Abstract
Synovial membrane has been shown to contain mesenchymal stem cells. We hypothesized that an enriched population of synovial fibroblasts would undergo chondrogenic differentiation and secrete cartilage extracellular matrix to a greater extent than would a mixed synovial cell population (MSCP). The optimum doses of transforming growth factor beta 1 (TGF-β1) and insulin-like growth factor 1 (IGF-1) for chondrogenesis were investigated. CD14-negative isolation was used to obtain a porcine cell population enriched in type-B synovial fibroblasts (SFB) from an MSCP. The positive cell surface markers in SFB were CD90, CD44, and cadherin-11. SFB and MSCP were cultured in the presence of 20 ng/mL TGF-β1 for 7 days, and SFB were demonstrated to have higher chondrogenic potential. Further dose–response studies were carried out using the SFB cells and several doses of TGF-β1 (2, 10, 20, and 40 ng/mL) and/or IGF-1 (1, 10, 100, and 500 ng/mL) for 14 days. TGF-β1 supplementation was essential for chondrogenesis and prevention of cell death, whereas IGF-1 did not have a significant effect on the SFB cell number or glycosaminoglycan production. This study demonstrates that the CD14-negative isolation yields an enhanced cell population SFB that is more potent than MSCP as a cell source for cartilage tissue engineering.
Introduction
Tissue engineering has emerged as an alternative approach to current treatments for cartilage defects, such as autologous chondrocyte implantation, microfracture, or prosthetic replacement. This approach generally employs in vitro cultivation of chondrocytes seeded on biocompatible polymers before implantation.1–3 While autologous chondrocytes have been used with relative success to generate cartilage-like tissues, one of the most important limitations of this cell source is the availability of adequate number of chondrocytes, which necessitates in vitro cell expansion, which is costly and can lead to loss of chondrogenic capacity.4 In addition, a new defect needs to be created in the uninvolved cartilage to collect cells, which has limited self-repair capacity and thus may lead to future problems. Alternative cell sources with chondrogenic potential include embryonic stem cells, mesenchymal progenitor cells derived from synovium, adipose tissue, and periosteum, among others.5–10
Synoviocytes have been shown to have chondrogenic potential,10–14 thus making them a suitable source for autologous chondrogenic precursor cells. Synoviocytes are located in the synovial joint lining within the joint capsule and are readily obtainable by surgical synovectomy. Synovectomy is a surgical procedure that can be accomplished with relatively low morbidity due to the high regenerative capacity of the synovium. Synoviocytes are typically supplemented with transforming growth factor-beta 1 (TGF-β1) to induce chondrogenesis.10,14 Other growth factors such as TGF-β3,15 insulin-like growth factor-1 (IGF-1),16,17 and bone morphogenetic proteins12,18 have also been used to induce chondrogenesis in synovial cells or other mesenchymal stem cells (MSCs). The dosing regimen for optimum chondrogenesis depends on each cell type; therefore, there is not an established universal dosing regimen for these growth factors.
The synovial membrane consists of type-A macrophage-derived synoviocytes and type-B synovial fibroblasts (SFB).19 Morphological and immunocytochemical analyses of SFB suggest similarities to MSC; therefore, it is plausible that they offer the multilineage differentiation potential of the synovium-derived MSC.20 In this study we hypothesized that a population enriched with SFB would be more chondrogenic than would a mixed cell population of synoviocytes. SFB can be purified by repeated passages that eliminate macrophages; however, cellular functions may change with increasing passages.21–24 Therefore, we used a negative isolation technique, which was previously demonstrated to isolate rheumatoid arthritis-SFB using anti-CD14 monoclonal antibodies.25 Dose–response studies were then carried out using TGF-β1 and IGF-1 alone and in combination to determine the optimum growth factor dosing regimen for chondrogenic differentiation of SFB.
Materials and Methods
Unless otherwise specified, all reagents were from Sigma-Aldrich (St. Louis, MO). Dulbecco's modified Eagle's medium (DMEM), phosphate buffered saline (PBS), and Dynabeads® CD14 were from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was from HyClone (Logan, UT).
Digestion of synovium
Primary cells were isolated from the synovial membranes of 4-month-old female pig knee joints by surgical synovectomy, mincing, and enzymatic digestion using 0.1% trypsin in PBS (30 min) and 0.1% collagenase II in 10% FBS/DMEM (2 h) at 37°C, followed by filtration with 70 μm cell strainers. Adipocytes were separated by centrifugation. The cells were expanded until 80% confluence (3 days) in high-glucose DMEM supplemented with 10% FBS, 1% ITS + Premix (BD Biosciences, San Jose, CA), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 2.5 μg/mL amphotericin-B. Nonadherent cells were removed by changing the medium every 2 days.
