Abstract
Safety of water was for a long time and still is one of the most pressing needs for many countries and different communities. Despite the fact that there are potentially many methods to evaluate water safety, finding a simple, rapid, versatile, and inexpensive method for detection of toxins in everyday items is still a great challenge. In this study, we extend the concept of composites obtained impregnation of porous fibrous materials, such as fabrics and papers, by single walled carbon-nanotubes (SWNTs) toward very simple but high-performance biosensors. They utilize the strong dependence of electrical conductivity through nanotubes percolation network on the width of nanotubes-nanotube tunneling gap and can potentially satisfy all the requirements outlined above for the routine toxin monitoring. An antibody to the microcystin-LR (MC-LR), one of the common culprits in mass poisonings, was dispersed together with SWNTs. This dispersion was used to dip-coat the paper rendering it conductive. The change in conductivity of the paper was used to sense the MC-LR in the water rapidly and accurately. The method has the linear detection range up to 10 nmol/L and non-linear detection up to 40 nmol/L. The limit of detection was found to be 0.6 nmol/L (0.6 ng/mL), which satisfies the strictest World Health Organization standard for MC-LR content in drinking water (1 ng/mL), and is comparable to the detection limit of traditional ELISA method of MC-LR detection, while drastically reducing the time of analysis by more than an order of magnitude, which is one of the major hurdles in practical applications. Similar technology of sensor preparation can also be used for a variety of other rapid environmental sensors.
Keywords: sensor, SWNT, nanotubes, environmental monitoring, liver cancer, microcystin, ELISA, water
INTRODUCTION
Microcystin-LR (MC-LR) is one of the most common and the most dangerous toxins produced by the cyanobacteria. Microcystin-LR and other microcystins represent one of the leading causes of water pollution and outbreaks of mass poisoning related to biological contaminants.2,3 The first documented event of mass poisoning with cyanobacterium-produced microcystin was in 1878 and related to a lake in Australia.4 Anecdotal evidence exist that the bloom of cyanobacterium was implicated in the death of soldiers in the army of General Zhu Ge-Ling who drank green water whilst crossing the river almost 2,000 years ago, and people around Monasterium Virdis Stagni (Monastery of Green Loch) in Scotland in 12th century5. Health problems including many deaths related to cyanobacterium contamination constantly occur in many places all over the world in both developing and developed countries in part because micocystins are not removed by the standard water processing protocols.
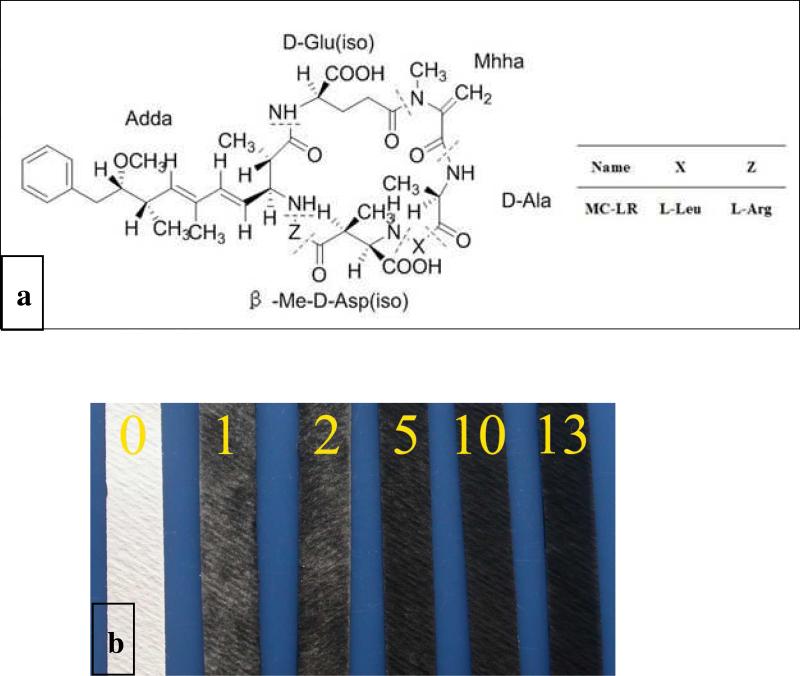
Chemically, MC-LR is a non-ribosomal cyclo-heptapeptides with molecular weight of 995.2 au (Figure 1a) which is strongly hepatotoxic to mammals by inhibiting activity of protein phosphatases responsible for cellular signaling. Besides causing acute liver failure in high doses, microcystins are suspected to promote the primary liver cancer when humans are exposed to them over a long period of time through daily drinking water.6-8
Figure 1.
