Abstract
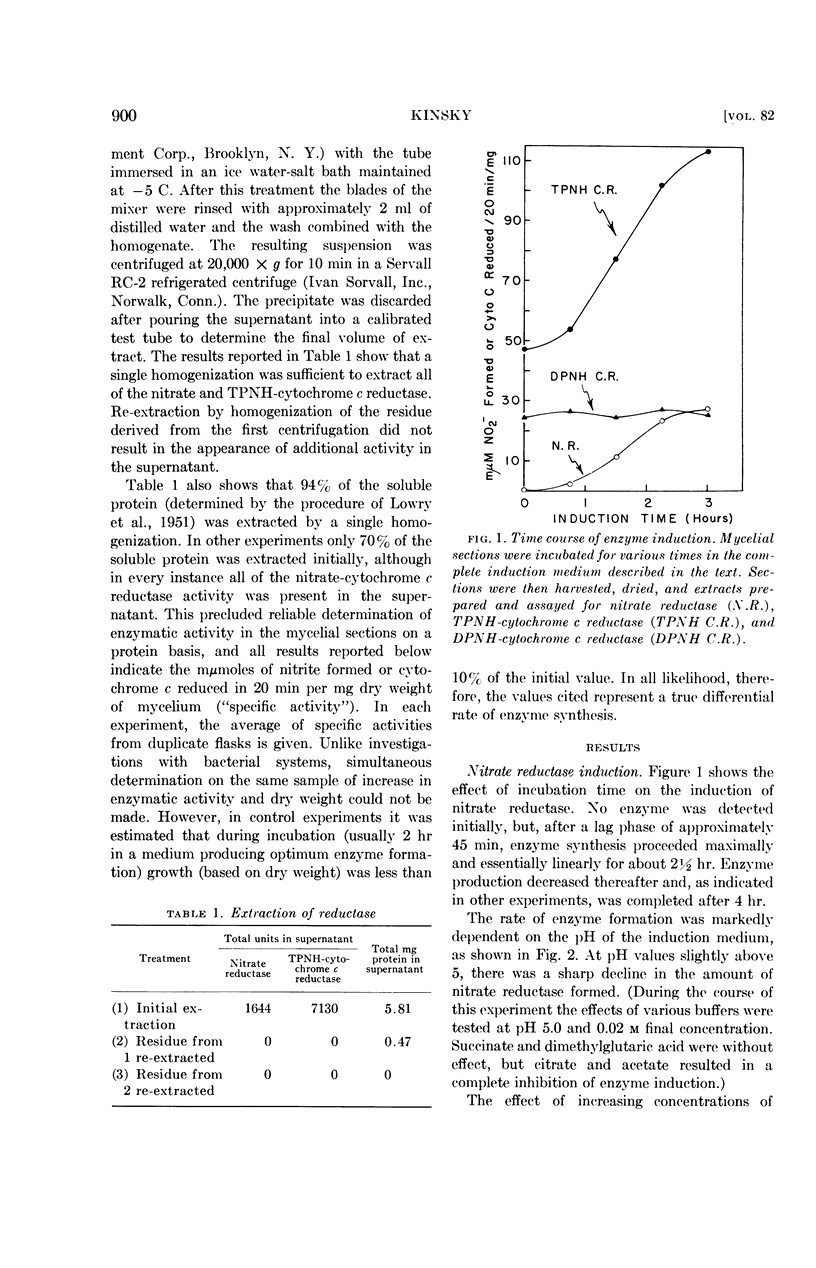
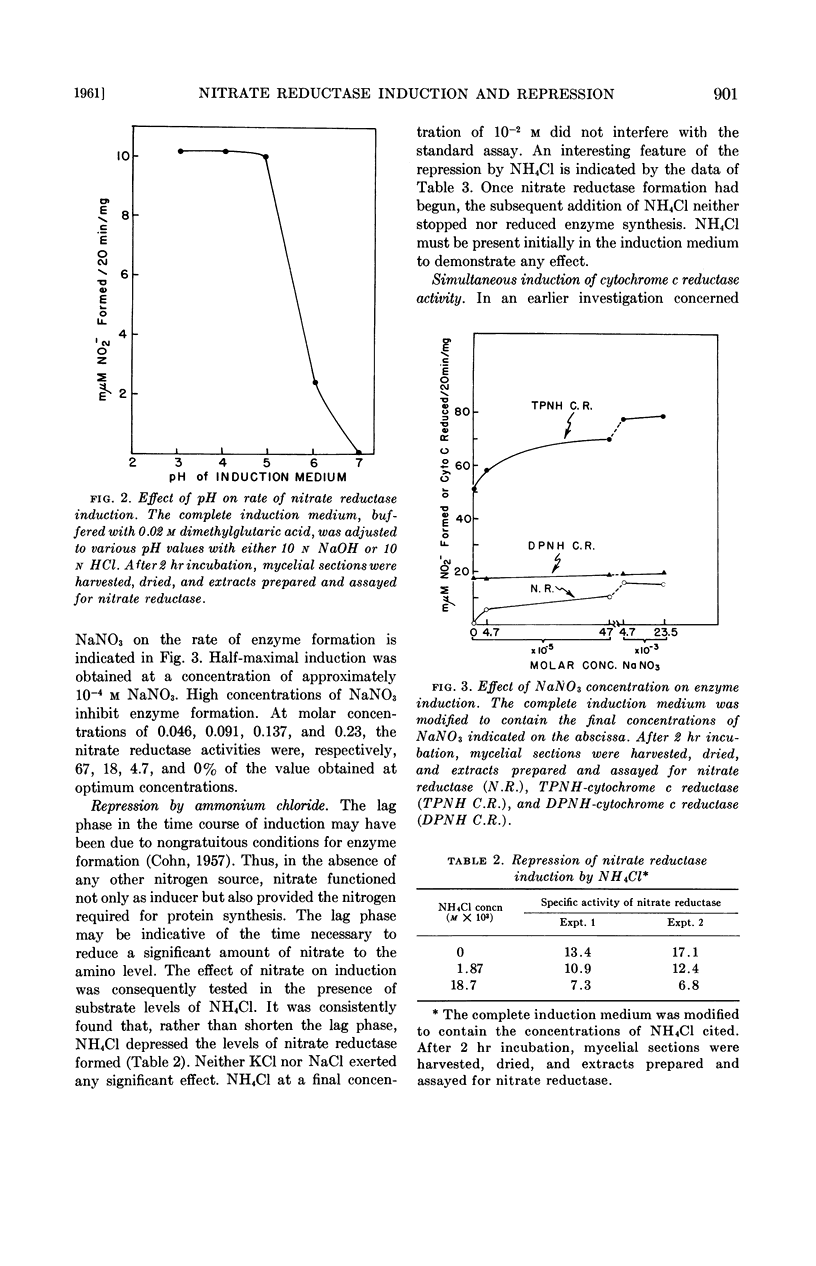
Kinsky, Stephen C. (Washington University, St. Louis, Mo.). Induction and repression of nitrate reductase in Neurospora crassa. J. Bacteriol. 82:898–904. 1961.—Techniques are described for studying induced enzyme formation in Neurospora crassa. The effects of various parameters (time, pH, nitrate concentration, etc.) on the induction of nitrate reductase were investigated. It was demonstrated that NH4+, which is the end product of the metabolic sequence initiated by nitrate reductase, repressed formation of the enzyme. Parallel to the formation of nitrate reductase there was an increase in reduced triphosphopyridine nucleotide (TPNH)-cytochrome c reductase activity. The relationship of these two activities to each other and to the constitutive TPNH-cytochrome c reductase present in noninduced mycelia is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHMANN B. J., BONNER D. M. Protoplasts from Neurospora crassa. J Bacteriol. 1959 Oct;78:550–556. doi: 10.1128/jb.78.4.550-556.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M. Contributions of studies on the beta-galactosidase of Escherichia coli to our understanding of enzyme synthesis. Bacteriol Rev. 1957 Sep;21(3):140–168. doi: 10.1128/br.21.3.140-168.