Negative isolation of SFB
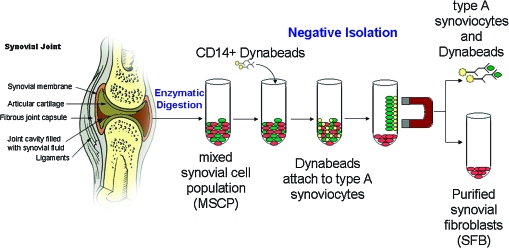
Synovium is the primary tissue, and the mixed synovial cell population (MSCP) contains type A-synovial cells and SFB. SFB were negatively isolated from MSCP by magnetic bead separation using Dynabeads CD1425 (Fig. 1a). Briefly, adherent MSCP were trypsinized (0.25% trypsin/0.2% EDTA), and 107/mL MSCP were incubated at 4°C for 1 h with washed 4 × 107/mL Dynabeads CD14 in 2% FBS/PBS. The CD14-positive cells (monocytes and macrophages) were collected using the Dynal Magnetic Particle Concentrator® and discarded as they were attached to the CD14-Dynabeads. The depleted supernatant was enriched with SFB.
FIG. 1.
Schematics of the negative isolation of type-B synovial fibroblasts (SFB) from a mixed synovial cell population (MSCP) using CD14-Dynabeads. Color images available online at www.liebertonline.com/ten.
Flow cytometry
Single-cell suspensions of MSCP and SFB cells were resuspended in PBS with 2% FBS and stained with directly conjugated monoclonal antibodies for CD44, CD90, Cadherin11, and CD68. Indirect flow cytometry was performed with monoclonal CD14 and then incubated with secondary antibody with FITC. The antibodies were purchased from Abcam (Cambridge, MA), eBioscience (SanDiego, CA), BD Biosciences, R&D Systems (Minneapolis, MN), and AbD Serotec (Raleigh, NC). Flow cytometry was performed with Becton Dickinson FACSort (San Jose, CA) and analyzed with FlowJo software (TreeStar, Asland, OR).
Pellet culture
About 5 × 105 cells (SFB or MSCP) were centrifuged (1200 rpm, 5 min) in 15 mL tubes to form a pellet.26 The pellets were cultured in 2 mL of a defined differentiation medium (high-glucose DMEM, 40 μg/mL proline, 100 nM dexamethasone, 0.1 mM ascorbic acid 2-phosphate, 100 U/mL penicillin, 100 mg/L streptomycin, and ITS+Premix (insulin [6.25 μg/mL], transferrin [6.25 μg/mL], selenous acid [6.25 μg/mL], linoleic acid [5.35 μg/mL], and bovine serum albumin [1.25 μg/mL])), with or without growth factor(s) TGF-β1 (2, 10, 20, 40 ng/mL) and/or IGF-1 (1, 10, 100, 500 ng/mL) (R&D Systems) at 37°C, 5% CO2. After 3 days, the pellets were transferred into 24-well plates on an orbital shaker for dynamic mixed culture and cultivated until harvest at 7 or 14 days.27 The medium was changed every 2 days.
Histology and immunohistochemistry
Pellets were fixed in 4% paraformaldehyde in PBS (pH 7.4) at 4°C for 24 h, alcohol-dehydrated, and paraffin-embedded. Five-micron sections were stained with safranin O/fast Green for glycosaminoglycan (GAG) and immunostained with monoclonal antibodies for type II collagen (NeoMarkers, Fremont, CA). Immunohistochemical sections were hydrated and incubated (30 min at room temperature [RT]) with 2 mg/mL testicular hyaluronidase in PBS (pH 5), rinsed with PBS, incubated for 30 min at RT with normal goat serum diluted at 1:10 in PBS followed by primary antibody for 1 h at RT, stained using diaminobenzidine (DAB) using Vectastain ABC kit (Burlingame, CA), and counterstained with hematoxylin.
Biochemical analyses
Pellets (n = 3 specimens per time point per group) were frozen and digested overnight at 60°C with 100 μL of 125 μg/mL papain and 10 mM cysteine in PBE buffer (100 mM phosphate and 10 mM EDTA, pH 6.5). DNA content was measured using Hoechst 33258 dye,28 and GAG was measured using 1–9-dimethylmethylene blue dye.29
Real-time quantitative reverse transcriptase–polymerase chain reaction
Samples were homogenized using an RNase-free pestle in TRIzol reagent (Life Technologies, Grand Island, NY), and RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA) following manufacturer's instructions. Quantification of mRNA was performed by real-time quantitative reverse transcriptase–polymerase chain reaction (PCR) with DNA Engine Opticon™ system (MJ Research, Waltham, MA). Porcine-specific PCR primers were designed according to the sequences available in GenBank using Gene-Tool software (BioTools, Edmonton, Alberta, Canada) (Table 1).
Table 1.