(A) Chemical structure of MC-LR. (B) Optical photographs of the SWNT-impregnated filter paper with a different number of the deposition cycles.
The World Health Organization (WHO) established the maximum concentrations for the microcystins in daily drinking water of 1 ng/mL9, but it is quite difficult to monitor microcystin levels with required frequency and accuracy. Development of a rapid, sensitive, simple, and inexpensive method to detect the toxins and chemicals in the water has attracted a lot of attention recently,10-15 and was proven to be quite challenging. The traditional methods, such as enzyme-linked immunosorbent assay (ELISA), high performance liquid chromatography (HPLC), mass spectrometry (MS), and magnetic resonance screening are having difficulties satisfying the tough requirements for routine pollution monitoring. For example, ELISA is quite sensitive, but time consuming and requires both special reagents and expertise to run the tests. Magnetic resonance screening requires expensive instrumentation as well as the specialized training, and so does HPLC-MS method. Neither of these techniques are portable.15 We believe that in order to obviate these difficulties we need to develop a method that combines the sensitivity and selectivity of immunoassay, such as ELISA, and simplicity, and portability of the electrochemical or electrical techniques. Optical and magnetic methods can provide low detection limits but are likely to be associated with high cost of testing devices and are often can be run only by professionals.
Carbon nanomaterials with different structures, for instance carbon nanotubes,16-18 carbon nanofiber,19-21 and carbon nanoparticles,22 have been used in rapid and sensitive determination of genotoxic analytes, proteins, and cells. Among them, carbon nanotubes (CNTs) earned special attention23 because of their superior chemical and electrical properties and excellent mechanical strength.24 The CNTs can be dispersed uniformly in some polymers such as the nafion®, poly(3-hexylthiophene), poly(sodium 4-styrene sulfonate) (PSS) and chitosan,25-28 which can be used in device manufacturing.29-30 Previously, our groups successfully demonstrated that single-walled carbon nanotubes (SWNTs) can be used to prepare smart electronic textiles with biosensing functionalities detecting a target protein very sensitively and specifically.1 This work is predicated on the hypothesis that similar technology can be used to satisfy the outlined requirements for toxin detection in food and water, attain the required detection limits, and be highly competitive in terms of quantification and specificity with well-established much more complex technologies. The data obtained in this work indicate that it is possible to use paper impregnated with SWNTs and antibodies as a foundation of very simple and low-cost method for the determination of the MC-LR toxin in the water. The prepared SWNT-paper sensor was able to detect MC-LR in the water sensitively and rapidly using electrochemical current-time (i-t) transients. In fact, the limit of detection (LOD) and detection range are comparable to those of ELISA, while the time of analysis was at least 28 times shorter, which makes it suitable for everyday environmental monitoring.
EXPERIMENTAL
The poly (sodium 4-styrene sulfonate) (PSS) was obtained from the Sigma and used as received. The antibodies to MC-LR were prepared in our laboratory through the immunization of the New Zealand rabbit. CNTs were purchased from the Organic Institute of Chinese Academy of Science and used without purification. The paper strips used for manufacturing of sensors were cut from the standard chemistry analytical filtration paper bought from Hangzhou Xinhua Paper Industry Co. Ltd. with the thickness of 0.2 mm and diameter of 160 mm.
