Abstract
This review summarizes microdialysis studies that address the question of which compounds serve as energy sources in the brain. Microdialysis was used to introduce 14C-labeled glucose, lactate, pyruvate, glutamate, glutamine and acetate into the interstitial fluid of the brain to observe their metabolism to 14CO2. Although glucose uptake from the systemic system supplies the carbon source for these compounds, compounds synthesized from glucose by the brain are subject to recycling, including complete metabolism to CO2. Therefore, the brain utilizes multiple compounds in its domain to provide the energy needed to fulfill its function. The physiological conditions controlling metabolism and the contribution of compartmentation into different brain regions, cell types and subcellular spaces are still unresolved. The aconitase inhibitor fluorocitrate, with a lower inhibition threshold in glial cells, was used to identify the proportion of lactate and glucose that was oxidized in glial cells versus neurons. The fluorocitrate data suggest that glial and neuronal cells are capable of utilizing both lactate and glucose for energy metabolism.
Keywords: energy metabolism, microdialysis, fluorocitrate, compartmentation, oxidation
Oxidative metabolism of 14C-labeled compounds with the formation of 14CO2 by brain tissue and cell cultures or extracts have indicated the wide range of substrates (glucose, lactate, pyruvate, glutamate, glutamine, acetate) that these preparations are able to metabolize for energy production (Bouzier-Sore et al. 2003; Cremer 1981; Dienel and Cruz, 2006; Edmond et al. 1987; Itoh et al. 2003; Larrabee 1995; McKenna et al. 1994; Peng et al. 1994; Poitry-Yamate et al. 1995; Roeder et al. 1984; Tildon et al. 1983; Zielke et al. 1996). These results may seem to be in contrast to the direct correlation of glucose and oxygen utilization by the intact brain that support the conclusion that glucose taken up by the brain from the blood can account for all the energy needs of the brain (Sokoloff 1992). One conclusion that might be drawn from these results is that the oxidation seen in vitro does not reflect the in vivo situation. A more nuanced conclusion is that brain energy metabolism is complex and that a wide range of compounds are formed in the brain from glucose as the carbon source. These compounds form the basis for the structural and physiological function of the brain. When these compounds are not needed to perform their basic function they are metabolized for reformation of brain constituents, released from the brain as by-products or oxidized for energy production.
Microdialysis has been adapted to both infuse radioactive substrates into the interstitial fluid of the rat brain and to sample the same fluid for the formation of 14CO2 (Huang et al. 1993). The methodology allows study of a large number of available 14C-labeled compounds by infusion of trace amounts into the interstitial space without drastically altering the physiological levels in the interstitial space.
Oxidation of compounds following infusion into the brain by microdialysis
14C-Labeled glucose, lactate, pyruvate, glutamate, glutamine, and acetate were tested as potential oxidative substrates and each was shown to be oxidized to 14CO2 (Table 1). The absolute oxidation rates for these substrates can not be calculated due to the limitations listed above. However, the data indicate that all listed compounds are actively oxidized and that the closer metabolically the compound is to the TCA cycle, the higher the rate of 14CO2 recovery. These results are consistent with oxidative capacity reported with in vitro cultured brain cells. Although readily oxidized following perfusion into the interstitial fluid, glutamate and pyruvate have a very low interstitial concentration. Consequently, the amounts present in the interstitial fluid can not serve as a significant reserve as energy sources, although the intracellular concentration of glutamate is high. In contrast glutamine and lactate are present in the interstitial fluid at concentrations ranging from 0.2 to 1 mmol/L (Kanamori and Ross 2004; Kuhr and Korf 1988). Consequently, they have the potential of serving as a mobile energy source. Since neither of these compounds is taken up from the periphery in adults, they are synthesized in the brain from glucose. Glutamine is an energy source for multiple types of intact and dissociated cells in culture (Tildon and Zielke 1988), including astrocytes (Huang and Hertz 1995) and neurons (Peng et al. 1994; Peng et al. 2007; Yu et al. 1995). The report of pyruvate recycling in cultured neurons is supportive of neuronal metabolism of glutamine to CO2 (Olstad et al. 2007).
Table 1.
Oxidation rate of substrate perfused into the hippocampus of the rat
| Substrate | µCi perfused/hr | Ave ± Stdev dpm/1µCi/hr |
n |
|---|---|---|---|
| [U-14C]Glucose | 3.55 | 194 ± 149 | 33 |
| [U-14C]Lactate | 1.48 | 539 ± 177 | 39 |
| [1-14C]Pyruvate | 2.05 | 2312 ± 1118 | 20 |
| [U-14C]Glutamate | 1.30 | 1015 ± 335 | 12 |
| [U-14C]Glutamine | 1.21 | 456 ± 179 | 18 |
| [U-14C]Acetate | 0.95 | 1204 ± 540 | 5 |
The recovery of 14CO2 is expressed as “Ave ± Stdev dpm/1µCi/hr.” The data were normalized to a perfusion rate of 1µCi/hr. No corrections were made for interstitial concentration, rate of cellular uptake or intracellular pool size. Reproduced with permission (Zielke et al. 2007a).