Nucleotide Sequences of Porcine-Specific Primers Used in Quantitative Polymerase Chain Reaction
| Target gene and GenBank accession number | Product size (bp) | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|---|
| Aggrecan AF201722b36 | 79 | TGCAGGTGACCATGGCC | CGGTAATGGAACACAACCCCT |
| Type II collagen AF20172437 | 106 | CCATCTGGCTTCCAGGGAC | CCACGAGGCCAGGAGCT |
| CD14 DQ079063 | 148 | CTGCACTCGGCCCTGGTCAAG | GCCCAAAGACAGCCATGACAAA |
| Vimentin AU05870736 | 144 | GGAAGGAGAAGAGAGCAGGATTTC | CCATCTCTGGTCTCAACCGTCT |
| 18S AY265350 | 180 | CGGCTACCACATCCAAGGAA | GCTGGAATTACCGCGGCT |
RNA (100–300 ng each) was used for oligo(dT)12–18-primed cDNA synthesis using SuperScript™ II RT (Invitrogen). The DyNAmo™ SYBR Green qPCR kit (MJ Research) was used for real-time analysis of cDNA samples from different groups. The cycle parameters were 95°C-10 min to activate the Tbr DNA polymerase, and then 39 cycles at 94°C-10 s denaturation, followed by 55°C-20 s annealing, and 72°C-20 s extension. The last extension was 72°C-5 min. The 18S RNA was amplified in parallel and used as an internal control. The cycle threshold values for 18S RNA and samples were measured (Perkin-Elmer, Wellesley, MA), and the relative transcription levels were calculated.30
Statistical analysis
Statistics in experiments comparing MSCP and SFB were assessed using one-way analysis of variance (ANOVA) with post Tukey-Kramer multiple comparisons test using InStat 3.0 (GraphPad Software, San Diego, CA). p < 0.05 was considered statistically significant.
ANOVA was used to model the differential changes associated with different doses of TGF-β1 over time (experiment A) and the degree to which different doses of IGF-1 moderate these effects (experiment B) using SAS version 9.2 (SAS Institute, Cary, NC). Experiment A crossed two factors: (1) four doses of TGF-β1 (2, 10, 20, and 40 ng/mL) and a control, by (2) three time points (3, 7, and 14 days). Experiment B crossed three factors: (1) one dose of TGF-β1 (10 ng/mL) and a control, (2) by four doses of IGF-1 (1, 10, 100, and 500 ng/mL) and a control, and (3) by three time points (3, 7, and 14 days). Each ANOVA included effects for each factor's main effect and all possible interactions. The highest-level statistically significant effects (three-way interaction > two-way interaction > main effect) were followed-up using orthogonal contrasts to test individual hypotheses. The overall alpha of the analysis was maintained at 0.05 across these multiple follow-up comparisons using the Holm test. GAG levels were positively skewed about their group means and so the GAG data were log (base 2) transformed before analysis, and the means and ±SE limits were back-transformed to their natural units for presentation (creating geometric means ± geometric SE ½ widths).
Results
Purification of synovial cells
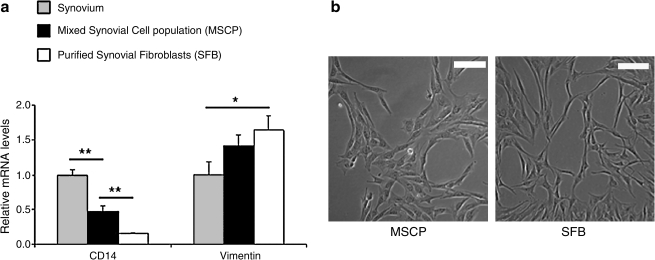
The negative isolation procedure resulted in an enriched SFB population. CD14, a cell surface marker expressed by monocytes and macrophages, was expressed less by SFB compared with both MSCP and the native synovial tissue (Fig. 2). Vimentin is the major subunit protein of the intermediate filaments of mesenchymal cells and is expressed highest in the enriched SFB cells, followed by MSCP (Fig. 2). As a result, the SFB were demonstrated to be an enriched population with increased Vimentin/CD14 expression ratio per cell.
FIG. 2.
(a) CD14 and Vimentin expression in synovium, MSCP, and purified SFB after negative isolation. Data are presented as mean ± SD; *p < 0.05, **p < 0.01; n = 3. SFB population has an increased Vimentin/CD14 expression ratio compared with that of MSCP. (b) 10 × images of MSCP and SFB; bar represents 100 μm.
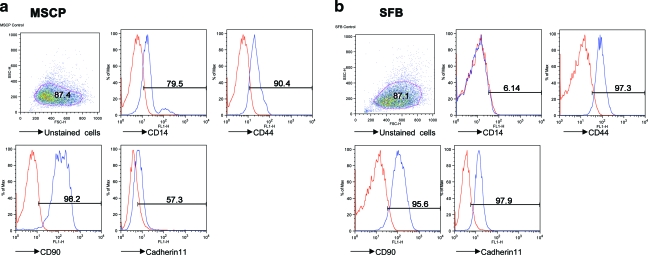
Figure 3 summarizes the surface markers used in the present study and shows their expression levels. The positive markers in MSCP were CD14, CD44, CD90, and cadherin-11. After purification, the macrophage marker CD14 disappeared, and MSC markers (CD44 [Hermes antigen, hyaluronan receptor], CD90 [Thy-1], and cadherin-11) showed increased expression levels.