Preparation of the CNT-coated paper sensors
Most of the time we used SWNTs for this work. The processing of MWNTs and corresponding paper electrodes from them (when applicable) followed the same protocol. SWNTs were dispersed in the PSS-water solution (weight ratio of SWNT to PSS 1:2) with the concentration of 50 mg/mL. Notably, PSS is an excellent stabilizer of the proteins, and thus, its use is particularly appropriate when proteins are incorporated between carbon nanotubes. This also insures their long shelf-life. 10 μl antibodies (Ab) to the MC-LR were added directly to the 6 ml as-prepared SWNT-PSS solution to obtain an Ab concentration of 10 μg/mL. Under these conditions, the SWNT/Ab ratio was about 5000:1. The filtration paper strips with the dimensions of 5cm×0.5cm were dipped into the CNT-Ab solution and freeze-dried under vacuum in order to minimize the antibody denaturation.
Detection of MC-LR
All experiments were performed using a CHI 760B Electrochemical Workstation (Chenhua Instrument Company, Shanghai). The i-t curves were recorded at an initial voltage of 2 V and a signal record interval time of 0.001 sec in a standard electrochemical cell with a platinum counter electrode and Hg2Cl2 reference electrode. After the addition of the analyte to the media around the working electrode, the current value was recorded against the time and the value of the current at the amperometric transient plateaus of the i-t curve was used as the criterion to signal change which was correlated with the concentration of MC-LR. In order to optimize the analytical parameters, different buffer solutions (0.1 M PBS, 0.1 M Tris-HCl, 0.1 M Borate buffer solution) at different pH values (PBS pH 6.9, pH 7.4, pH 7.9, pH 8.4), and at different reaction temperatures (4°C, 25 °C, 37 °C) were used. Different concentrations of MC-LR were obtained by dilution of the stock MC-LR solution. The detection range was started from zero. Then 5μL aliquots of the MC-LR solutions of different concentrations were added to the starting volume. The i-t curves were recorded after immunoreaction time of 300 s in a standard electrochemical cell. The control experiments were carried out by adding the non-target analyte solution into the starting volume in the same way. The limit of the detection (LOD) was calculated as LOD=3.3SD/S, where SD is the standard deviation of the response well known from statistics and S is the slope of the calibration curve in the initial stages. Sample simulating actual environmental tests were prepared by spiking the real water samples from the Tai Lake in Wuxi, China with the different concentrations of the MC-LR standard solutions. All the determination results were carried out at least three times followed by the calculation of average values. The original quantity of the MC-LR in the Tai Lake was determined by the traditional ELISA (Details of the ELISA in Supporting Information).
Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM) characterization
The CNT coated paper electrode before and after the immune detection was coated with gold and imaged with the JSM 6380LV scanning electron microscope and Veeco /Digital Instruments Multimode IIIa atomic force microscope.
RESULTS AND DISCUSSION
We have previously reported the possibility of the SWNT coated cotton yarns to detect proteins in solutions, such as blood protein albumin, which is important for the design of a new generation of smart clothes for the first responders, people in high risk professions, and biomedical devices for monitoring of different diseases.1 It would be fundamentally interesting as well as practically important to establish whether the similar method of analysis can be applied to the environmental needs and food safety. With this idea in mind, we have prepared and characterized the SWNT coated paper as the sensor for MC-LR toxin in water. MWNTs were also tested in the analogous process but the corresponding MWNT-paper composites displayed lower stability under current, conductivity, and overall robustness rather than SWNT electrodes. We attribute it to greater flexibility of SWNTs leading to stronger adherence to paper; it originates in strong non-covalent cooperative interactions between the polyelectrolytes and cellulose. The great effect of flexibility of nanoscale wires on mechanical properties of the resulting materials and adhesion of the components to each other was demonstrated before for cellulose.31 It is also probably relevant to mention that, even under high electrical current, no detachment of nanotubes from the SWNT-modified paper electrode was observed.
Regular filter paper strips were dip-coated with the SWNT and dried in air; dip-dry cycles were repeated until the desirable electrical parameters of the sensor were obtained. The number of the cycles is treated as the number of SWNT layers deposited. The deposition of SWNTs can be observed by the change in color from white to black (Figure 1B). Typically for the preparation of sensors, 13 deposition cycles were used. SEM images of the SWNT-coated paper indeed indicate the typical paper morphology, presence of the finely integrated nanotubes, and excellent physical integrity of the material. (See details in the Supporting Information)
As expected the conductivity of the produced material increases with increasing the SWNT contents and the number of layers of SWNT/PSS dispersion deposited (Supporting Information). The gradual increase of conductivity is quite important because in perspective the conductivity of the paper electrode needs to be within a specific range of values depending on the parameters of electrical circuit being used in order to get the best noise-to-signal ratio and the detection linearity for sensing in aqueous environments. Here we used the SWNT-paper composite with a conductivity of 8.33 mS/cm. The Ab in the electrode was also optimized to get the best noise-to-signal result of the detection (see Supporting Information).