Compartmentation of oxidative metabolism of glutamate and glutamine
Metabolic compartmentation in the brain was first established with the observation that radiolabeled acetate labeled glutamine more extensively than glutamate, its immediate precursor (Berl and Clarke 1969). Subsequent studies have contributed to the understanding of glutamate and glutamine metabolic compartmentation by establishing that astrocytes are the site of the enzyme glutamine synthetase (Norenberg and Martinez-Hernandez 1979) and that neurons have a much greater amount of phosphate activated glutaminase than astrocytes (Hogstad et al. 1988).
To ascertain if glutamine or glutamate is extensively oxidized in brain and in which compartment or cell type, it was necessary to determine their specific activity in the interstitial fluid during the oxidation study. Initial microdialysis studies (Huang et al. 1993; Zielke et al. 1997) indicated that the production of 14CO2 was linear with time for more than 6 hours once equilibrium had been established between the perfusate and the interstitial fluid. These data suggested that a steady state specific activity had been established 1 hr after the 14C-labeled compounds was perfused into the interstitial fluid. Once the steady state was achieved, the radioactive amino acid in the perfusate was replaced with artificial CSF and eluates from the microdialysis probe were collected every 2 minutes for HPLC analysis followed by determination of radioactivity in glutamate or glutamine. The specific activity dropped rapidly with time. Extrapolation of the specific activity back to the end of the oxidation study provided an estimate of the interstitial specific activity during the oxidation study (Zielke et al. 1998). Based on the extrapolated extracellular specific activities it was calculated that glutamine was oxidized at 5 times the rate of glutamate (Table S1). Since glutamine is metabolized via glutamate, the data support the conclusion that glutamine and glutamate are oxidized in separate compartments. Based on the primary localization of glutaminase in neurons, it suggests that glutamine is a significant oxidative substrate in neurons. In vivo NMR studies by Pascual et al. (1998) indicated that both glutamate and glutamine were oxidative substrate under normoxia and that their oxidative metabolism increased dramatically during ischemic conditions. These authors calculated oxidative rates, based in part on extracellular concentration that favored glutamate oxidation over glutamine. Differences between these two studies may be related to assumption of different interstitial glutamine concentrations.
Effect of potential competitive or stimulatory metabolites
The addition of a non-radioactive substrate to oxidation experiments along with a 14C-labeled substrate provides information about whether two compounds share the same uptake system, if they are intermediates in a common metabolic pathway, or if they serve as alternate energy sources. Addition of non-radioactive lactate to 14C-pyruvate significantly reduced the recovery of 14CO2 from the dialysate during in vivo oxidative studies and the reciprocal seemed also to be the case (Fig. S1). A minimal conclusion is that they are oxidized in the same compartment(s). The reciprocal inhibitory effects may also suggest competitive uptake by the same carrier. This is supported by the observation that α-cyano-4-hydroxycinnamate, an inhibitor of the mitochondrial monocarboxylate transporter (Halestrap and Denton, 1974) inhibited the oxidation of both 14C-pyruvate and 14C-lactate (Zielke et al. 2007a).
Addition of non-labeled pyruvate had a small but not significant effect on the amount of 14CO2 recovery from studies with 14C-glucose (Fig. S1) that was similar to a large and highly significant observation in cultured astrocytes (Hertz, 2004). Lactate had no effect in similar experiments (Zielke et al. 2007a). An additional study is consistent with glucose and lactate oxidation by both neurons and astrocytes (Zielke et al. 2007b).