FIG. 3.
Characterization of the (a) MSCP and (b) purified SFB using flow cytometry. Color images available online at www.liebertonline.com/ten.
TGF-β1–induced chondrogenesis
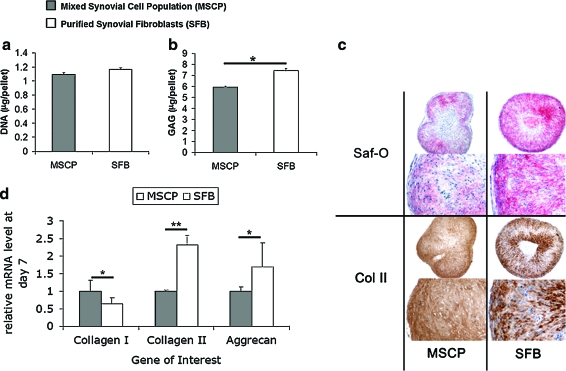
To compare the chondrogenic potential of MSCP and SFB, cell pellets were cultivated for 7 days in the presence of 20 ng/mL TGF-β1, a chondrogenic growth factor. The DNA content capacity of the two cell populations was identical (Fig. 4a). The GAG production in SFB cells was higher than that of MSCP at the end of 7 days (7.4 ± 0.2 μg vs. 5.9 ± 0.1 μg) (Fig. 4b), and these results were confirmed by qualitative observations of denser safranin-O staining in the SFB-pellet cross sections (Fig. 4c). Type II collagen staining was observed in both groups (Fig. 4c). The relative expression of chondrogenic genes of interest (aggrecan and type II collagen) was greater in the purified SFB cells, and the expression of type I collagen was less in SFB compared with those by MSCP (Fig. 4d).
FIG. 4.
Comparison of the proliferative and chondrogenic potential of MSCP with the purified SFB cultivated for 7 days with 20 ng/mL TGF-β1. (a) DNA and (b) GAG per pellet (c) histological cross sections of MSCP pellets and SFB pellets depicting staining for GAG (safranin-O/fast green) and type II collagen (Immunohistochemistry). Scale bars are 200 μm. (d) Relative mRNA expression of aggrecan, type II collagen, and type I collagen genes in the SFB pellets with respect to those in the MSCP pellets. Data are presented as mean ± SD; *p < 0.05, **p < 0.01; n = 3. TGF-β1, transforming growth factor beta 1; GAG, glycosaminoglycan. Color images available online at www.liebertonline.com/ten.
Dose–response studies with TGF-β1
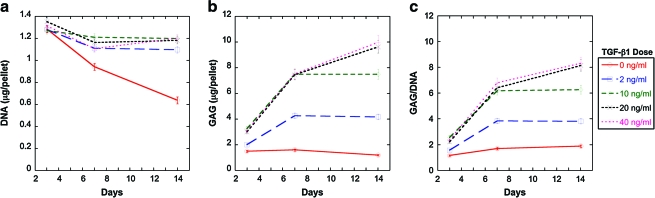
After demonstrating the chondrogenic potential in SFB cells in the presence of 20 ng/mL TGF-β1, a study was carried out to determine the optimum TGF-β1 concentration for chondrogenesis. The SFB cells were cultivated in pellet cultures for 14 days with 0, 2, 10, 20, or 40 ng/mL TGF-β1. The DNA content decreased significantly over time (p < 0.0001) without any supplementation. TGF-β1 supplementation maintained the DNA content at 3, 7, and 14 days; the DNA contents per pellet at day 3 were not significantly different between groups; however, the group without TGF-β1 supplementation had significantly less DNA than those supplemented with TGF-β1 at day 7 (p < 0.01) and day 14 (p < 0.001). No differences among doses of 2–40 ng/mL TGF-β1 were observed at day 14 (Fig. 5a).
FIG. 5.
Dose–response of SFB pellets to 0, 2, 10, 20, and 40 ng/mL TGF-β1: (a) DNA, (b) GAG, and (c) GAG/DNA contents of pellets cultivated for 14 days are listed as geometric mean ± SE, n = 3. Color images available online at www.liebertonline.com/ten.