For sensing, we employed the standard three-electrode electrochemical station to measure changes in electrical properties of the SWNT-paper strips, which were used as work electrodes. Pt wire and the saturated Hg2Cl2 were used as a counter and referenced electrodes, respectively. The standard electrochemical set-up gives more accurate results than a simple clamping of the SWNT-paper material between two electrodes due to interfacial potential drops at electrode-SWNT interfaces of different nature including the Schottky barrier.32 Variations in these voltages can cause errors in determination of the conductivity of the material and hence, analyte concentration.
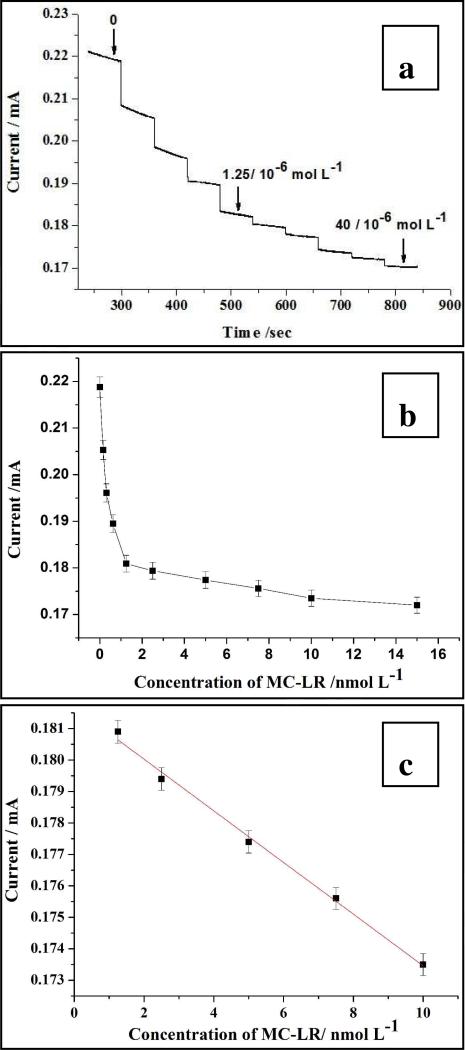
Different concentrations of the MC-LR were obtained by dilution of a stock solution of 0.156 nmol/L, 0.313 nmol/L, 0.625 nmol/L, 1.25 nmol/L, 2.5 nmol/L, 5 nmol/L, 10 nmol/L, 20 nmol/L, 40 nmol/L, 100 nmol/L. Aliquots of this solution were added into the reaction cell one by one at specific time points to obtain the trend from low to high concentrations. After each addition at least 300 s was allowed to pass to make sure that immunoreaction has enough time to proceed before the corresponding i-t curve was recorded. The reaction time of 300 s adopted here was based on average time requirements for antibody-antigen complex formation. The current values in the region of amperometric plateau in i-t transient, i.e. in the “flat” portion of the curve with t=150-200 s ( see Supporting Information) were used as the analytical signals to be correlated to the concentration of MC-LR.
From the detection results, it is clear that that the presence of the target analyte, i.e. MC-LR in this case reduces the current through the electrode, and hence, the conductivity of SWNT-paper composite. This is quite different than the observations made for SWNT and anti-albumin Ab on cotton, where the current increased when antigen was present in solution. For albumin detection, the transduction mechanism was described as the removal of Ab from the SWNT layers, resulting in shrinking of nanotubes-nanotube gaps and related to that improvement of charge transport. In the case of electrodes described here, a different mechanism is apparently at play. Antigen penetrates through the SWNT polymer layer on the surface of paper fibers and forms the immunocomplex with Ab within the material. The Ab-antigen assembly spreads apart neighboring SWNTs, increases the nanotubes-nanotube contact resistance, and hence, reduces the current passing through the material.