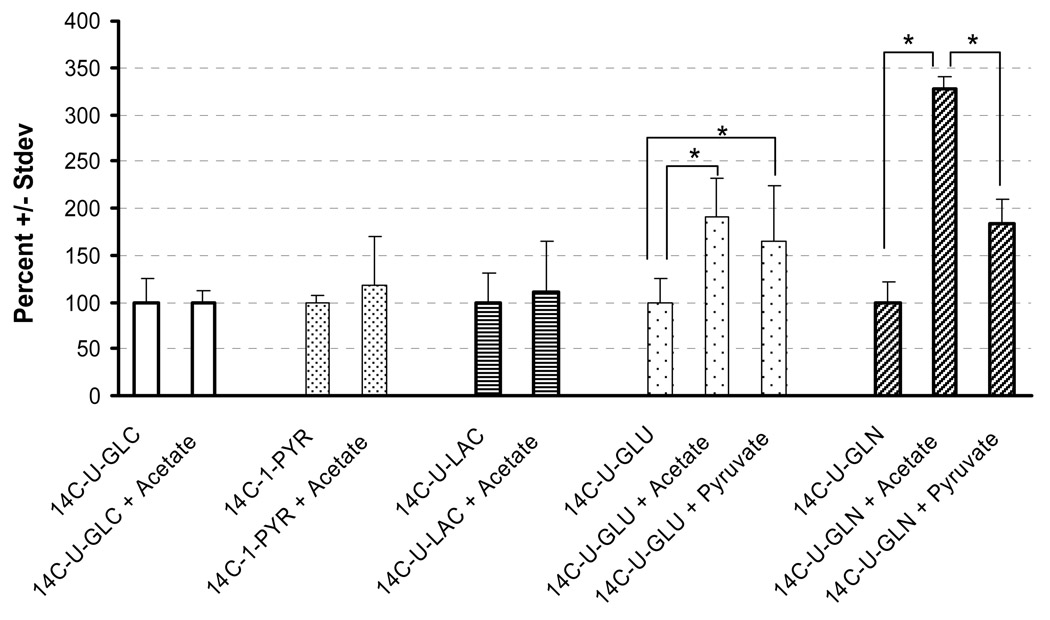
Co-infusion of acetate, and to a lesser degree pyruvate, stimulated 14C-glutamate and 14C-glutamine oxidation, but had no effect on 14C-glucose, 14C-pyruvate or 14C-lactate oxidation (Fig. 1). Acetate is preferentially transported into astrocytes (Waniewski and Martin, 1998) and converted to acetyl-CoA, suggesting that this effect occurs primarily in this cell type. An increase of acetyl-CoA may potentially enhance TCA cycle activity. Pyruvate could function in the same manner, but a buildup of acetyl-CoA is expected to decrease pyruvate dehydrogenase activity (Sugden and Holness, 2003), resulting in a self-limiting stimulatory effect. Pyruvate may also enhance oxidation of glutamate and glutamine by serving as an amino group acceptor (Hodgkins et al. 1999). Inclusion of another amino group acceptor, α-ketoisocaproate, increased two fold the rate of 14C-glutamate oxidation (Zielke et al., 1997). In addition, carboxylation of pyruvate to oxalacetate may dilute the specific activity of TCA cycle intermediates, decreasing the apparent stimulatory effect of pyruvate compared to acetate.
Fig. 1.
Effect of acetate and pyruvate on in vivo oxidation. The mean ± stdev.is expressed as percent, normalized to an infusion rate of 1 µCi/hr. The 100% values for each compound, expressed as dpm/1µCi/hr, are: 14C-U-glucose 391 ± 96, n=5; 14C-1-pyruvate, 3,349 ± 198, n=5; 14C-U-lactate, 713 ± 220, n=7; 14C-U-glutamate, 1,775 ± 438, n=5; and 14C-U-glutamine, 573 ± 126, n=4 (*P<0.05). From Zielke et al. 2007a with permission.
Lactate Metabolism
A current issue in brain metabolism is the role of lactate in brain, and specifically in neuronal metabolism. The astrocyte-neuron shuttle hypothesis (ANLS) was proposed in 1994 to interrelate neurotransmitter glutamate uptake by astrocytes, enhanced glycolysis and lactate release by astrocytes (Pellerin and Magistretti 1994). In the extreme, proponents proposed that neurons did not utilize glucose, but rather relied solely on lactate. Multiple studies and reviews with different conclusions have resulted (Ames 2000; Chih et al. 2001; Dienel and Cruz 2004; Dienel and Hertz 2001; Hertz 2004; Pellerin 2003; Aubert et al. 2005). Because the methodology is not available to measure oxidative metabolism during a single synaptic event, we addressed the issue of glucose and lactate oxidation over a longer time frame in the intact in vivo brain using microdialysis and inhibition of TCA cycle activity in glial cells using the aconitase inhibitor fluorocitrate (Paulsen et al. 1987; Clarke 1991).