The GAG deposition in the pellets at 3 days was lower with 0 and 2 ng/mL TGF-β1 supplementation than that with 10–40 ng/mL TGF-β1 supplementation (p < 0.05) (Fig. 5b). The GAG content per pellet at 7 days remained the lowest in pellets without TGF-β1 supplementation (p < 0.0001 compared with any dose of TGF-β1). From 3 to 7 days, 2, 10, and 20 ng/mL TGF-β1 supplementation increased the GAG content 2.1-, 2.3-, and 2.5-fold (p < 0.00001) with respect to GAG contents in day 3. From 3 to 14 days, 2, 10, and 20 ng/mL TGF-β1 supplementation increased the GAG content 2.6-, 2.9-, and 4.0-fold (p < 0.00001), respectively. No significant differences were observed among 10–40 ng/mL at 7 days and 20–40 ng/mL at 14 days. Doubling the TGF-β1 dose from 10 to 20 ng/mL increased the GAG content by only 28% at 14 days (p < 0.01).
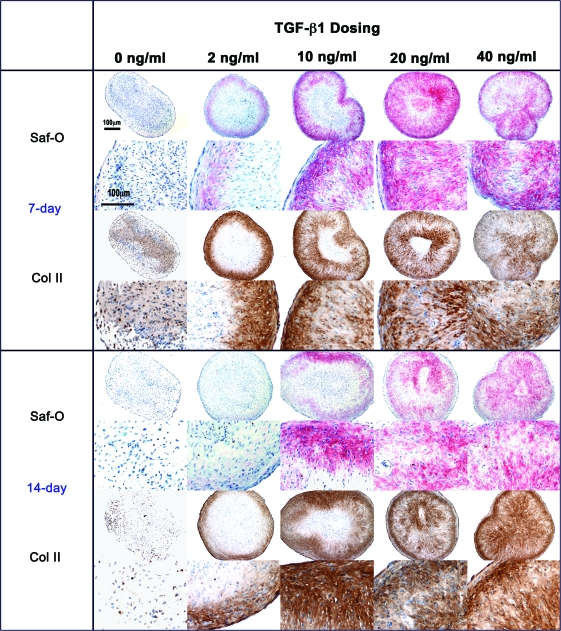
Qualitative observation of the histological cross sections supports the biochemical results that supplementation with TGF-β1 enhances extracellular matrix (ECM) production (Fig. 6). The pellets that were cultivated without TGF-β1 or with 2 ng/mL TGF-β1 exhibited little or no positive staining, while TGF-β1 doses of 10–40 ng/mL demonstrated GAG presence (safranin-O) and type II collagen. The center of the pellets had little or no staining that may possibly indicate diffusion limitations. Some of the 7-day samples (e.g., 2 and 20 ng/mL TGF-β1) appear to have more intense GAG and type II collagen staining than the 14-day samples in Figure 6, contrary to the biochemical analyses that indicate that GAG content increases with time. These samples depict the staining of a single pellet, which may have more or less staining than the other pellets used for the calculation of mean GAG content. Therefore, the histological analyses were used just as a qualitative method to confirm the presence of staining.
FIG. 6.
Histological cross sections of SFB pellets cultivated in the presence of 0, 2, 10, 20, and 40 ng/mL TGF-β1. Sections were stained with safranin-O/fast green for GAG and immunostained using type II collagen monoclonal antibodies. Scale bars are 100 μm. Color images available online at www.liebertonline.com/ten.
Dose–response studies with IGF-1
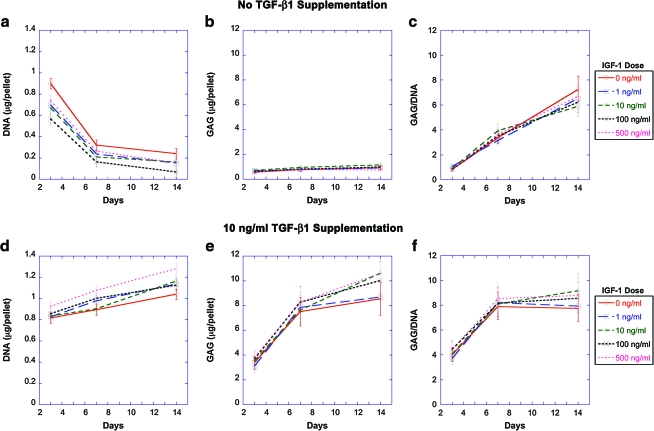
Pellets were cultivated with 0, 1, 10, 100, and 500 ng/mL IGF-1 with or without the presence of 10 ng/mL TGF-β1. Without TGF-β1 supplementation, the DNA content of pellets decreased with time from day 3 to 7 (p < 0.0001) and the decrease slowed from day 7 to 14 (p < 0.01), regardless of the concentration of IGF-1 (Fig. 7a). The GAG contents of the pellets were not affected by IGF-1 concentration and remained constantly low over time without TGF-β1 supplementation (Fig. 7b). GAG/DNA contents of the pellets were not influenced by IGF-1 and significantly increased over time (Fig. 7c).
FIG. 7.