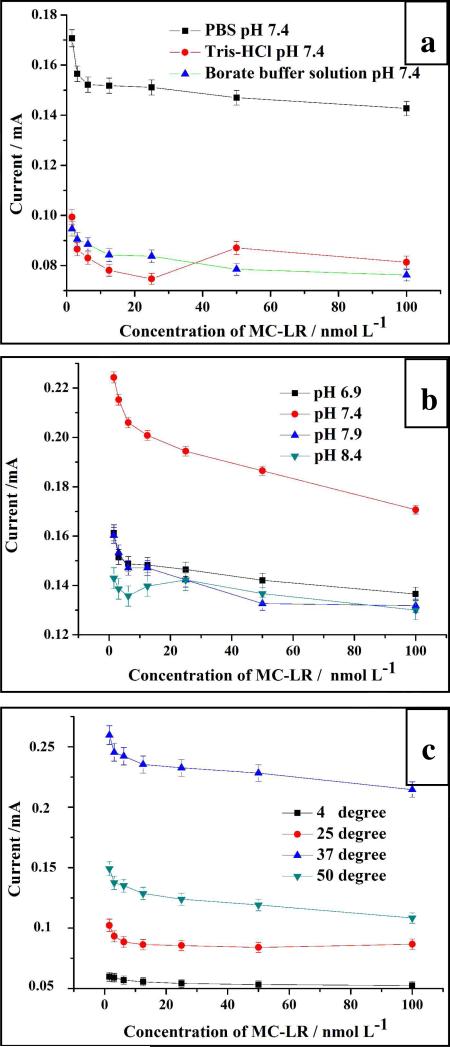
Sensitivity and selectivity of electrochemical detection can be influenced very strongly by media conditions, and thus, the basic variables, namely the buffer composition, pH, and temperature need to be optimized before the evaluation of the method's performance is undertaken especially for comparison with other techniques. Note that the optimal conditions cannot be replicated from other well known electrical and electrochemical systems due to the fundamental difference of signal transduction mechanism in our system. So, we tested the behavior of the SWNT-paper electrodes in three different buffer solutions at the same pH (Figure 2a). As one can see, the signal from MC-LR was the strongest in PBS. So, this buffer was chosen for all the subsequent tests.
Figure 2.
The optimization of the sensing conditions for MC-LR.
The pH value of the buffer should also be optimized and most likely to be maintained around physiological values to preserve the activity of the antibody. The maximum response for all the concentrations of MC-LR For pH 7.4 PBS we observed (Figure 2b).
Temperature affects many parameters related to both electron transport in the SWNT composite, antibody-antigen reaction, and ionic currents between the fibers of the paper. Usually, three temperatures, i.e. 4°C, 25 °C, 37 °C, are adopted for detection schemes based on immunocomplexes. We also included and additional temperature of 50°C to have a more comprehensive representation of the temperature effect. At first, the intensity of the signal increases with temperature due to the enhancement of the activity of the antibodies. Among all the temperatures tested, the strongest signal was obtained at 37 °C at pH 7.4 PBS, which indicates that antigen-antibody reaction in the SWNT-paper electrode proceeds with the highest efficiency under these conditions. When the temperature surpassed 37 °C and reached 50 °C, the intensity of the signal dropped again for denaturation of the antibodies set on (Figure 2c). So, 37 °C was adopted for all the following experiments.
Now let us evaluate MC-LR sensing for these moderately optimized conditions established above. Overall, the analytical parameters of the system in respect to MC-LR exceeded our expectations. The SWNT-paper electrode can sense even the minor change of the MC-LR in the solution with LOD of 0.6 nmol/L, which corresponds to 0.6 ng/mL, which is better than that for liquid chromatography-mass spectrometry (LC-MS), and a sensitivity of 7*102 A L mol−1 (Figure 3c). We believe that further optimization of several other available parameters, such as the number of dip cycles, the quality of nanotubes, and others can be still substantially improved. The detection is also highly specific. The control sample of ochratoxin, which belongs to the family of micotoxints and is also a carcinogen, produced only slight amperometric variations in the current probably due to manipulations with the solution but no systematic correlation with the concentration of the control sample was observed (See Supporting Information).