Fluorocitrate has been utilized in vitro at concentrations of 5–100 µmol/L (Hassel et al. 1995) to inhibit glial but not neuronal aconitase activity. Recent studies with hippocampal slices utilized 10 and 20 mmol/L fluoroacetate to demonstrate that synaptic transmission was inhibited, but electrogenic membrane function was unaffected (Canals et al. 2008). The lactate dehydrogenase activity in the medium remained unaffected after a 24 h exposure to fluorocitrate indicating that cell disruption had not occurred even at this elevated level of inhibitor. In vivo inhibition of glial aconitase activity was achieved by the injection of 1 nmol into the brain (Paulsen et al. 1987). The range of fluorocitrate concentrations that inhibited glial aconitase activity and not neuronal activity was quite narrow. If the quantity injected was increased to 2 nmol, neuronal damage was also observed. A one time injection approach was not suitable for the desired oxidation studies needed to address questions about cellular metabolic activity because the requirement of pre-implantation of the guide cannula and the desire to assure inhibition throughout the oxidation study. Using studies with in vitro concentrations of 5–100 µmol/L as a guide (Hassel et al. 1995), the studies by Zielke et al. (2007b) were performed at 5, 20 and 100 µmol/L fluorocitrate in the microdialysis perfusate. Based on an estimated equilibration of 15% of the fluorocitrate across the dialysis membrane, Zielke et al. (2007b) proposed that the brain would be exposed to fluorocitrate concentrations that had been shown in vitro to only affect astrocytes. The interstitial level of two astrocytic products, glutamine and lactate, was used as determinant of astrocyte TCA cycle inhibition. Their concentrations in the interstitial fluid were a function of increasing fluorocitrate levels in the perfusate.
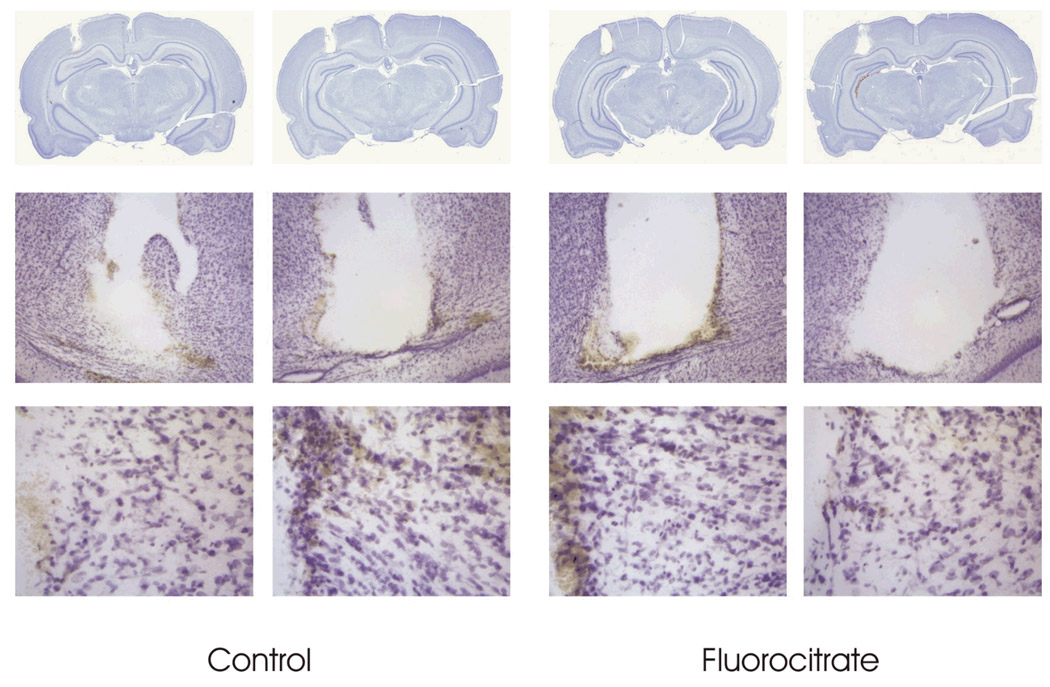
Studies were performed to ascertain if 100 µmol/L fluorocitrate caused cellular damage. Rats were perfused by microdialysis for 6 hours with 100 µmol/L fluorocitrate and the animals were euthanized 24 hours later. Nissl stained brain sections showed no evidence of neuronal damage (Fig. 2). The results were interpreted as indicating that minimal blockage of the TCA cycle had occurred in neurons. The behavior of rats perfused with 100 µM fluorocitrate ranged from sleep to being awake. When rats were perfused with 300 µmol/L fluorocitrate, the animals were hyper active and had bursts of rapid activity, circling rapidly in their enclosure.
Fig. 2.
Nissl stain of rat brain 24 hrs after perfusion with 100 µM fluorocitrate for 6 hrs. The brains were perfused in situ with 4% paraformaldehyde and processed for Nissl staining (FD Neurotechnologies, Inc, Ellicott City, MD).
Potential alterations of glial and neuronal metabolism due to fluorocitrate were further assessed by measuring the effect on oxidation of compounds that enter the TCA cycle after the aconitase reaction. Oxidation studies were performed using [14C]glutamate and [14C]glutamine which enter the TCA cycle via α-ketoglutarate. Oxidation of neither of these compounds was affected by 100 µmol/L fluorocitrate (Fig. S2) indicating that cellular metabolism was not grossly affected by microdialysis perfusion at this fluorocitrate concentration. We concluded that 100 µmol/L fluorocitrate should be considered the upper level that primarily affects the glial compartment and that the optimum concentration is probably 20–50 µmol/L.