Dose–response of SFB pellets cultivated in the presence of 0, 1, 10, 100, and 500 ng/mL IGF-1 supplemented (a–c) without any TGF-β1 and (d–f) with 10 ng/mL TGF-β1. (a, d) DNA, (b, e) GAG, and (c, f) GAG/DNA contents of pellets cultivated for 14 days are listed as geometric mean ± SE, n = 3. Color images available online at www.liebertonline.com/ten.
When 10 ng/mL TGF-β1 was supplied with IGF-1, the DNA content increased with time (p < 0.0001) (Fig. 7d). The GAG and GAG/DNA contents increased significantly from day 3 to 7 (p < 0.001) and the increase slowed down from day 7 to 14 (p < 0.05) (Fig. 7e, f). IGF-1 did not significantly influence the DNA and GAG contents of the pellets.
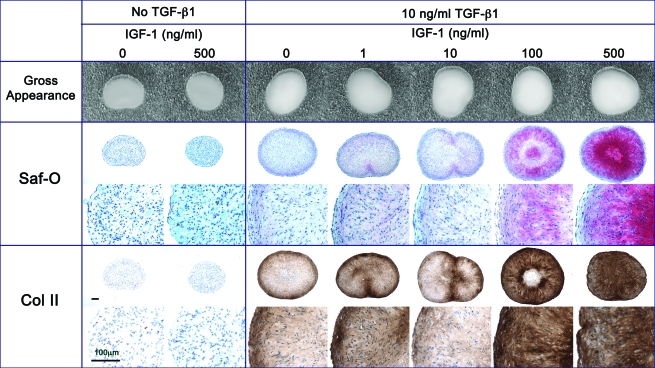
Qualitative observations of the histological cross sections of pellets revealed that the pellets cultivated without TGF-β1 appeared smaller than those cultured with TGF-β1 and had no visible staining for safranin-O or type II collagen, regardless of the IGF-1 dosing. However, it appeared that 500 ng/mL IGF-1 with 10 ng/mL TGF-β1 supplementation resulted in denser staining for both safranin-O and type II collagen antibodies than in pellets cultivated without TGF-β1 regardless of the presence of IGF-1 and those cultivated with TGF-β1 and lower doses of IGF-1 (1,10,100 ng/mL) (Fig. 8).
FIG. 8.
Morphology of SFB pellets cultivated for 14 days in the presence of 0, 1, 10, 100, and 500 ng/mL IGF-1 supplemented with or without 10 ng/mL TGF-β1. Sections were stained with safranin-O/fast green for GAG and immunostained using type II collagen monoclonal antibodies. Scale bars are 100 μm. Color images available online at www.liebertonline.com/ten.
Discussion
In this study, we employed a negative isolation procedure to obtain an enriched population of SFB. In vitro observations suggest that the type-A cells do not proliferate as quickly as the SFB cells and the SFB cells generally outgrow MSCP after expansion for several passages31,32; characterization of MSCP during in vitro expansion procedures reveals that cells appear to develop a stronger gene expression of vimentin and no expression of CD14 (a monocyte/macrophage marker) after three passages.10 Hence, an enriched SFB population could be obtained by simply passaging the cells at least three times; however, this is a lengthy and expensive procedure compared to the CD14-negative isolation procedure, which can be used to isolate SFB immediately after the digestion of synovium tissue. Our results are in agreement with those previous studies that increased vimentin and decreased CD14 expression occur in SFB after negative isolation. The surface marker characterization of the SFB population revealed that it was enriched with cells expressing MSC-related markers CD44 (a hyaluronic acid receptor), CD90 (Thy-1 cell surface antigen), and cadherin-11 (an upregulator of osteoblastic differentiation in MSCs). Other advantages of the negative isolation method include the prevention of adherence of the CD14-antibody–magnetic bead complex to the cell population of interest, that is, SFB, since the recovery of cells that adhere to the magnetic beads is not feasible due to possible functional alterations of cells.25 Finally, this technique provides an adequate number of cells and eliminates the risk of cellular dedifferentiation of the synovial MSC within monolayer cultures during passaging. Such a quick isolation technique may be beneficial in therapeutic tissue engineering applications to repair cartilage and bone defects.
The isolated SFB population demonstrated a greater chondrogenic capacity than did the MSCP, as evidenced by the biochemical and histological assessment of 7-day pellet cultures, as well as by the changes in expression of the chondrogenic marker genes. We believe that this increased potency stems from the removal of CD14-positive monocytes and macrophages, that is, type-A synoviocytes, which do not undergo chondrogenic differentiation, enriching the SFB population used for the in vitro experiments.
