Abstract
Objective
To determine if differences in short-term virologic failure among commonly used ART regimens translate to differences in clinical events in antiretroviral-naïve patients initiating ART.
Design
Observational cohort study of patients initiating ART between January 2000 and December 2005.
Setting
The Antiretroviral Therapy Cohort Collaboration (ART-CC) is a collaboration of 15 HIV cohort studies from Canada, Europe, and the United States.
Subjects, participants
A total of 13,546 antiretroviral-naïve HIV-positive patients initiating ART with efavirenz (EFV), nevirapine (NVP), lopinavir/ritonavir (LPV/r), nelfinavir (NFV), or abacavir (ABC) as third drugs in combination with a zidovudine and lamivudine NRTI backbone.
Main outcome measures
Short-term (24-week) virologic failure (>500 copies/mL) and clinical events within 2 years of ART initiation (incident AIDS-defining event, death, and a composite measure of these two outcomes).
Results
Compared with EFV as initial third drug, short-term virologic failure was more common with all other third drugs evaluated; NVP (adjusted odds ratio=1.87, 95%CI=1.58,2.22), LPV/r (1.32, 95%CI=1.12–1.57), NFV (3.20, 95%CI=2.74,3.74), and ABC (2.13, 95%CI=1.82,2.50). However, the rate of clinical events within 2 years of ART initiation appeared higher only with NVP (adjusted hazard ratio for composite outcome measure 1.27, 95%CI=1.04,1.56) and ABC (1.22, 95%CI=1.00,1.48).
Conclusions
Among antiretroviral-naïve patients initiating therapy, between-ART regimen differences in short-term virologic failure do not necessarily translate to differences in clinical outcomes. Our results should be interpreted with caution because of the possibility of residual confounding by indication.
Keywords: Adolescent; Adult; Anti-Retroviral Agents; therapeutic use; Disease-Free Survival; Drug Interactions; Drug Resistance, Viral; Drug Therapy, Combination; Epidemiologic Methods; Female; HIV Infections; drug therapy; immunology; virology; HIV-1; Humans; Male; Middle Aged; Odds Ratio; RNA, Viral; metabolism; Reverse Transcriptase Inhibitors; therapeutic use; Treatment Outcome; Viral Load; Young Adult
Keywords: HIV, AIDS, Antiretroviral Therapy, Highly Active, Cohort analysis, Viral load, AIDS-related Opportunistic Infections, Mortality
INTRODUCTION
Combination antiretroviral therapy (ART) has resulted in dramatic reductions in HIV-associated morbidity and mortality for persons with access to treatment. Preferred initial ART regimens have changed over time based largely on evidence from randomized controlled clinical trials (RCTs). However, clinical trials are usually powered to detect between regimen differences in short-term suppression of plasma HIV RNA. Due to the rarity, in recent years, of clinical events such as incident AIDS-defining events and death [1–3], clinical trials are typically underpowered to detect differences in these clinical outcome measures.
Collaborations of observational cohort studies, a complementary study design to RCTs, may have the statistical power to evaluate between-regimen differences in clinical outcomes. However, results from such studies are likely to be affected by confounding because of the non-randomized selection of initial ART regimens in clinical practice [4], often referred to as “confounding by indication [5].” Statistical methods can adjust for measured imbalances between treatment groups, but unmeasured confounding can never be fully excluded [6]. Recognizing these limitations, observational HIV cohort studies can play an important role in providing evidence that is not available from RCTs [7, 8].
Previous observational studies have evaluated between-regimen differences in short-term virologic failure in treatment-naïve patients initiating ART in clinical practice settings, often yielding findings consistent with RCT results [9–21]. However, these studies have largely been underpowered to evaluate between-regimen differences in clinical outcomes. Previously, the ART Cohort Collaboration (ART-CC) evaluated between-regimen differences in virologic and clinical outcomes in patients initiating ART between 1996–2002 [22]. However, several regimens evaluated in that study are no longer widely used in clinical practice. Furthermore, ritonavir-boosted (RTV) protease inhibitors (PIs) (amprenavir, lopinavir, saquinavir, and indinavir) were evaluated together due to the low number of such regimens in the database at the time of the study. Heterogeneity of outcomes between boosted-PI regimens may have affected the overall findings for these regimens, which were found to be inferior for both virologic and clinical outcomes, relative to efavirenz (EFV). Here, we analyse data from an updated ART-CC database, to evaluate between-regimen differences in short-term virologic failure and rates of clinical events among treatment-naïve patients initiating ART between 2000 and 2005 conducting all analyses at the level of the individual regimen. We hypothesized that between-ART regimen differences in short-term virologic failure would not fully predict the effects on clinical endpoints.
METHODS
Cohorts
The Antiretroviral Therapy Cohort Collaboration (ART-CC) is a collaboration of 15 HIV cohort studies from Canada, Europe and the United States that was established in 2001. The collaboration has been described in detail elsewhere [23–26]. Briefly, prospective cohort studies were eligible for participation if they had enrolled at least 100 HIV-1–infected patients aged ≥16 years who had: (1) not previously received antiretroviral treatment, (2) started ART with a combination of at least three antiretroviral drugs after 1996, and (3) been followed for a median duration of at least one year after ART initiation. The database was updated in 2007 to additionally include patients who had started ART up until 31 December 2005 with follow up until 1 July 2006. All cohorts have been approved by their local ethics committees or institutional review boards, use standardised methods of data collection, and schedule follow-up visits at least once every six months.
The cohorts in the dataset for this analysis are: French Hospital Database on HIV (FHDH) ANRS CO4 [27] and the Aquitaine Cohort ANRS CO3 (France) [28], the AIDS Therapy Evaluation project Netherlands (ATHENA) [29], Italian Cohort of Antiretroviral-Naive Patients (ICONA) [30], Swiss HIV Cohort Study (SHCS) [31], Frankfurt HIV Cohort [32] and Köln/Bonn Cohort (Germany) [33], the EuroSIDA study (20 countries in Europe and Argentina) [34], the Collaborations in HIV Outcomes Research US (CHORUS) [35], the University of Alabama at Birmingham 1917 Clinic Cohort [36] and the Veterans Aging Cohort Study (VACS) (USA) [37], the Royal Free Hospital Cohort (United Kingdom) [38], the British Columbia Centre for Excellence in HIV/AIDS [39] and the South Alberta Clinic (Canada) [40], and PISCIS, Catalonia and Balearic Islands (Spain) [41].
Data collection
Patient selection and data extraction were performed at the data centres of the participating cohorts. Anonymised data on a predefined set of demographic, laboratory, and clinical variables were pooled and analyzed centrally. Cohort data managers from EuroSIDA were asked to provide a unique study ID for each record as EuroSIDA patients may also be members of other cohort studies.