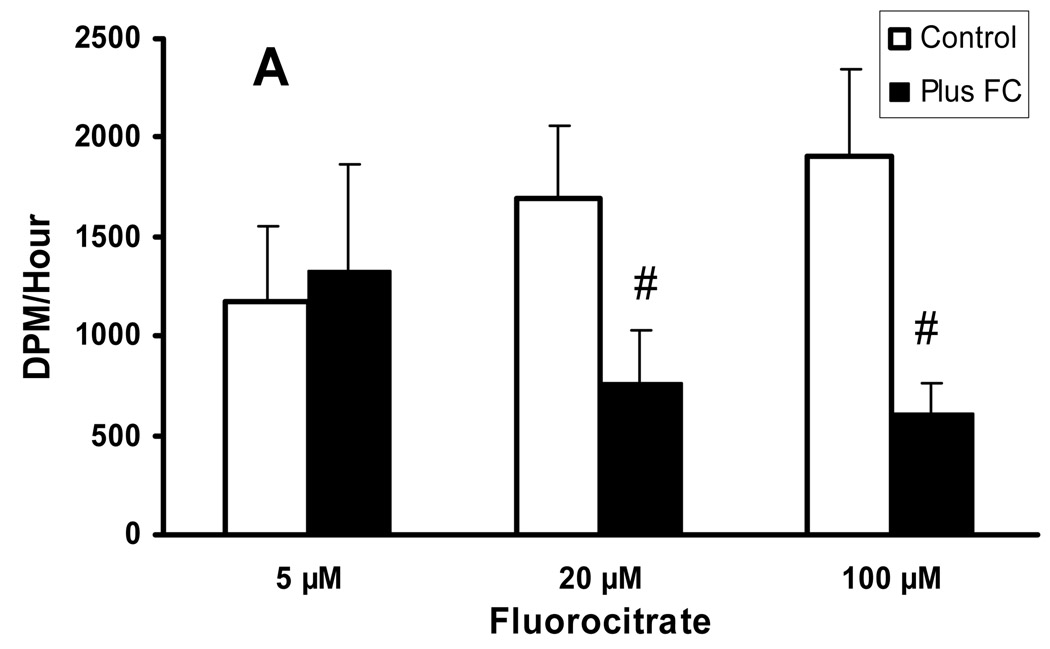
Oxidative studies were performed on the effect of fluorocitrate on lactate and glucose oxidation. The 5 µmol/L fluorocitrate had no effect on either lactate or glucose oxidation while 20 and 100 µmol/L fluorocitrate reduced production of 14CO2 from 14C-lactate by 55% and 68%, respectively (Fig 3). These results support the conclusion that glial cells are capable of oxidizing lactate as well as producing lactate. As a corollary, Zielke et al. (2007b) proposed that the portion of lactate oxidation that was not inhibited by fluorocitrate occurred in the less fluorocitrate sensitive compartment, neurons. In comparable experiments, 14C-glucose oxidation was reduced in a non-dose dependent manner, 50% and 24%, respectively (Fig. 4). Using analogous reasoning, a significant portion of glucose is oxidized in both the glial and neuronal cells. These indicated that a threshold level of inhibitor had been reached at near 20 µmol/L fluorocitrate.
Fig. 3.
Concentration effect of fluorocitrate on 14CO2 recovery following perfusion with [U-14C]lactate. Mean ± stdev. (0 µmol/L, n= 13; 5 µmol/L, n=4; 20 µmol/L, n=5; 100 µmol/L, n=4; #p<0.001). From Zielke et al. 2007b with permission.
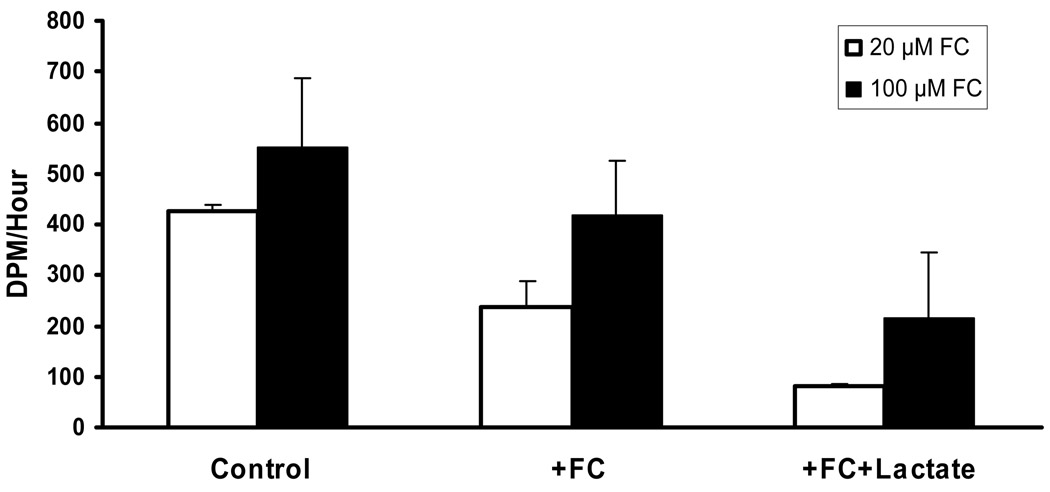
Fig. 4.