Figure 3.
The sensing results of MC-LR (a) and the calibration curve of the determination (b) and (c).
The calibration curve for MC-LR on SWNT-paper electrodes in the range of 0-40 nmol/L has a prevalent L-shape. In the most important range of 0-10 μmol/L, where the concentration of the toxin in polluted water should be expected in majority of tests, the calibration curve displayed excellent linearity with R2 of 0.99426 (Figure 3c), but for greater concentrations it deviated from the line with the expected upward trend, which finally levels off at ca. 40μmol/L (Figure 3a). Such behavior is indicative of the saturation phenomena when most of the antibodies in the SWNT-paper electrode formed complexes with MC-LR. Depending on the requirements the range of detection and linearity can be certainly expanded by tuning the SWBT/Ab ratio, number of the layers deposited, and polymers used to prepare the SWNT dispersion.
According WHO requirements, the content of the MC-LR in the daily water should be less than 1ng/mL, which corresponds to 1 nmol/L (MrMC-LR = 995.2 au). From this point of view, the method based on SWNT-paper could be used to monitor the quality of the drinking water for safety control. Comparing with the traditional ELISA method, the newly developed method has detection range and LOD similar to those of ELISA, but with much shorter detection time. It is also much easier to set-up and perform. The LOD and detection range for MC-LR detection by ELISA are 0.1 ng/mL and 50 ng/mL, respectively. The time necessary for the analysis by ELISA usually exceeds 14 hours. In case of our method, the entire analysis with sample preparation takes no longer than 30 min, which corresponds to 28 fold reduction of required time. This is much more suitable for the task of everyday monitoring of water supply.
Besides the sensitivity and specificity confirmation of the developed method, the evaluation of repeatability of the method and its applicability to the water samples from the potential place of pollution was carried out. The water from Tai lake was spiked with MC-LR As shown in Table 1, the technique affords excellent recoveries of the spiked samples and acceptable relative standard deviation (n=3). Overall, superb correlation between the MC-LR concentration values obtained by ELISA and SWNT-paper method was observed.
Table 1.
Detection of microcystin in the water of Tai lake, Wuxi, China
| Water sample | Original Concentrationa (ng/mL) | Spiked concentrationb (ng/mL) | Detected concentrationc Mean±SD (ng/mL) | Recovery (%) Mean±SD |
|---|---|---|---|---|
| 1.04 | 0.1 | 1.09±0.03 | 95.61±2.6 | |
| Water from Tai | 1.69 | 1 | 2.65±0.05 | 98.51±1.9 |
| Lake | 2.87 | 2 | 4.58±0.19 | 94.05±3.9 |
| 5.61 | 5 | 10.75±0.23 | 101.32±2.2 |
Polluted water samples are from different areas of Tai lake. Original concentrations of MC-LR in the were determined by the standard ELISA protocol.
Different concentrations of MC-LR standard were added to the water samples. After thorough mixing and standing for at least 2 h, the samples were extracted by solid phase extraction to removing potentially interfering substances prior to assay. Samples were analyzed blind.
SD was calculated based on three experiment for each sample.
Conclusions
To meet the urgent demand of monitoring the quality of drinking water, a very simple, rapid, sensitive, and inexpensive electrical sensor based on material obtained by impregnation of common filtration papers with carbon nanotubes and antibodies has been developed. The mechanism of sensing is predicated on the formation of Ab-antigen complex between carbon nanotubes forming a dense percolation network. Due to high sensitivity of SWNT-SWNT charge transfer to the distance between the nanotubes, the conductivity of the network exhibits strong dependence on the presence of analytes. This hypothesis was evaluated by making a sensor to highly toxic MC-LR cyclopeptide often present in water polluted by cyanobacteria. The detection limit, sensitivity, specificity and the repeatability of the developed analytical method can be compared to that of ELISA while the sensor is much easier to use. The time necessary for detection was reduced by at least 28 times. It is believed that the developed could be a potential and powerful method for the monitoring of the environmental water resource. Importantly, this method is very versatile and can be easily extended to many other harmful chemicals or toxins in the water or food by changing the antibody in the sensor electrode.