Statistical analyses
Analyses were restricted to HIV-1 positive subjects aged 16 years or older, who first started antiretroviral therapy in the period 1 January 2000 - 31 December 2005 and had at least 6 months of potential follow-up before the cohort-specific database close date. Because of the focus on more recent ART regimens, study inclusion criteria required initiation of efavirenz (EFV), nevirapine (NVP), lopinavir/ritonavir (LPV/r), nelfinavir (NFV) or abacavir (ABC) as “third drugs.” We evaluated short-term (24-week) virologic failure (HIV RNA >500 copies/mL) and longer-term clinical outcomes (incident AIDS-defining event, death from any cause, and a composite measure of these two outcomes) by third drug, in patients who were taking zidovudine and lamivudine (ZDV and 3TC) as the NRTI backbone. This approach was taken to focus on differences in virologic failure and clinical outcomes between third drugs given in combination with the same NRTI pair, such that potential differences in prescribing patterns for NRTI backbones across third drugs was not a factor. ZDV and 3TC were chosen as the NRTI backbone as this combination represented the most commonly prescribed NRTI pair (68% of regimens). All centres used the 1993 US Centers for Disease Control and Prevention criteria and guidelines for the definitive or presumptive diagnosis of AIDS-defining events [42]. Only new AIDS diagnosis, defined as the first occurrence of each AIDS-defining condition was considered to be an incident event; recurrences of conditions were not considered. Change in regimen at six months after initiating ART was a secondary outcome.
To evaluate short-term virologic failure, logistic regression models were used to estimate crude and adjusted odds ratios (OR) of detectable 24-week plasma HIV-1 RNA, i.e. >500 copies/mL, among patients with an available measurement at that time (+/− 3 month window). For this analysis, patients who died prior to 24 weeks and those with missing 24-week plasma HIV-1 RNA values were excluded. Logistic regression models were also used to estimate the crude and adjusted OR of not being on the initial regimen at 24 weeks, a secondary outcome measure. Sensitivity analyses were conducted evaluating 24-week virologic failure and initial ART regimen change for all patients, including those with missing 24-week measurements. For these analyses patients with missing 24-week plasma HIV RNA measures (+/− 3 months), including those who died, were considered treatment failures (“missing equals failure”).
To evaluate clinical outcomes, we measured time from the date of initiating ART to earlier of the date that clinical endpoints occurred and the date of censoring (end of follow-up). In patients free of events, follow-up was censored on the date of the most recent visit plus half the usual visit interval (usually 3 months) for AIDS and the combined endpoint (incident AIDS event or death from any cause). For mortality, the censoring date was extended to the date the patient was last known to be alive in cohorts that could assert complete vital registration; otherwise, as above. Because the proportion of patients remaining on their initial regimen decreases over time, effects of initial regimen become increasingly diluted by regimen changes with increasing time since initiation of ART, Follow-up was therefore censored at 2 years after starting ART or at the cohort-specific close of database date, if either of these occurred sooner. Additional analyses, removing the 2-year censoring, were conducted to evaluate longer-term differences in clinical events according to initial ART regimen.
Weibull proportional hazards regression models were used to model the association of initial treatment regimen and other prognostic factors with disease progression as measured by clinical outcome measures. We estimated crude and adjusted hazard ratios (HR) comparing other third drugs with EFV when taken in combination with ZDV and 3TC. Analyses followed an “intent to continue initial therapy” principle, in that eligible subjects were analyzed according to initial regimen, regardless of whether they later discontinued or modified their therapeutic regimen.
All multivariable models were adjusted for age at initiation of therapy (16–29, 30–39, 40–49 >50 years), sex, transmission risk group (injection drug user (IDU), non-IDU), clinical stage (A/B, C), baseline CD4 cell count (<25, 25–49, 50–99, 100–199, 200–349, ≥350 cells/μL), baseline plasma HIV-1 RNA (<1000, 1000–9,999 10,000–99,999 and ≥100,000 copies/mL), year of starting ART and cohort. Sensitivity analyses were restricted to patients who started ART with a CD4 count ≤200 cells/μL, and to those whose reported transmission risk group was non-IDU.
AIDS-free survival up to two years after initiation of therapy by third drug was plotted using the estimated probability of survival free of an incident AIDS-defining event or death from the adjusted Weibull model with covariates set at their average value across the population of patients and for the cohort with median survival.
RESULTS
Overall, 13,546 patients in the ART-CC initiated ART with the third drugs of interest (EFV, NVP, LPV/r, NFV, ABC) paired with ZDV and 3TC during the study period. Among study participants, 69% were male, 13% reported a history of IDU, and 20% had CDC clinical stage C disease (Table 1). Overall, the median age (inter-quartile range) was 38 years (31 – 45), and the median CD4 count and plasma log10 HIV RNA levels were 218 cells/μL (104 – 329) and 4.9 (4.4 – 5.3), respectively, at the time of ART initiation. During the study period, EFV was the most commonly prescribed third drug (28%), followed by LPV/r (21%), ABC (19%), NFV (16%), and NVP (16%). Temporal trends in prescribing patterns measured as the proportion of overall ART prescriptions in a given year represented by each antiretroviral drug indicated increased use of LPV/r and decreased use of all other third drugs over the course of the observation period (Figure 1). Compared with other third drugs, LPV/r was more commonly prescribed to patients with lower CD4 counts (median 150 cells/μL) and higher plasma HIV RNA levels (5.1 log 10 copies/mL), while the opposite was observed for ABC (median 251 cells/μL and 4.7 log 10 copies/mL) and NVP (median 260 cells/μL and 4.7 log 10 copies/mL) (Table 1).
Table 1.
Patient characteristics of 13,546 antiretroviral naïve HIV-infected patients in the Antiretroviral Therapy Cohort Collaboration (ART-CC) initiating ART with zidovudine and lamivudine stratified by 3rd drug, 2000 – 2005.
| Overall | EFV | NVP | LPV/r | NFV | ABC | |
|---|---|---|---|---|---|---|
| N (Row %) | ||||||
| Frequency 3rd drug | 13,546 (100) | 3,788 (28) | 2,151 (16) | 2,875 (21) | 2,217 (16) | 2,515 (19) |
| Characteristic by 3rd drug | N (Column %) | |||||
| Male | 9,368 (69) | 2,967 (78) | 1,306 (61) | 2,088 (73) | 1,229 (55) | 1,778 (71) |
| IDU | 1,740 (13) | 492 (13) | 261 (12) | 232 (8) | 321 (14) | 434 (17) |
| Clinical CDC stage C | 2,674 (20) | 848 (22) | 255 (12) | 766 (27) | 443 (20) | 362 (14) |
| Median (IQR) | ||||||
| Age (years) | 38 (31 – 45) | 39 (33 – 47) | 36 (30 – 43) | 38 (32 – 46) | 36 (29 – 44) | 38 (32 – 46) |
| CD4 count (cells/μL) | 218 (104 –329) | 207 (94 – 320) | 260 (171 – 366) | 150 (55 – 264) | 214 (92 – 351) | 251 (163 – 354) |
| HIV RNA (log copies/mL) | 4.9 (4.4 – 5.3) | 4.9 (4.5 – 5.4) | 4.7 (4.2 – 5.1) | 5.1 (4.7 – 5.5) | 4.8 (4.2 – 5.3) | 4.7 (4.2 – 5.1) |
EFV, efavirenz; NVP, nevirapine; LPV/r, lopinavir/ritonavir; NFV, nelfinavir; ABC, abacavir; ZDV, zidovudine; 3TC, lamivudine.
Figure 1. Temporal trends in antiretroviral prescribing patterns among 13,546 antiretroviral naïve HIV-infected patients in the Antiretroviral Therapy Cohort Collaboration (ART-CC) initiating ART with ZDV/3TC and stratified by 3rd drug, 2000 – 2005.