Effect of fluorocitrate and lactate on recovery of 14CO2 following perfusion of [U-14C]glucose into the interstitial space by microdialysis. Three one hour dialysate fractions were collected as described in Material and Methods to establish the baseline (Control) rate of 14CO2 recovery from [U-14C]glucose. Perfusion was continued with [U-14C]glucose plus 20 or 100 µmol/L fluorocitrate (+FC). The perfusate was then changed to [U-14C]glucose plus fluorocitrate plus 50 mmol/L lactate (+FC+Lactate). In studies with 20 µmol/L fluorocitrate each set of samples was significantly different from each other. In studies with 100 µmol/L fluorocitrate, control values were significantly different only from (+FC+Lactate) samples. (Analysis of variance and Tukey’s test; p<0.05; n=4). From Zielke et al. 2007b with permission.
Addition of non-radioactive lactate in addition to 14C-glucose and 20 or 100 µmol/L fluorocitrate further decreased the rate of glucose oxidation by 31% and 38%, respectively (Fig. 4). These data further support the conclusion that neurons oxidize lactate. Based on these findings we propose that under normal physiological circumstances, both neurons and astrocytes utilize glucose and lactate for energy. The quantitative values from these studies differ in magnitude with in vitro studies that indicated a almost 4-fold higher contribution from lactate metabolism than glucose metabolism to oxidative metabolism in cultured cortical neurons (Bouzier-Sore et al. 2004). This may reflect the differences between pure in vitro cultures versus an intact brain with a mixed population of cells.
Concluding remarks
The use of microdialysis for oxidative studies shares a common advantage with all other types of microdialysis studies. Namely, that one obtains in vivo results from a non-anaesthetized animal that more closely reflects the functioning of a normal brain. However, not all of metabolic interactions are revealed since the data reflects the results in the interstitial fluid rather than in the individual cells. Glucose, lactate, pyruvate, acetate, glutamate and glutamine are some of the multiple compounds that were oxidized to 14CO2 by brain cells when perfused into the interstitial fluid. It is further concluded that equal amounts of lactate are oxidized in neurons and astrocytes, whereas oxidative metabolism of glucose accounts for 2/3 of total glucose metabolism in neurons and 1/3 in astrocytes (Fig S3). The data support the conclusion that both astrocytes and neurons utilize the enzymatic mechanisms present in their cells to obtain energy. Because of the difference in time frame, the current studies do not directly address the energetics during synaptic transmission.
Supplementary Material
Effect of pyruvate, lactate and acetate on oxidation. The mean ± stdev.is expressed as percent, normalized to an infusion rate of 1 µCi/hr. The 100% values for each compound, expressed as dpm/1µCi/hr, are: [U-14C]glucose, 509 ± 305, n=14; [1-14C]pyruvate, 3031 ± 1109, n=7; [U-14C]lactate, 854± 192, n=7. * = p&<0.05. From Zielke et al. 2007a with permission.
Effect of 100 µM fluorocitrate on the oxidation of [U-14C]glutamate and [U-14C]glutamine. Mean ± stdev., n= 5 for glutamate and n=8 for glutamine.
Summary of the effect of fluorocitrate on glucose and lactate oxidation in the brain of a rat. The interstitial pools of lactate and glucose were labeled in separate experiments. The percent inhibition of [U-14C]lactate and [U-14C]glucose oxidation by fluorocitrate was attributed to lactate and glucose oxidation, respectively, by astrocytes. The percent oxidation by neurons was calculated by subtraction. Modified, from Zielke et al. 2007b with permission.
Starting and extrapolated specific activities of infused [U-14C]glutamate and [U-14C]glutamine in the interstitial space of the rat brain
Acknowledgements
This work was supported in part by NIH grant 16596.
