankin H.J. Synovial joint degeneration and the syndrome of osteoarthritis. Instr Course Lect. 2000;49:481. [PubMed] [Google Scholar]
- 9.Hunziker E.B. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage. 1999;7:15. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 10.Li W.J. Jiang Y.J. Tuan R.S. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12:1775. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 11.Chung C. Erickson I.E. Mauck R.L. Burdick J.A. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Eng Part A. 2008;14:1121. doi: 10.1089/ten.tea.2007.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kisiday J. Jin M. Kurz B. Hung H. Semino C. Zhang S. Grodzinsky A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng K.W. Wang C.C. Mauck R.L. Kelly T.A. Chahine N.O. Costa K.D. Ateshian G.A. Hung C.T. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J Orthop Res. 2005;23:134. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Mouw J.K. Case N.D. Guldberg R.E. Plaas A.H. Levenston M.E. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Mauck R.L. Nicoll S.B. Seyhan S.L. Ateshian G.A. Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 16.Seidel J.O. Pei M. Gray M.L. Langer R. Freed L.E. Vunjak-Novakovic G. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41:445. [PubMed] [Google Scholar]
- 17.Kisiday J.D. Jin M. DiMicco M.A. Kurz B. Grodzinsky A.J. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Grodzinsky A.J. Levenston M.E. Jin M. Frank E.H. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 19.Blunk T. Sieminski A.L. Gooch K.J. Courter D.L. Hollander A.P. Nahir A.M. Langer R. Vunjak-Novakovic G. Freed L.E. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002;8:73. doi: 10.1089/107632702753503072. [DOI] [PubMed] [Google Scholar]
- 20.Gooch K.J. Blunk T. Courter D.L. Sieminski A.L. Vunjak-Novakovic G. Freed L.E. Bone morphogenetic proteins-2, -12, and -13 modulate in vitro development of engineered cartilage. Tissue Eng. 2002;8:591. doi: 10.1089/107632702760240517. [DOI] [PubMed] [Google Scholar]
- 21.Mauck R.L. Wang C.C. Oswald E.S. Ateshian G.A. Hung C.T. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Kisiday J.D. Kurz B. DiMicco M.A. Grodzinsky A.J. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 2005;11:141. doi: 10.1089/ten.2005.11.141. [DOI] [PubMed] [Google Scholar]
- 23.Williams G.M. Klein T.J. Sah R.L. Cell density alters matrix accumulation in two distinct fractions and the mechanical integrity of alginate-chondrocyte constructs. Acta Biomater. 2005;1:625. doi: 10.1016/j.actbio.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 26.Awad H.A. Wickham M.Q. Leddy H.A. Gimble J.M. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 27.Coleman R.M. Case N.D. Guldberg R.E. Hydrogel effects on bone marrow stromal cell response to chondrogenic growth factors. Biomaterials. 2007;28:2077. doi: 10.1016/j.biomaterials.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Sahoo S. Chung C. Khetan S. Burdick J.A. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules. 2008;9:1088. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W.J. Tuli R. Huang X. Laquerriere P. Tuan R.S. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Mauck R.L. Yuan X. Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Ponticiello M.S. Schinagl R.M. Kadiyala S. Barry F.P. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52:246. doi: 10.1002/1097-4636(200011)52:2<246::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 32.Huang A.H. Yeger-McKeever M. Stein A. Mauck R.L. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C.Y. Reuben P.M. D'Ippolito G. Schiller P.C. Cheung H.S. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec A Discov Mol Cell Evol Biol. 2004;278:428. doi: 10.1002/ar.a.20010. [DOI] [PubMed] [Google Scholar]
- 34.Park H. Temenoff J.S. Tabata Y. Caplan A.I. Mikos A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hui T.Y. Cheung K.M. Cheung W.L. Chan D. Chan B.P. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials. 2008;29:3201. doi: 10.1016/j.biomaterials.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Kavalkovich K.W. Boynton R.E. Murphy J.M. Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002;38:457. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Tuli R. Tuli S. Nandi S. Huang X. Manner P.A. Hozack W.J. Danielson K.G. Hall D.J. Tuan R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 38.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S. Ateshian G.A. Hung C.T. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byers B.A. Mauck R.L. Chiang R.L. Tuan R.S. Temporal exposure of TGF-beta3 under serum-free conditions enhances biomechanical and biochemical maturation of tissue-engineered cartilage. Trans Orthop Res Soc. 2006;31:43. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caterson E.J. Nesti L.J. Li W.J. Danielson K.G. Albert T.J. Vaccaro A.R. Tuan R.S. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57:394. doi: 10.1002/1097-4636(20011205)57:3<394::aid-jbm1182>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Mehlhorn A.T. Schmal H. Kaiser S. Lepski G. Finkenzeller G. Stark G.B. Sudkamp N.P. Mesenchymal stem cells maintain TGF-beta-mediated chondrogenic phenotype in alginate bead culture. Tissue Eng. 2006;12:1393. doi: 10.1089/ten.2006.12.1393. [DOI] [PubMed] [Google Scholar]
- 42.Ballock R.T. Heydemann A. Wakefield L.M. Flanders K.C. Roberts A.B. Sporn M.B. TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993;158:414. doi: 10.1006/dbio.1993.1200. [DOI] [PubMed] [Google Scholar]
- 43.Moioli E.K. Hong L. Guardado J. Clark P.A. Mao J.J. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006;12:537. doi: 10.1089/ten.2006.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moioli E.K. Hong L. Mao J.J. Inhibition of osteogenic differentiation of human mesenchymal stem cells. Wound Repair Regen. 2007;15:413. doi: 10.1111/j.1524-475X.2007.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stegemann H. Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 46.Neuman R.E. Logan M.A. The determination of hydroxyproline. J Biol Chem. 1950;184:299. [PubMed] [Google Scholar]
- 47.Huang A.H. Yeger-McKeever M. Stein A. Mauck R.L. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthritis Cartilage. 2008;16:1074. doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oberlender S.A. Tuan R.S. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120:177. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 49.Tacchetti C. Tavella S. Dozin B. Quarto R. Robino G. Cancedda R. Cell condensation in chondrogenic differentiation. Exp Cell Res. 1992;200:26. doi: 10.1016/s0014-4827(05)80067-9. [DOI] [PubMed] [Google Scholar]
- 50.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Kimura J.H. Hunziker E.B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 51.Quinn T.M. Schmid P. Hunziker E.B. Grodzinsky A.J. Proteoglycan deposition around chondrocytes in agarose culture: construction of a physical and biological interface for mechanotransduction in cartilage. Biorheology. 2002;39:27. [PubMed] [Google Scholar]
- 52.Mueller M.B. Tuan R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jukes J.M. Both S.K. Leusink A. Sterk L.M. van Blitterswijk C.A. de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:6840. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelttari K. Winter A. Steck E. Goetzke K. Hennig T. Ochs B.G. Aigner T. Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 55.Connelly J.T. Garcia A.J. Levenston M.E. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28:1071. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Kisiday J.D. Kopesky P.W. Evans C.H. Grodzinsky A.J. McIlwraith C.W. Frisbie D.D. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008;26:322. doi: 10.1002/jor.20508. [DOI] [PubMed] [Google Scholar]
- 57.Mauck R.L. Byers B.A. Yuan X. Tuan R.S. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 58.Mouw J.K. Connelly J.T. Wilson C.G. Michael K.E. Levenston M.E. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells. 2007;25:655. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.