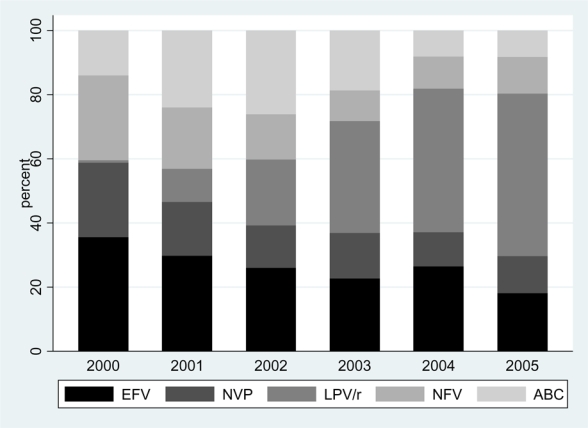
EFV, efavirenz; NVP, nevirapine; LPV/r, lopinavir/ritonavir; NFV, nelfinavir; ABC, abacavir Shaded bars represent the proportion of ART-CC patients initiating HAART with each of the listed 3rd drugs during each of the calendar years from 2000 – 2005.
In multivariable logistic regression analyses among patients who had 24-week plasma HIV RNA measures (n=11,194, 83%), detectable 24-week plasma HIV RNA (>500 copies/mL) was more common with all other third drugs than with EFV (Table 2). Findings were most pronounced for NFV (adjusted OR=3.20, 95%CI=2.74, 3.74), while patients treated with NVP (1.87, 95%CI=1.58, 2.22) and ABC (2.13, 95%CI=1.82, 2.50) had roughly twice the odds of short-term virologic failure. There was a modest increase in the odds of 24-week virologic failure for LPV/r (adjusted OR=1.32, 95%CI=1.12, 1.57). Among those with available 24-week data (n=11,338, 84% of cohort), patients receiving NVP (adjusted OR=1.30, 95%CI=1.13, 1.51) and NFV (1.39, 95%CI=1.20, 1.60) were less likely to be treated with their initial regimen at 24 weeks than those receiving EFV (Table 2).
Table 2.
Short term (24-week) regimen durability and virologic failure among 11,338 antiretroviral naïve HIV-infected patients in the Antiretroviral Therapy Cohort Collaboration (ART-CC, plasma HIV RNA >500 copies/mL) initiating ART with zidovudine and lamivudine stratified by 3rd drug, 2000 – 2005.
| Odds ratio (95%CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3rd Drug | Obs. | No longer on initial regimen at 24 weeks | Obs. | 24 week virologic failure ART-CC (plasma HIV RNA >500 c/mL) | |||||
| N (%) | Crude | Adjusteda | N (%) | Crude | Adjusteda | ||||
| EFV | 3,258 | 679 (21) | 1 | 1 | 3,087 | 383 (12) | 1 | 1 | |
| NVP | 1,759 | 448 (25) | 1.30 (1.13, 1.49) | 1.30 (1.13, 1.51) | 1,776 | 348 (20) | 1.72 (1.47, 2.02) | 1.87 (1.58, 2.22) | |
| LPV/r | 2,518 | 622 (25) | 1.25 (1.10, 1.41) | 1.04 (0.91, 1.19) | 2,457 | 365 (15) | 1.23 (1.06, 1.44) | 1.32 (1.12, 1.57) | |
| NFV | 1,715 | 490 (29) | 1.52 (1.33, 1.74) | 1.39 (1.20, 1.60) | 1,829 | 593 (32) | 3.39 (2.93, 3.92) | 3.20 (2.74, 3.74) | |
| ABC | 2,088 | 432 (21) | 0.99 (0.87, 1.13) | 0.94 (0.81, 1.08) | 2,045 | 486 (24) | 2.20 (1.90, 2.55) | 2.13 (1.82, 2.50) | |
| Table 2a. Sensitivity analysis of short-term (24-week) regimen durability and virologic failure among 13,546 antiretroviral naïve HIV-infected patients in the Antiretroviral Therapy Cohort Collaboration (ART-CC, plasma HIV RNA >500 copies/mL) initiating ART with zidovudine and lamivudine stratified by 3rd drug (missing data = failure), 2000 – 2005. | |||||||
|---|---|---|---|---|---|---|---|
| Odds ratio (95%CI) | |||||||
| 3rd Drug | Obs. | No longer on initial regimen at 24 weeks | 24 week virologic failure ART-CC (plasma HIV RNA >500 c/mL) | ||||
| N (%) | Crude | Adjusteda | N (%) | Crude | Adjusteda | ||
| EFV | 3,788 | 1,209 (32) | 1 | 1 | 1,084 (29) | 1 | 1 |
| NVP | 2,151 | 840 (39) | 1.37 (1.22, 1.53) | 1.29 (1.14, 1.45) | 723 (34) | 1.26 (1.13, 1.42) | 1.44 (1.27, 1.63) |
| LPV/r | 2,875 | 979 (34) | 1.10 (0.99, 1.22) | 1.04 (0.92, 1.16) | 783 (27) | 0.93 (0.84, 1.04) | 1.03 (0.91, 1.16) |
| NFV | 2,217 | 992 (45) | 1.73 (1.55, 1.92) | 1.54 (1.37, 1.72) | 981 (44) | 1.98 (1.77, 2.21) | 1.98 (1.76, 2.22) |
| ABC | 2,515 | 859 (34) | 1.11 (0.99, 1.23) | 1.01 (0.90, 1.14) | 956 (38) | 1.53 (1.37, 1.70) | 1.53 (1.36, 1.72) |
EFV, efavirenz; NVP, nevirapine; LPV/r, lopinavir/ritonavir; NFV, nelfinavir; ABC, abacavir
Multivariable logistic regression models adjusted for age, sex, IDU, entry CD4, entry HIV RNA, year starting ART, and cohort.
In general, sensitivity analyses including all patients (n=13,546) and utilising a “missing equals failure” approach gave similar results to those observed in primary analyses, but with odds ratios that were attenuated towards one (Table 2a). In these analyses the odds of virologic failure were similar for LPV/r relative to EFV (adjusted OR=1.03, 95%CI=0.91, 1.16). This is in contrast to the primary analyses in which the odds of 24-week virologic failure were higher with LPV/r compared with EFV. Sensitivity analyses of 24-week change of initial ART regimen yielded similar results to primary analyses, and parameter estimates were of comparable magnitude (Table 2a).
In Weibull proportional hazards analysis of clinical outcomes, rates of incident AIDS events (adjusted HR=1.28, 95%CI=1.02, 1.60), death (1.54, 95%CI=1.09, 2.19) and the combined clinical outcome (1.27, 95%CI=1.04, 1.56) appeared higher for NVP than for EFV (Table 3). Because baseline CD4 counts tended to be higher for patients initiating NVP (median baseline CD4 count 260 cells/μL) than those starting EFV (median 207 cells/μL), adjusted hazard ratios for this comparison were markedly greater than crude hazard ratios. In contrast, for the comparison of LPV/r (median baseline CD4 count 150 cells/μL) with EFV adjusted hazards were attenuated towards one after controlling for patient characteristics at baseline. In adjusted analyses there was little evidence that rates of clinical events in patients on LPV/r differed from those in patients receiving EFV (AIDS-event adjusted HR=1.14, 95%CI=0.93, 1.39, death 1.12, 95%CI=0.80, 1.57, combined outcome measure 1.13, 95%CI=0.94, 1.36) (Table 3). Figure 2 shows that by two years after starting ART there was an estimated difference of about 2% in the cumulative probability of AIDS-free survival, between patients initiating ART on NFV and those initiating on NVP.