The TGF-β superfamily, including bone morphogenetic proteins, has been widely used to induce chondrogenesis in MSC.12,26,33 Our data also suggest that at least 10 ng/mL TGF-β1 is essential for maintaining the DNA content and secretion of GAG. Longobardi et al.17 report that IGF-1 modulates MSC chondrogenesis by stimulating proliferation, regulating cell apoptosis, and inducing expression of type II collagen and sox9 in MSC; however, our data suggest that IGF-1 alone does not influence chondrogenesis nor cell proliferation in SFB cells, demonstrated by decreasing DNA content over time and very low levels of GAG. When TGF-β1 was supplied with IGF-1, DNA and GAG contents increased over time, but the IGF-1 dosing did not have a significant effect. It is important to note that the study by Longobardi et al.17 was conducted in the absence of insulin, whereas our culture medium contained insulin. The fact that IGF-1 alone does not increase ECM production, and the synergy of IGF-1 and TGF-β1 was previously reported in in vitro studies using MSC13; Worster et al.16 showed that pretreatment of MSC with TGF-β1 before IGF-1 treatment was more conducive to chondrogenesis. While our histological sections depicted increased GAG staining with 500 ng/mL IGF-1 and 10 ng/mL TGF-β1, analyses of the DNA and GAG contents of the SFB pellets with three-way ANOVA detected no significant interaction between TGF-β1 and IGF-1 (p = 0.26). Sakimura et al.34 supplemented cultures of synovial cells seeded on PGA scaffolds with 10 ng/mL TGF-β1 and 100 ng/mL IGF-1, and reported increased ECM production with IGF-1 after 8 weeks of cultivation. While some studies suggest that IGF-1 enhances ECM production in chondrocytes,35 it is plausible that MSC are less likely to experience the effects of IGF-1 when they have not undergone chondrogenic differentiation.
In conclusion, our results suggest that the negative isolation technique is a feasible method for the quick isolation of SFB from synovial membrane, that SFB have a higher chondrogenic potential than do MSCP, and that TGF-β1 (10 ng/mL) is vital for chondrogenesis in SFB, while IGF-1 is ineffective. Gene expression analysis comparing SFB and MSCP responses to IGF-1 and other growth factors would aid in better understanding the effects of these growth factors. Further studies are required to test the effects of other chondrogenic growth factor sequences on SFB and optimization of the purification of SFB using MSC surface markers. This study demonstrates that the SFB cells represent a promising cell source for orthopedic tissue engineering applications, and translational studies using SFB and optimum doses of growth factors in vivo are required to assess the repair capacity of these cells in cartilage defects.
Acknowledgments
This work was supported by the Aircast Foundation, Brown University VP Research Seed Grant, and the Office of Research and Development, RR&D Service, U.S. Department of Veterans Affairs, and COBRE (NIH 1P20-RR024484) Center grant from the National Institutes of Health. The authors would like to acknowledge Jason T. Machan, Ph.D., for statistical analyses of the growth factor dose–response experiments and Sara Spangenberger (Core Research Laboratories, Rhode Island Hospital) for flow cytometry.
Disclosure Statement
No competing financial interests exist.
References
- 1.Freed L.E. Marquis J.C. Nohria A. Emmanual J. Mikos A.G. Langer R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 2.Vacanti C.A. Langer R. Schloo B. Vacanti J.P. Synthetic polymers seeded with chondrocytes provide a template for new cartilage formation. Plast Reconstr Surg. 1991;88:753. doi: 10.1097/00006534-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Nehrer S. Breinan H.A. Ramappa A. Hsu H.P. Minas T. Shortkroff S. Sledge C.B. Yannas I.V. Spector M. Chondrocyte-seeded collagen matrices implanted in a chondral defect in a canine model. Biomaterials. 1998;19:2313. doi: 10.1016/s0142-9612(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 4.Dell'Accio F. De Bari C. Luyten F.P. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44:1608. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H. Murphy C.L. Murphy C. Kimura M. Kawai S. Polak J.M. Chondrogenic differentiation of murine embryonic stem cells: effects of culture conditions and dexamethasone. J Cell Biochem. 2004;93:454. doi: 10.1002/jcb.20171. [DOI] [PubMed] [Google Scholar]
- 6.Erickson G.R. Gimble J.M. Franklin D.M. Rice H.E. Awad H. Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 7.Tuli R. Nandi S. Li W.J. Tuli S. Huang X. Manner P.A. Laquerriere P. Noth U. Hall D.J. Tuan R.S. Human mesenchymal progenitor cell-based tissue engineering of a single-unit osteochondral construct. Tissue Eng. 2004;10:1169. doi: 10.1089/ten.2004.10.1169. [DOI] [PubMed] [Google Scholar]
- 8.Guilak F. Awad H.A. Fermor B. Leddy H.A. Gimble J.M. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41:389. [PubMed] [Google Scholar]