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEWSON C. A., NICHOLAS D. J. Utilization of nitric oxide by micro-organisms and higher plants. Nature. 1960 Dec 3;188:794–796. doi: 10.1038/188794a0. [DOI] [PubMed] [Google Scholar]
- GORINI L., MAAS W. K. The potential for the formation of a biosynthetic enzyme in Escherichia coli. Biochim Biophys Acta. 1957 Jul;25(1):208–209. doi: 10.1016/0006-3002(57)90450-x. [DOI] [PubMed] [Google Scholar]
- KINSKY S. C., McELROY W. D. Neurospora nitrate reductase: the role of phosphate flavine and cytochrome c reductase. Arch Biochem Biophys. 1958 Feb;73(2):466–483. doi: 10.1016/0003-9861(58)90290-x. [DOI] [PubMed] [Google Scholar]
- LEVIN A. P., MAGASANIK B. The effect of purines on the formation of two enzymes involved in purine biosynthesis. J Biol Chem. 1961 Jan;236:184–188. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- NASON A., EVANS H. J. Triphosphopyridine nucleotide-nitrate reductase in Neurospora. J Biol Chem. 1953 Jun;202(2):655–673. [PubMed] [Google Scholar]
- NICHOLAS D. J., NASON A. Mechanism of action of nitrate reductase from Neurospora. J Biol Chem. 1954 Nov;211(1):183–197. [PubMed] [Google Scholar]
- YATES R. A., PARDEE A. B. Control by uracil of formation of enzymes required for orotate synthesis. J Biol Chem. 1957 Aug;227(2):677–692. [PubMed] [Google Scholar]