Table 3.
Clinical outcomes (Incident AIDS-defining event, death, and composite measure of these 2 outcomes) within 2 years of ART initiation among 13,546 antiretroviral naïve HIV-infected patients in the Antiretroviral Therapy Cohort Collaboration (ART-CC) initiating ART with zidovudine and lamivudine stratified by 3rd drug, 2000 – 2005.
| Hazard ratio (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3rd Drug | Obs. | Incident AIDS event | Death | Incident AIDS event or Death | ||||||
| N | Crude | Adjusteda | N | Crude | Adjusteda | N | Crude | Adjusteda | ||
| EFV | 3,788 | 259 | 1 | 1 | 98 | 1 | 1 | 320 | 1 | 1 |
| NVP | 2,151 | 117 | 0.90 (0.72, 1.12) | 1.28 (1.02, 1.60) | 54 | 1.16 (0.82, 1.64) | 1.54 (1.09, 2.19) | 147 | 0.91 (0.74, 1.11) | 1.27 (1.04, 1.56) |
| LPV/r | 2,875 | 208 | 1.39 (1.15, 1.68) | 1.14 (0.93, 1.39) | 72 | 1.46 (1.06, 2.02) | 1.12 (0.80, 1.57) | 249 | 1.39 (1.16, 1.65) | 1.13 (0.94, 1.36) |
| NFV | 2,217 | 146 | 1.02 (0.83, 1.26) | 0.98 (0.79, 1.21) | 47 | 0.88 (0.62, 1.25) | 0.89 (0.63, 1.28) | 167 | 0.95 (0.79, 1.15) | 0.92 (0.76, 1.11) |
| ABC | 2,515 | 130 | 0.85 (0.69, 1.06) | 1.12 (0.89, 1.40) | 62 | 1.21 (0.87, 1.68) | 1.41 (1.01, 1.99) | 175 | 0.95 (0.79, 1.15) | 1.22 (1.00, 1.48) |
| Table 3a. Sensitivity analysis restricted to 6,235 patients with baseline CD4 counts ≤200 cells/μL at ART initiation. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | ||||||||||
| 3rd Drug | Obs. | Incident AIDS event | Death | Incident AIDS event or Death | ||||||
| N | Crude | Adjusted | N | Crude | Adjusted | N | Crude | Adjusted | ||
| EFV | 1,837 | 206 | 1 | 1 | 69 | 1 | 1 | 244 | 1 | 1 |
| NVP | 697 | 66 | 0.96 (0.73, 1.28) | 1.13 (0.85, 1.50) | 37 | 1.70 (1.12, 2.58) | 1.99 (1.31, 3.03) | 86 | 1.05 (0.82, 1.36) | 1.24 (0.96, 1.59) |
| LPV/r | 1,771 | 178 | 1.13 (0.91, 1.39) | 1.08 (0.86, 1.36) | 57 | 1.23 (0.85, 1.79) | 1.02 (0.69, 1.51) | 207 | 1.13 (0.93, 1.38) | 1.06 (0.86, 1.30) |
| NFV | 1,059 | 118 | 1.03 (0.81, 1.29) | 0.94 (0.74, 1.19) | 28 | 0.73 (0.47, 1.15) | 0.72 (0.46, 1.14) | 133 | 0.98 (0.79, 1.22) | 0.91 (0.73, 1.14) |
| ABC | 871 | 87 | 1.03 (0.79, 1.33) | 1.14 (0.87, 1.49) | 40 | 1.59 (1.06, 2.39) | 1.63 (1.07, 2.47) | 115 | 1.18 (0.94, 1.49) | 1.29 (1.01, 1.63) |
| Table 3b. Sensitivity analysis restricted to 11,806 patients with mode of HIV acquisition recorded as non-IDU. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | ||||||||||
| 3rd Drug | Obs. | Incident AIDS event | Death | Incident AIDS event or Death | ||||||
| N | Crude | Adjusteda | N | Crude | Adjusteda | N | Crude | Adjusteda | ||
| EFV | 3,247 | 224 | 1 | 1 | 74 | 1 | 1 | 270 | 1 | 1 |
| NVP | 1,807 | 94 | 0.84 (0.65, 1.07) | 1.18 (0.92, 1.52) | 41 | 1.13 (0.76, 1.67) | 1.55 (1.04, 2.33) | 117 | 0.86 (0.68, 1.07) | 1.20 (0.95, 1.50) |
| LPV/r | 2,211 | 188 | 1.36 (1.11, 1.66) | 1.09 (0.88, 1.35) | 62 | 1.57 (1.10, 2.25) | 1.14 (0.78, 1.66) | 221 | 1.36 (1.13, 1.64) | 1.08 (0.89, 1.32) |
| NFV | 1,754 | 129 | 1.09 (0.87, 1.36) | 1.02 (0.81, 1.28) | 32 | 0.83 (0.55, 1.27) | 0.88 (0.57, 1.34) | 142 | 1.00 (0.81, 1.23) | 0.95 (0.77, 1.18) |
| ABC | 1,870 | 96 | 0.77 (0.60, 0.98) | 0.99 (0.77, 1.28) | 45 | 1.24 (0.85, 1.82) | 1.62 (1.09, 2.42) | 130 | 0.89 (0.72, 1.11) | 1.16 (0.92, 1.45) |
| Table 3c. Longer-term clinical outcomes removing the 2 year censoring with follow-up through 1 July 2006 or cohort specific database close date. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | ||||||||||
| 3rd Drug | Obs. | Incident AIDS event | Death | Incident AIDS event or Death | ||||||
| N | Crude | Adjusteda | N | Crude | Adjusteda | N | Crude | Adjusteda | ||
| EFV | 3,788 | 312 | 1 | 1 | 152 | 1 | 1 | 399 | 1 | 1 |
| NVP | 2,151 | 143 | 0.91 (0.74, 1.12) | 1.24 (1.01, 1.52) | 74 | 1.02 (0.77, 1.36) | 1.29 (0.96, 1.72) | 185 | 0.92 (0.77, 1.10) | 1.22 (1.02, 1.47) |
| LPV/r | 2,875 | 223 | 1.43 (1.20, 1.72) | 1.18 (0.97, 1.43) | 85 | 1.39 (1.05, 1.85) | 1.06 (0.79, 1.43) | 268 | 1.40 (1.19, 1.64) | 1.15 (0.97, 1.37) |
| NFV | 2,217 | 193 | 1.10 (0.92, 1.32) | 1.05 (0.87, 1.26) | 83 | 0.95 (0.73, 1.25) | 0.93 (0.70, 1.22) | 231 | 1.03 (0.87, 1.21) | 0.98 (0.83, 1.16) |
| ABC | 2,515 | 155 | 0.87 (0.72, 1.07) | 1.11 (0.91, 1.37) | 86 | 1.14 (0.87, 1.50) | 1.32 (1.00, 1.75) | 214 | 0.97 (0.82, 1.16) | 1.21 (1.02, 1.45) |
EFV, efavirenz; NVP, nevirapine; LPV/r, lopinavir/ritonavir; NFV, nelfinavir; ABC, abacavir
Multivariable Weibull regression models adjusted for age, sex, IDU, entry CD4, entry HIV RNA, and year starting ART and stratified on cohort. Follow up restricted to 2 years.