References
- Ames A., III CNS energy metabolism as related to function. Brain Res. Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Magistretti PJ, Pellerin L. Brain lactate kinetics: modeling evidence for neuronal lactate uptake upon activation. Proc. Natl. Acad. Sci., USA. 2005;102:16448–16453. doi: 10.1073/pnas.0505427102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl S, Clarke DD. Metabolic compartmentation of glutamate in the CNS. In: Lajtha A, editor. Handbook of Neurochemistry: Chemical and Cellular Architecture. Vol. 1. New York: Plenum Press; 1969. pp. 447–472. [Google Scholar]
- Bouzier-Sore A-K, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose in culture. J. Cereb. Blood Flow. Met. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- Canals S, Larrosa B, Pintor J, Mena MA, Herreras O. Metabolic challenge to glia activates an adenosine-mediated safety mechanism that promotes neuronal survival by delaying the onset of spreading depression waves. J. Cer. Blood Flow & Met. 2008;28:1835–1844. doi: 10.1038/jcbfm.2008.71. [DOI] [PubMed] [Google Scholar]
- Chih C-P, Lipton P, Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose? Trends in Neurosci. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Clarke DD. Fluorocitrate and fluorocitrate: mechanism of action. Neurochem. Res. 1991;16:1055–1058. doi: 10.1007/BF00965850. [DOI] [PubMed] [Google Scholar]
- Cremer JE. Nutrients for the brain: problems in supply. Early Hum. Develop. 1981;5:117–132. doi: 10.1016/0378-3782(81)90043-8. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem. Intern. 2004;45:321–351. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Astrocyte activation in working brain: energy supplied by minor substrates. Neurochem. Intern. 2006;48:586–595. doi: 10.1016/j.neuint.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. Neurochem. Int. 2001;66:824–838. doi: 10.1002/jnr.10079. [DOI] [PubMed] [Google Scholar]
- Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J. Neurosci. Res. 1987;18:551–561. doi: 10.1002/jnr.490180407. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Denton RM. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes α-cynao-4-hydroxycinnamate. Biochem. J. 1974;38:313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B, Westergaard N, Schousboe A, Fonnum F. Metabolic differences between primary cultures of astrocytes and neurons from cerebellum and cerebral cortexEffect of fluorocitrate. Neurochem. Res. 1995;20:413–420. doi: 10.1007/BF00973096. [DOI] [PubMed] [Google Scholar]
- Hertz L. The astrocyte-neuron lactate shuttle: a challenge of a challenge. J. Cereb. Blood Flow Metab. 2004;24:1242–1248. doi: 10.1097/00004647-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Hodgkins PS, Wu H-Q, Zielke HR, Schwarcz R. 2-Oxoacids regulate kynurenic acid production in the rat brain: studies in vitro and in vivo. J. Neurochem. 1999;72:643–651. doi: 10.1046/j.1471-4159.1999.0720643.x. [DOI] [PubMed] [Google Scholar]
- Hogstad S, Svenneby G, Torgner IA, Kvamme E, Hertz L, Schousboe A. Glutaminase in neurons and astrocytes cultured from mouse brain: kinetic properties and effects of phosphate, glutamate, and ammonia. Neurochem. Res. 1988;13:383–388. doi: 10.1007/BF00972489. [DOI] [PubMed] [Google Scholar]
- Huang R, Hertz L. Noradrenaline-induced stimulation of glutamine metabolism in primary cultures of astrocytes. J. Neurosci. Res. 1995;41:677–683. doi: 10.1002/jnr.490410514. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zielke CL, Tildon JT, Zielke HR. Monitoring in vivo oxidation of 14C-labelled substrates to 14CO2 by brain microdialysis. Dev. Neurosci. 1993;15:233–239. doi: 10.1159/000111339. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc. Natl. Acad. Sci., USA. 2003;100:4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori K, Ross BD. Quantitative determination of extracellular glutamine concentration in the rat brain, and its elevation in vivo by system A transport inhibitor, α-(methylamino)isobutyrate. J. Neurochem. 2004;90:203–210. doi: 10.1111/j.1471-4159.2004.02478.x. [DOI] [PubMed] [Google Scholar]
- Kuhr WG, Korf J. Extracellular lactic acid as an indicator of brain metabolism: Continuous on-line measurement in conscious, freely moving rats with intrastriatal dialysis. J. Cereb. Blood Flow. Met. 1988;8:130–137. doi: 10.1038/jcbfm.1988.17. [DOI] [PubMed] [Google Scholar]
- Larrabee MG. Lactate metabolism and its effects on glucose metabolism in an excised neural tissue. J. Neurochem. 1995;64:1734–1741. doi: 10.1046/j.1471-4159.1995.64041734.x. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Tildon JT, Stevenson JH, Hopkins IB. Energy metabolism in cortical synaptic terminals from weanling and mature rat brain: evidence for multiple compartments of tricarboxylic acid cycle activity. Dev. Neurosci. 1994;16:291–300. doi: 10.1159/000112122. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez a. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Olstad E, Olsen GM, Qu H, Sonnewald U. Pyruvate recycling in cultured neurons from cerebellum. J. Neurosci. Res. 2007;85:3318–3325. doi: 10.1002/jnr.21208. [DOI] [PubMed] [Google Scholar]