Supplementary Material
Supporting Information
Simple, Rapid, Sensitive, and Versatile SWNT-Paper Sensor for Environmental Toxin Detection Competitive with ELISA
Libing Wang,1# Wei Chen,1, 2# Dinghua Xu,1# Bong Sup Shim,2 Yingyue Zhu,1 Fengxia Sun,1 Liqiang Liu,1 Chifang Peng,1 Zhengyu Jin,1 Chuanlai Xu,1, 2* Nicholas A. Kotov2*
1School of Food Science and Technology, State Key Lab of the Food Science & Technology, Jiangnan University, Wuxi, Jiangsu Province, 214122, China;
2Department of Chemical Engineering, Department of Materials Science and Engineering, Department of Biomedical Engineering, University of Michigan, Ann Arbor, Michigan 48109, USA.
Figure S1. Optical photographs of the MWNT and SWNT coated paper electrodes.
*To Whom correspondence should be addressed. xcl@jiangnan.edu.cn; kotov@umich.edu
#These three authors contribute equally to this paper.
Figure S2. The conductivity of SWNT-impregnated filter paper
Figure S3. The SEM images of (a) the face and (b) the edge of the 13 deposition cycles paper electrode.
Figure S4. Amperometric i-t traces for sensing of the target samples of MC-LR
Figure S5. Amperometric i-t traces for sensing of the control samples of ochratoxin.
Figure S6. The sensing results for the control samples of ochratoxin.
The detailed process of the ELISA is as follows:
1. Coat each well in a 96-well plate (Costar #9018) with 100 μL of a coating antigen solution.
2. Cover and rock overnight in an incubator at 4°C.
3. Wash 3 times with PBS-Tween 20 in vacuum-apparatus and pat dry.
4. The plate was blocked with 100μL (0.5%, w/v) OVA solution in PBS solution for 2 h at 37 °C.
5. Wash 3times with PBS-Tween 20.
6. Add 100 μL/well MC-LR at different dilutions or samples with 100μL/well pAb then incubate for 0.5 hour at 37 °C.
7. Wash 3times with PBS-Tween 20 and pat dry.
8. Dilute horseradish peroxidase-conjugated goat anti-rabbit IgG 1 : 3000 in PBS-Tween 20 for 0.5 hour and incubate as before.
9. Wash 6times as before and pat dry.
10. Prepare color substrate (TMB) and add 100 μL/well for 15 min in dark at room temperature.
11. H2SO4 (2 mol/L) was added to stop the reaction and record the absorption in a micro plate reader at 450nm.
ACKNOWLEDGMENTS
The authors herein want to express the great appreciation for the financial support by the National Science Foundation of China (Nos. 20675035, 20871060, 20835006) and the 11th Five Years Key Programs for Science and Technology Development of China (Ns. 200810099, 200810219, 2008ZX08012-001) and supported by 111 project-B07029 and PCSIRT0627. NAK wants to acknowledge the support of AFOSR (No. FA9550-05-1-043), NSF (Nos. CMS-0528867, ECS-0601345), and NIH (NIH No. 5R01EB007350-02).