Figure 2. Estimated AIDS free survival among 13,546 antiretroviral naïve HIV-infected patients in the Antiretroviral Therapy Cohort Collaboration (ART-CC) initiating ART with ZDV/3TC stratified by 3rd drug, 2000 – 2005.
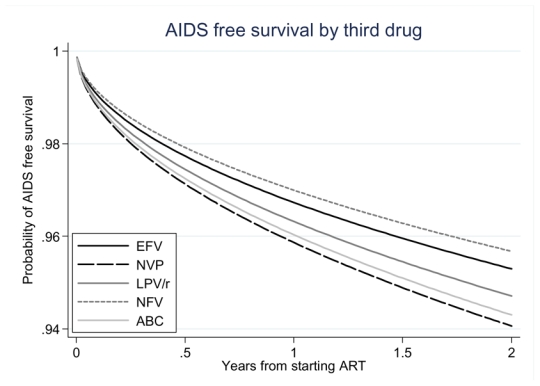
Survival curves shown are estimated from a Weibull model with follow-up censored at 2 years, with covariates set at the average value across the population of patients and for the cohort with median survival. Corresponding adjusted hazard ratios are shown in Table 3.
Relative to EFV, hazards of AIDS-events (adjusted HR=0.98, 95%CI=0.79, 1.21), death (0.89, 95%CI=0.63, 1.28), and the combined clinical endpoint (0.92, 95%CI=0.76, 1.11) were slightly lower for NFV (Table 3). In adjusted analyses, hazards of death (adjusted HR=1.41, 95%CI=1.01, 1.99) and the combined clinical endpoint (1.22, 95%CI=1.00, 1.48) appeared higher for ABC relative to EFV. Such differences were not apparent in unadjusted analyses, in part because the median baseline CD4 count was higher for patients on ABC (median 251 cells/μL) than for those on EFV (median 207 cells/μL).
In general, sensitivity analyses of the combined clinical outcome measure (incident AIDS event or death) restricted to patients with baseline CD4 counts ≤200 cells/μL when initiating ART yielded parameter estimates of similar magnitude for each third drug relative to the primary analyses, although confidence intervals were wider (Table 3a). However, larger shifts in hazards ratios relative to primary analyses were observed when AIDS events and death were modeled separately, relative to models of the composite clinical outcome measure. On the whole, sensitivity analyses of clinical outcomes restricted to non-IDU patients yielded similar findings to primary analyses (Table 3b). Evaluation of longer-term clinical events by initial ART regimen removing 2 year censoring yielded similar results to primary analyses although, as expected, hazard ratios were attenuated towards one (Table 3c).
DISCUSSION
Among antiretroviral-naïve patients initiating ART in clinical practice settings, short-term (24-week) virologic failure was more common for all third drugs evaluated (NVP, LPV/r, NFV, and ABC) relative to EFV when given in combination with ZDV and 3TC. However, compared with EFV, estimated rates of AIDS and death appeared higher only with NVP and ABC. For LPV/r and NFV, we found little evidence that rates of AIDS and death differed from those on EFV. Taken together, these findings suggest that between ART regimen differences in short-term virologic failure do not necessarily translate to differences in clinical outcomes.
While suppression of plasma HIV RNA is an important goal of treatment to avoid the emergence of viral resistance among other reasons, the ultimate aim of antiretroviral therapy is to prevent clinical progression and death. Preferred initial ART regimens change frequently, based on differential rates of virologic suppression observed in clinical trials. Our study suggests that such differences in virologic suppression between ART regimens may not translate to differences in clinical events among patients receiving treatment in a clinical practice setting. This observation may relate, in part, to the many available antiretroviral treatment options: patients failing treatment at 24-weeks may subsequently switch to other effective ART regimens. A recent study recognized the association of longitudinal CD4 count and plasma HIV RNA responses in contributing to long-term clinical outcomes in patients initiating modern ART, regardless of specific initial regimen [43].
Most previous observational studies (like RCTs) have lacked statistical power to analyze between-regimen differences in clinical events among patients initiating ART [9–20]. The ART-CC makes such comparisons possible through the collaborative efforts of multiple observational HIV cohort studies. The current study advances the findings of an earlier report from the ART-CC [22], by focusing on more recent ART regimens at the level of the third drug among patients receiving the same NRTI backbone (ZDV and 3TC). Importantly, the current study allowed for the evaluation of LPV/r individually, and not grouped with other RTV-boosted protease inhibitors (amprenavir, saquinavir, and indinavir) as done in the earlier analysis due to the relatively small frequency of LPV/r use during the earlier evaluation period (1996–2002).
In contrast with our earlier study, in which RTV-boosted protease inhibitors were associated with increased rates of both short-term virologic failure and clinical outcomes compared with EFV [22], LPV/r was associated only with 24-week virologic failure in the current study. The AIDS Clinical Trial Group (ACTG) 5142 study found a higher frequency of virologic failure among ARV-naïve patients treated with 2 NRTIs and LPV/r compared to those treated with 2 NRTIs and EFV [44]. While EFV outperformed LPV/r in achieving plasma HIV RNA levels <50 copies/mL and showed a trend for superiority at <200 copies/mL, increases in CD4 counts were greater for patients receiving LPV/r than for those receiving EFV in that randomized clinical trial. In the current study similar 24-week CD4 responses were observed for patients treated with LPV/r and EFV (median CD4 increase 110 cells/μL vs. 100 cells/μL, respectively). These similar CD4 responses among ART-CC patients treated with LPV/r and EFV may have contributed to comparable hazards of clinical outcomes, despite a higher frequency of 24-week virologic failure in patients treated with LPV/r.
Another possible explanation for the apparent lack of difference in clinical endpoints between EFV and LPV/r may relate to varying resistance patterns emerging upon treatment failure between different initial ART regimens. Recently, it was shown that patients failing a first-line NNRTI containing regimen harboured viruses with higher numbers of IAS-USA drug resistance mutations and resistance to more antiretroviral drug classes when compared to patients initiating therapy with ritonavir-boosted PI containing regimens [45]. Thus, EFV-based regimens, although more virologically effective as shown in this study, may result in more HIV resistance upon failure making it more difficult to generate potent successive ART regimens. In contrast, it might be easier to find effective salvage regimens for patients failing an initial boosted-PI regimen due to the lower number of drug resistance mutations observed. Furthermore, another study found the emergence of resistance to NNRTIs was associated with a greater risk of subsequent death than was the emergence of PI resistance [46].