- Pascual JM, Carceller F, Roda JM, Cerdán S. Glutamate, glutamine, and GABA as substrates for the neuronal and glial compartments after focal cerebral ischemia in rats. Stroke. 1998;29:1048–1057. doi: 10.1161/01.str.29.5.1048. [DOI] [PubMed] [Google Scholar]
- Paulsen RE, Constestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J. Neurochem. 1987;48:1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem. Intern. 2003;43:331–338. doi: 10.1016/s0197-0186(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci., USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Zhang X, Hertz L. High extracellular potassium concentrations stimulate oxidative metabolism in a glutamatergic neuronal culture and glycolysis in cultured astrocytes but have no stimulatory effect in a GABAergic neuronal culture. Brain Res. 1994;663:168–172. doi: 10.1016/0006-8993(94)90475-8. [DOI] [PubMed] [Google Scholar]
- Peng L, Gu L, Zhang H, Huang X, Hertz, Hertz L. Glutamine as an energy substrate in cultured neurons during glucose deprivation. J. Neurosci. Res. 2007;85:3480–3486. doi: 10.1002/jnr.21262. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J. Neurosci. 1995;15:5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder LM, Tildon JT, Stevenson JH. Competition among oxidizable substrates in brains of young and adult ratsWhole homogenates. Biochem. J. 1984;219:125–130. doi: 10.1042/bj2190125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. The brain as a chemical machine. In: Yu ACH, Hertz L, Norenberg MD, Sykova E, Waxman SG, editors. Progress in Brain Research. vol 94. Amsterdam: Elsevier Science; 1992. pp. 19–33. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. Endocrinol. Metab. 2003;284:E855–E862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- Tildon JT, Merrill S, Roeder LM. Differential substrate oxidation by dissociated brain cells and homogenates during development. Biochem. J. 1983;216:21–25. doi: 10.1042/bj2160021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tildon JT, Zielke HR. Glutamine: an energy source for mammalian tissues. In: Kvamme E, editor. Glutamine and Glutamate in Mammals. Vol 1. Boca Raton: CRC Press, Inc.; 1988. pp. 167–182. [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J. Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AC, Fisher TE, Hertz E, Tildon JT, Schousboe A, Hertz L. Metabolic fate of [14C]-glutamine in mouse cerebral neurons in primary culture. J. Neurosci. Res. 1994;11:351–357. doi: 10.1002/jnr.490110403. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Collins RM, Jr, Baab PJ, Huang Y, Zielke CL, Tildon JT. Compartmentation of [14C]glutamate and [14C]glutamine oxidative metabolism in the rat hippocampus as determined by microdialysis. J. Neurochem. 1998;71:1315–1320. doi: 10.1046/j.1471-4159.1998.71031315.x. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Huang Y, Baab PJ, Collins RM, Jr, Zielke CL, Tildon JT. Effect of α-ketoisocaproate and leucine on the in vivo oxidation of glutamate and glutamine in the rat brain. Neurochem. Res. 1997;22:1159–1164. doi: 10.1023/a:1027325620983. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Tildon JT, Zielke CL. Use of intracellular versus extracellular specific activities in calculation of glutamine metabolism in astrocytes: effect of dibutyryl cyclic AMP. Develop. Neurosci. 1996;18:224–230. doi: 10.1159/000111410. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ. Oxidation of 14C-labeled compounds perfused by microdialysis in the brains of free-moving rats. J. Neurosci. Res. 2007a;85:3145–3149. doi: 10.1002/jnr.21424. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ, Tildon JT. Effect of fluorocitrate on the oxidation of lactate and glucose in the brain of free moving rats. J. Neurochem. 2007b;101:9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of pyruvate, lactate and acetate on oxidation. The mean ± stdev.is expressed as percent, normalized to an infusion rate of 1 µCi/hr. The 100% values for each compound, expressed as dpm/1µCi/hr, are: [U-14C]glucose, 509 ± 305, n=14; [1-14C]pyruvate, 3031 ± 1109, n=7; [U-14C]lactate, 854± 192, n=7. * = p&<0.05. From Zielke et al. 2007a with permission.
Effect of 100 µM fluorocitrate on the oxidation of [U-14C]glutamate and [U-14C]glutamine. Mean ± stdev., n= 5 for glutamate and n=8 for glutamine.
Summary of the effect of fluorocitrate on glucose and lactate oxidation in the brain of a rat. The interstitial pools of lactate and glucose were labeled in separate experiments. The percent inhibition of [U-14C]lactate and [U-14C]glucose oxidation by fluorocitrate was attributed to lactate and glucose oxidation, respectively, by astrocytes. The percent oxidation by neurons was calculated by subtraction. Modified, from Zielke et al. 2007b with permission.
Starting and extrapolated specific activities of infused [U-14C]glutamate and [U-14C]glutamine in the interstitial space of the rat brain