- 9.De Bari C. Dell'Accio F. Luyten F.P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.De Bari C. Dell'Accio F. Tylzanowski P. Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Iwata H. Ono S. Sato K. Sato T. Kawamura M. Bone morphogenetic protein-induced muscle- and synovium-derived cartilage differentiation in vitro. Clin Orthop Relat Res. 1993;296:295. [PubMed] [Google Scholar]
- 12.Park Y. Sugimoto M. Watrin A. Chiquet M. Hunziker E.B. BMP-2 induces the expression of chondrocyte-specific genes in bovine synovium-derived progenitor cells cultured in three-dimensional alginate hydrogel. Osteoarthritis Cartilage. 2005;13:527. doi: 10.1016/j.joca.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Shirasawa S. Sekiya I. Sakaguchi Y. Yagishita K. Ichinose S. Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura K. Solchaga L.A. Caplan A.I. Yoo J.U. Goldberg V.M. Johnstone B. Chondroprogenitor cells of synovial tissue. Arthritis Rheum. 1999;42:2631. doi: 10.1002/1529-0131(199912)42:12<2631::AID-ANR18>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki T. Muneta T. Sakaguchi Y. Nimura A. Yokoyama A. Koga H. Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 16.Worster A.A. Brower-Toland B.D. Fortier L.A. Bent S.J. Williams J. Nixon A.J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 17.Longobardi L. O'Rear L. Aakula S. Johnstone B. Shimer K. Chytil A. Horton W.A. Moses H.L. Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21:626. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 18.Majumdar M.K. Wang E. Morris E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 19.Edwards J.C. The nature and origins of synovium: experimental approaches to the study of synoviocyte differentiation. J Anat. 1994;184(Pt 3):493. [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenabeele F. De Bari C. Moreels M. Lambrichts I. Dell'Accio F. Lippens P.L. Luyten F.P. Morphological and immunocytochemical characterization of cultured fibroblast-like cells derived from adult human synovial membrane. Arch Histol Cytol. 2003;66:145. doi: 10.1679/aohc.66.145. [DOI] [PubMed] [Google Scholar]
- 21.Bonab M.M. Alimoghaddam K. Talebian F. Ghaffari S.H. Ghavamzadeh A. Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crisostomo P.R. Wang M. Wairiuko G.M. Morrell E.D. Terrell A.M. Seshadri P. Nam U.H. Meldrum D.R. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 23.Allen K.D. Athanasiou K.A. Effect of passage and topography on gene expression of temporomandibular joint disc cells. Tissue Eng. 2007;13:101. doi: 10.1089/ten.2006.0094. [DOI] [PubMed] [Google Scholar]
- 24.Kang S.W. Yoo S.P. Kim B.S. Effect of chondrocyte passage number on histological aspects of tissue-engineered cartilage. Biomed Mater Eng. 2007;17:269. [PubMed] [Google Scholar]
- 25.Zimmermann T. Kunisch E. Pfeiffer R. Hirth A. Stahl H.D. Sack U. Laube A. Liesaus E. Roth A. Palombo-Kinne E. Emmrich F. Kinne R.W. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture—primary culture cells markedly differ from fourth-passage cells. Arthritis Res. 2001;3:72. doi: 10.1186/ar142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 27.Bilgen B. Orsini E. Aaron R.K. Ciombor D.M. FBS suppresses TGF-beta-1-induced chondrogenesis in synoviocyte pellet cultures while dexamethasone and dynamic stimuli are beneficial. J Tissue Eng Regen Med. 2007;1:436. doi: 10.1002/term.56. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y.-J. Sah R.L.Y. Doong J.-Y. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 29.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 30.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi Y. Sekiya I. Yagishita K. Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 32.Rosengren S. Boyle D.L. Firestein G.S. Acquisition, culture, and phenotyping of synovial fibroblasts. Methods Mol Med. 2007;135:365. doi: 10.1007/978-1-59745-401-8_24. [DOI] [PubMed] [Google Scholar]
- 33.Kurth T. Hedbom E. Shintani N. Sugimoto M. Chen F.H. Haspl M. Martinovic S. Hunziker E.B. Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthritis Cartilage. 2007;15:1178. doi: 10.1016/j.joca.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Sakimura K. Matsumoto T. Miyamoto C. Osaki M. Shindo H. Effects of insulin-like growth factor I on transforming growth factor beta1 induced chondrogenesis of synovium-derived mesenchymal stem cells cultured in a polyglycolic acid scaffold. Cells Tissues Organs. 2006;183:55. doi: 10.1159/000095509. [DOI] [PubMed] [Google Scholar]
- 35.Fortier L.A. Mohammed H.O. Lust G. Nixon A.J. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 36.Chen J. Baer A.E. Paik P.Y. Yan W. Setton L.A. Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem Biophys Res Commun. 2002;293:932. doi: 10.1016/S0006-291X(02)00314-5. [DOI] [PubMed] [Google Scholar]
- 37.Upton M.L. Chen J. Setton L.A. Region-specific constitutive gene expression in the adult porcine meniscus. J Orthop Res. 2006;24:1562. doi: 10.1002/jor.20146. [DOI] [PubMed] [Google Scholar]