This updated analysis of the ART-CC found higher odds of 24-week virologic failure and hazards of clinical endpoints with NVP compared to EFV in analyses adjusted for covariates (Table 3). The findings regarding virologic failure are in contrast to the 2NN clinical trial [47], but consistent with other observational studies comparing these NNRTIs [18–20]. While we are not able to determine the reasons for the observed inferior virologic and clinical outcomes associated with NVP use in the current study, it is possible that EFV outperformed NVP in a clinical practice setting. It is also possible that unmeasured confounders associated with NVP selection in clinical practice, confounding by indication, contributed to the inferior outcomes for NVP in the current study. Notably, shifts in parameter estimates for both NVP (increased) and LPV/r (decreased) for clinical outcome measures were observed between unadjusted and adjusted analyses attributable to differential patient profiles (e.g., baseline CD4 count and plasma HIV viral load) among patients stratified by third drug receipt (Table 1).
The impact of confounding by indication in the selection of third drugs was more apparent in the evaluation of clinical outcomes than observed in analyses of short-term virologic failure; more marked shifts in parameter estimates between crude and adjusted analyses were observed for the clinical outcomes models (Tables 2 and 3). Prior studies have shown the importance of baseline CD4 counts at the time of ART initiation on subsequent clinical events [24, 48]. Taking into account the drastically different median CD4 counts among patients initiating ART observed in this study (e.g., LPV/r 150 cells/μL, NVP 260 cells/μL, and ABC 251 cells/μL), it would be expected that multivariable models controlling for these differences would lead to shifts in estimates observed for crude analyses, as was seen. While the impact of confounding by indication is observed across analyses in this study, it is notable that sensitivity analyses of adjusted models largely yielded consistent findings to those observed in primary analyses.
The findings of our study must be interpreted with regard to the study limitations. The potential for confounding is inherent to all observational studies. The impact of confounding by indication is demonstrated and discussed in this manuscript, but it is possible that other unmeasured confounders not included in adjusted statistical models may have contributed to observed study findings. As with prior studies of the ART-CC, we have adjusted for factors associated with clinical events (e.g., baseline CD4 count), but cannot rule out the possibility of unmeasured confounding. For example, it is possible that a provider’s selection of initial ART regimen was influenced by their expectations of a patient’s adherence to their antiretroviral medications. Such prescribing bias may represent unmeasured confounding that contributed to the between regimen differences in outcomes observed in this study. Furthermore, between-provider differences (e.g., experience) may also have contributed to differential outcomes. Finally, while the ART-CC has broad geographic representation from Europe and North America, findings of this study may not apply to other geographic settings.
In summary, among patients initiating ART from 2000–2005 in clinical practice settings with a ZDV and 3TC backbone, those receiving third drugs other than EFV (NVP, LPV/r, NFV, and ABC) were more likely to experience short-term (24-week) virologic failure. However, such differences were not as prominent in the evaluation of clinical events, which were more common (relative to EFV) in patients receiving NVP and ABC as the third drug of their initial ART regimen, but with little evidence of such differences for those receiving NFV and LPV/r. This study clearly demonstrates the impact of confounding by indication: such confounding, as well as the potential for unmeasured confounding should be taken into account when conducting, evaluating, and reviewing studies utilizing this methodology [6, 49]. Because of the limited available evidence from randomized trials on the impact of initial ART regimens on rates of clinical events, findings from well-designed observational cohort studies may serve a complementary role to findings from clinical trials in informing clinical practice.
Acknowledgments
We are grateful to all patients, doctors, nurses and other persons who were involved with the participating cohort studies. We would like to thank participating cohort members who provided thoughtful review and feedback on the content of this manuscript, particularly Colette Smith, Bruno Ledergerber, Peter Reiss, and Caroline Sabin.
Funding
The ART Cohort Collaboration is supported by the UK Medical Research Council grant RD1564. Sources of funding of individual cohorts include the Agence Nationale de Recherche contre le SIDA (ANRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the French, Italian, Spanish and Swiss Ministries of Health, The Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (Grant No. 33CSC0- 108787) the Stichting HIV Monitoring, the European Commission, the British Columbia and Alberta Governments, the Michael Smith Foundation for Health Research, the Canadian Institutes of Health Research, the VHA Office of Research and Development and unrestricted grants from GlaxoSmithKline, Roche and Boehringer-Ingelheim. Supported in part by the “Spanish Network for AIDS Research (RIS; ISCIII-RETIC RD06/006).
The Antiretroviral Cohort Collaboration (ART-CC) Study Group
Steering Committee
Jordi Casabona (PISCIS), Geneviève Chêne (Aquitaine), Dominique Costagliola (FHDH), François Dabis (Aquitaine), Antonella D’Arminio Monforte (ICONA), Julia del Amo (CoRIS-MD), Frank de Wolf (ATHENA), Matthias Egger (SHCS), Gerd Fätkenheuer (Koln/Bonn), John Gill (South Alberta Clinic), Jodie Guest (HAVACS), Robert Hogg (BCCfE-HIV), Amy Justice (VACS), Mari Kitahata (Washington), Fiona Lampe (Royal Free), Bruno Ledergerber (SHCS), Amanda Mocroft (EuroSIDA), Peter Reiss (ATHENA), Michael Saag (Alabama), Schlomo Staszewski (Frankfurt).
Coordinating Team
Matthias Egger, Margaret May, Ross Harris, Jonathan Sterne (Principal Investigator)
a A complete listing of the ART-CC steering committee, coordinating team, and contributing cohorts follows the text. Writing committee: Michael J. Mugavero,1 Margaret May,2 Ross Harris,2 Michael S. Saag,1 Dominique Costagliola,3 Matthias Egger,4 Andrew Phillips,5 Huldrych F. Günthard,6 Francois Dabis,7 Robert Hogg,8 Frank de Wolf,9 Gerd Fatkenheuer,10 M. John Gill,11 Amy Justice,12 Antonella D’Arminio Monforte,13 Fiona Lampe,5 Jose M. Miró,14 Schlomo Staszewski,15 Jonathan A. C. Sterne2
1Division of Infectious Disease, Department of Medicine, University of Alabama at Birmingham, Birmingham, USA
2Department of Social Medicine, University of Bristol, Bristol, UK.
3INSERM U720, UPMC Paris 06, Paris, France.
4Department of Social and Preventive Medicine, University of Bern, Bern, Switzerland.
5Department of Primary Care and Population Sciences Royal Free and University College Medical School, London, UK.
6Divison of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland.
7INSERM U593, Université Victor Segalen Bordeaux, France
8Division of Epidemiology and Population Health, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, Canada.
9Academisch Medisch Centrum bij de Universiteit van Amsterdam, Amsterdam. The Netherlands.
10 Department of Internal Medicine, University of Cologne, Germany.
11 Division of Infectious Diseases, University of Calgary, Calgary, Canada.
12Yale University School of Medicine, New Haven, CT, USA and VA Connecticut Healthcare System, West Haven, CT, USA.
13Clinic of Infectious Diseases & Tropical Medicine, “San Paolo” Hospital, University of Milan, Italy.
14 Hospital Clinic – IDIBAPS. University of Barcelona. Barcelona, Spain.
15 Zentrum der Inneren Medizin, J. W. Goethe Universität, Frankfurt, Germany.
Footnotes
Author Contributions:
Study conception & design: Robert Hogg, Michael J. Mugavero, Margaret May, Michael S. Saag, Matthias Egger, Jonathan A. C. Sterne
Data analysis: Margaret May, Ross Harris
Acquisition of data and/or interpretation of data: Dominique Costagliola, Frank de Wolf, Amy Justice, Matthias Egger, Huldrych F. Günthard, Antonella D’Arminio Monforte, Jose M. Miró, Schlomo Staszewski, Andrew Phillips, Robert Hogg, Francois Dabis, Fiona Lampe, Gerd Fatkenheuer, M. John Gill, Michael J. Mugavero, Michael S. Saag, Margaret May, Ross Harris, Jonathan A. C. Sterne
Drafting the manuscript: Michael J. Mugavero, Margaret May, Michael S. Saag, Jonathan A. C. Sterne
Critical revision of the manuscript for important intellectual content: Ross Harris, Dominique Costagliola, Matthias Egger, Andrew Phillips, Huldrych F. Günthard, Francois Dabis, Robert Hogg, Frank de Wolf, Gerd Fatkenheuer, M. John Gill, Amy Justice, Antonella D’Arminio Monforte, Fiona Lampe, Jose M. Miró, Schlomo Staszewski
Final manuscript approval: Michael J. Mugavero, Margaret May, Ross Harris, Michael S. Saag, Dominique Costagliola, Matthias Egger, Andrew Phillips, Huldrych F. Günthard, Francois Dabis, Robert Hogg, Frank de Wolf, Gerd Fatkenheuer, M. John Gill, Amy Justice, Antonella D’Arminio Monforte, Fiona Lampe, Jose M. Miró, Schlomo Staszewski, Jonathan A. C. Sterne
A listing of ART-CC cohorts contributing to this study may be found in an online appendix.
Presented in part: 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, CA; February 2007, abstract 527.
CITATIONS
- 1.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Sabin CA, Smith CJ, Youle M, Lampe FC, Bell DR, Puradiredja D, et al. Deaths in the era of HAART: contribution of late presentation, treatment exposure, resistance and abnormal laboratory markers. Aids. 2006;20:67–71. doi: 10.1097/01.aids.0000196178.73174.24. [DOI] [PubMed] [Google Scholar]
- 3.van Sighem AI, van de Wiel MA, Ghani AC, Jambroes M, Reiss P, Gyssens IC, et al. Mortality and progression to AIDS after starting highly active antiretroviral therapy. Aids. 2003;17:2227–2236. doi: 10.1097/00002030-200310170-00011. [DOI] [PubMed] [Google Scholar]
- 4.Hughes MD. Initial treatment of HIV infection: randomized trials with clinical end points are still needed. J Infect Dis. 2006;194:542–544. doi: 10.1086/506369. [DOI] [PubMed] [Google Scholar]
- 5.Walker AM. Confounding by indication. Epidemiology. 1996;7:335–336. [PubMed] [Google Scholar]
- 6.D’Agostino RB, Jr, D’Agostino RB., Sr Estimating treatment effects using observational data. Jama. 2007;297:314–316. doi: 10.1001/jama.297.3.314. [DOI] [PubMed] [Google Scholar]
- 7.Hughes MD, Williams PL. Challenges in using observational studies to evaluate adverse effects of treatment. N Engl J Med. 2007;356:1705–1707. doi: 10.1056/NEJMp078038. [DOI] [PubMed] [Google Scholar]
- 8.Smeeth L, Douglas I, Hubbard R. Commentary: we still need observational studies of drugs--they just need to be better. Int J Epidemiol. 2006;35:1310–1311. doi: 10.1093/ije/dyl134. [DOI] [PubMed] [Google Scholar]
- 9.De Luca A, Cozzi-Lepri A, Antinori A, Zaccarelli M, Bongiovanni M, Di Giambenedetto S, et al. Lopinavir/ritonavir or efavirenz plus two nucleoside analogues as first-line antiretroviral therapy: a non-randomized comparison. Antivir Ther. 2006;11:609–618. [PubMed] [Google Scholar]
- 10.Easterbrook PJ, Newson R, Ives N, Pereira S, Moyle G, Gazzard BG. Comparison of virologic, immunologic, and clinical response to five different initial protease inhibitor-containing and nevirapine-containing regimens. J Acquir Immune Defic Syndr. 2001;27:350–364. doi: 10.1097/00126334-200108010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Friedl AC, Ledergerber B, Flepp M, Hirschel B, Telenti A, Furrer H, et al. Response to first protease inhibitor- and efavirenz-containing antiretroviral combination therapy. The Swiss HIV Cohort Study. Aids. 2001;15:1793–1800. doi: 10.1097/00002030-200109280-00008. [DOI] [PubMed] [Google Scholar]
- 12.Lucas GM, Chaisson RE, Moore RD. Comparison of initial combination antiretroviral therapy with a single protease inhibitor, ritonavir and saquinavir, or efavirenz. Aids. 2001;15:1679–1686. doi: 10.1097/00002030-200109070-00011. [DOI] [PubMed] [Google Scholar]
- 13.Phillips AN, Grabar S, Tassie JM, Costagliola D, Lundgren JD, Egger M. Use of observational databases to evaluate the effectiveness of antiretroviral therapy for HIV infection: comparison of cohort studies with randomized trials. EuroSIDA, the French Hospital Database on HIV and the Swiss HIV Cohort Study Groups. Aids. 1999;13:2075–2082. doi: 10.1097/00002030-199910220-00010. [DOI] [PubMed] [Google Scholar]
- 14.Potard V, Rey D, Mokhtari S, Frixon-Marin V, Pradier C, Rozenbaum W, et al. First-line highly active antiretroviral regimens in 2001–2002 in the French Hospital Database on HIV: combination prescribed and biological outcomes. Antivir Ther. 2007;12:317–324. [PubMed] [Google Scholar]
- 15.Pulido F, Arribas JR, Miro JM, Costa MA, Gonzalez J, Rubio R, et al. Clinical, virologic, and immunologic response to efavirenz-or protease inhibitor-based highly active antiretroviral therapy in a cohort of antiretroviral-naive patients with advanced HIV infection (EfaVIP 2 study) J Acquir Immune Defic Syndr. 2004;35:343–350. doi: 10.1097/00126334-200404010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Weiser SD, Guzman D, Riley ED, Clark R, Bangsberg DR. Higher rates of viral suppression with nonnucleoside reverse transcriptase inhibitors compared to single protease inhibitors are not explained by better adherence. HIV Clin Trials. 2004;5:278–287. doi: 10.1310/LNHD-K1R7-HQP5-HJCQ. [DOI] [PubMed] [Google Scholar]
- 17.Wood E, Hogg RS, Heath KV, de la Rosa R, Lee N, Yip B, et al. Provider bias in the selection of non-nucleoside reverse transcriptase inhibitor and protease inhibitor-based highly active antiretroviral therapy and HIV treatment outcomes in observational studies. Aids. 2003;17:2629–2634. doi: 10.1097/00002030-200312050-00010. [DOI] [PubMed] [Google Scholar]
- 18.Braithwaite RS, Kozal MJ, Chang CC, Roberts MS, Fultz SL, Goetz MB, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. Aids. 2007;21:1579–1589. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews GV, Sabin CA, Mandalia S, Lampe F, Phillips AN, Nelson MR, et al. Virological suppression at 6 months is related to choice of initial regimen in antiretroviral-naive patients: a cohort study. Aids. 2002;16:53–61. doi: 10.1097/00002030-200201040-00008. [DOI] [PubMed] [Google Scholar]
- 20.Phillips AN, Pradier C, Lazzarin A, Clotet B, Goebel FD, Hermans P, et al. Viral load outcome of non-nucleoside reverse transcriptase inhibitor regimens for 2203 mainly antiretroviral-experienced patients. Aids. 2001;15:2385–2395. doi: 10.1097/00002030-200112070-00006. [DOI] [PubMed] [Google Scholar]
- 21.Chou R, Fu R, Huffman LH, Korthuis PT. Initial highly-active antiretroviral therapy with a protease inhibitor versus a non-nucleoside reverse transcriptase inhibitor: discrepancies between direct and indirect meta-analyses. Lancet. 2006;368:1503–1515. doi: 10.1016/S0140-6736(06)69638-4. [DOI] [PubMed] [Google Scholar]
- 22.Rates of disease progression according to initial highly active antiretroviral therapy regimen: a collaborative analysis of 12 prospective cohort studies. J Infect Dis. 2006;194:612–622. doi: 10.1086/506362. [DOI] [PubMed] [Google Scholar]
- 23.Chene G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 25.May M, Porter K, Sterne JA, Royston P, Egger M. Prognostic model for HIV-1 disease progression in patients starting antiretroviral therapy was validated using independent data. J Clin Epidemiol. 2005;58:1033–1041. doi: 10.1016/j.jclinepi.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 26.May M, Royston P, Egger M, Justice AC, Sterne JA. Development and validation of a prognostic model for survival time data: application to prognosis of HIV positive patients treated with antiretroviral therapy. Stat Med. 2004;23:2375–2398. doi: 10.1002/sim.1825. [DOI] [PubMed] [Google Scholar]
- 27.Grabar S, Le Moing V, Goujard C, Leport C, Kazatchkine MD, Costagliola D, Weiss L. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 28.Binquet C, Chene G, Jacqmin-Gadda H, Journot V, Saves M, Lacoste D, Dabis F. Modeling changes in CD4-positive T-lymphocyte counts after the start of highly active antiretroviral therapy and the relation with risk of opportunistic infections: the Aquitaine Cohort, 1996–1997. Am J Epidemiol. 2001;153:386–393. doi: 10.1093/aje/153.4.386. [DOI] [PubMed] [Google Scholar]
- 29.Wit FW, van Leeuwen R, Weverling GJ, Jurriaans S, Nauta K, Steingrover R, et al. Outcome and predictors of failure of hi ghly active antiretroviral therapy: one-year follow-up of a cohort of human immunodeficiency virus type 1-infected persons. J Infect Dis. 1999;179:790–798. doi: 10.1086/314675. [DOI] [PubMed] [Google Scholar]
- 30.d’Arminio Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, et al. Insights into the reasons for disconti nuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. Aids. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study. Bmj. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brodt HR, Kamps BS, Gute P, Knupp B, Staszewski S, Helm EB. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. Aids. 1997;11:1731–1738. doi: 10.1097/00002030-199714000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Fatkenheuer G, Theisen A, Rockstroh J, Grabow T, Wicke C, Becker K, et al. Virological treatment failure of protease inhibitor therapy in an unselected cohort of HIV-infected patients. Aids. 1997;11:F113–116. doi: 10.1097/00002030-199714000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Lundgren JD, Phillips AN, Vella S, Katlama C, Ledergerber B, Johnson AM, et al. Regional differences in use of antiretroviral agents and primary prophylaxis in 3122 European HIV-infected patients. EuroSIDA Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:153–160. doi: 10.1097/00042560-199711010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Becker SL, Raffanti SR, Hansen NI, Fusco JS, Fusco GP, Slatko GH, et al. Zidovudine and stavudine sequencing in HIV treatment planning: findings from the CHORUS HIV cohort. J Acquir Immune Defic Syndr. 2001;26:72–81. doi: 10.1097/00126334-200101010-00011. [DOI] [PubMed] [Google Scholar]
- 36.Chen RY, Accortt NA, Westfall AO, Mugavero MJ, Raper JL, Cloud GA, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 37.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study ( VACS): Overview and description. Med Care. 2006;44:S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mocroft A, Barry S, Sabin CA, Lepri AC, Kinloch S, Drinkwater A, et al. The changing pattern of admissions to a London hospital of patients with HIV: 1988–1997. Royal Free Centre for HIV Medicine. Aids. 1999;13:1255–1261. doi: 10.1097/00002030-199907090-00016. [DOI] [PubMed] [Google Scholar]
- 39.Rhone SA, Hogg RS, Yip B, Sherlock C, Conway B, Schechter MT, et al. The antiviral effect of ritonavir and saquinavir in combination amongst HIV-infected adults: results from a community-based study. Aids. 1998;12:619–624. doi: 10.1097/00002030-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Mocroft A, Gill MJ, Davidson W, Phillips AN. Predictors of a viral response and subsequent virological treatment failure in patients with HIV starting a protease inhibitor. Aids. 1998;12:2161–2167. doi: 10.1097/00002030-199816000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Jaen A, Casabona J, Esteve A, Miro JM, Tural C, Ferrer E, et al. Clinical-epidemiological characteristics and antiretroviral treatment trends in a cohort of HIV infected patients. The PISCIS Project. Med Clin (Barc) 2005;124:525–531. doi: 10.1157/13073938. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definitions for AIDS among adolescents and adults. MMWR. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 43.Olsen CH, Gatell J, Ledergerber B, Katlama C, Friis-Moller N, Weber J, et al. Risk of AIDS and death at given HIV-RNA and CD4 cell count, in relation to specific antiretroviral drugs in the regimen. Aids. 2005;19:319–330. [PubMed] [Google Scholar]
- 44.Riddler SA, Haubrich R, DiRienzo G, Peeples L, Powderly WG, Klingman KL, et al. A prospecitve randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV-infection - ACTG 5142. Program and Abstracts of the XVI International AIDS Conference; 13–18 August 2006; Toronto, CA. abstract THLB0204. [Google Scholar]
- 45.von Wyl V, Yerly S, Boni J, Burgisser P, Klimkait T, Battegay M, et al. Emergence of HIV-1 drug resistance in previously untreated patients initiating combination antiretroviral treatment: a comparison of different regimen types. Arch Intern Med. 2007;167:1782–1790. doi: 10.1001/archinte.167.16.1782. [DOI] [PubMed] [Google Scholar]
- 46.Hogg RS, Bangsberg DR, Lima VD, Alexander C, Bonner S, Yip B, et al. Emergence of drug resistance is associated with an increased risk of death among patients first starting HAART. PLoS Med. 2006;3:e356. doi: 10.1371/journal.pmed.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–1263. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 48.Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O’Shaughnessy MV, Montaner JS. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. Jama. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 49.Pocock SJ, Elbourne DR. Randomized trials or observational tribulations? N Engl J Med. 2000;342:1907–1909. doi: 10.1056/NEJM200006223422511. [DOI] [PubMed] [Google Scholar]