Footnotes
SUPPORTING INFORMATION AVAILABLE
Additional information as noted in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Shim BS, Chen W, Doty C, Xu CL, Kotov NA. Nano Letters. 2008;8:4151–4157. doi: 10.1021/nl801495p. [DOI] [PubMed] [Google Scholar]
- 2.Nidhi G, Pant SC, Vijayaraghavan, Lakshman R. Toxicology. 2003;188:285–296. doi: 10.1016/s0300-483x(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 3.Maria P, Silvia P, Angeles J, Ana MC. Toxicon. 2009;54:161–169. [Google Scholar]
- 4.Francis G. Nature. 1878;18:11–12. [Google Scholar]
- 5.Chorus I, Bartram J. Toxic cyanobacteria in water; A guide to their public health consequences, monitoring and management. E & FN Spon; London: 1999. [Google Scholar]
- 6.U.S. EPA . Toxicological Reviews of Cyanobacterial Toxins: Microcystins LR, RR, YR and LA (External Review Draft) U.S. Environmental Protection Agency; Washington, DC: 2006. EPA/600/R-06/139. [Google Scholar]
- 7.Yu SZ. Drinking water and primary liver cancer. In: Tang ZY, Wu MC, Xia SS, editors. Primary Liver Cancer. Spring-Verlag; Berlin: 1989. pp. 30–37. [Google Scholar]
- 8.Zhou L, Yu H, Chen K. Biomed. Environ. Sci. 2002;15:166–171. [PubMed] [Google Scholar]
- 9.WHO Guidelines for drinking water quality. World Health Organization; 1998. [Google Scholar]
- 10.Hao XL, Kuang H, Li YL, Yuan Y, et al. Journal of Agricultural and Food Chemistry. 2009;57:3033–3039. doi: 10.1021/jf803807b. [DOI] [PubMed] [Google Scholar]
- 11.Peng CF, Chen YW, Chen W, Xu CL, et al. Food Chemistry. 2008;109:647–653. [Google Scholar]
- 12.Peng CF, Li ZK, Zhu YY, Chen W, et al. Biosensors and Bioelectronics. 2009 DOI: 10.1016/j.bios.2009.05.031. [Google Scholar]
- 13.Xie HL, Liu LQ, Xu CL. Anal. Chim. Acta. 2009;634:129–133. doi: 10.1016/j.aca.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Peng CF, Jin ZY, Qiao RR, et al. Biosensors and Bioelectronics. 2009;24:2051–2056. doi: 10.1016/j.bios.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Ma W, Chen W, Qiao RR, Liu CY, Yang CH, Li ZK, Xu DH, Peng CF, Jin ZY, Xu CL, Zhu SF, Wang LB. Biosensors and Bioelectronics. 2009 DOI: doi:10.1016/j.bios.2009.06.020. [Google Scholar]
- 16.Heller DA, Jin H, Martinez BM, Patel D, Miller BM, et al. Nat. Nanotechnol. 2009;4:114–120. doi: 10.1038/nnano.2008.369. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Tabakman SM, Goodwin AP, Kattah MG, Daranciang D, Wang X, Zhang G, Li X, Liu Z, Utz PJ, Jiang K, Fan S, Dai H. Nat. Biotechnol. 2008;26:1285–1292. doi: 10.1038/nbt.1501. [DOI] [PubMed] [Google Scholar]
- 18.Satishkumar BC, Brown LO, Gao Y, Wang CC, Wang HL, Doorn SK. Nat. Nanotechnol. 2007;2:560–564. doi: 10.1038/nnano.2007.261. [DOI] [PubMed] [Google Scholar]
- 19.Shim BS, Starkovich J, Kotov N. Composites Science and Technology. 2006;66(9):1174–1181. [Google Scholar]
- 20.Koziol K, Vilatela J, Moisals A, Motta M, Cunniff P, Sennett M, Windle A. Science. 2007;318:1892–1895. doi: 10.1126/science.1147635. [DOI] [PubMed] [Google Scholar]
- 21.Hao C, Ding L, Zhang X, Ju H. Anal. Chem. 2007;79:4442–4447. doi: 10.1021/ac062344z. [DOI] [PubMed] [Google Scholar]
- 22.Fu CC, Lee HY, Chen K, Lim TS, Wu HY, Lin PK, Wei PK, Tsao PH, Chang HC, Fann W. Proc. Natl. Acad. Sci. USA. 2007;104:727–732. doi: 10.1073/pnas.0605409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iijima S. Nature. 1991:354–356. [Google Scholar]
- 24.Baughman RH, Zakhidov AA, de Heer WA. Science. 2002;297:787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Fernando KAS, Lin Y, Meziani MJ, Veca LM, Cao L, Zhang P, Kimani MM, Sun YJ. Am. Chem. Soc. 2008;130:1415–1419. doi: 10.1021/ja0768035. [DOI] [PubMed] [Google Scholar]
- 26.Pradhan B, Batabyal SK, Pal AJ. J. Phys. Chem. B. 2006;110:8274–8277. doi: 10.1021/jp060122z. [DOI] [PubMed] [Google Scholar]
- 27.Lau C, Cooney MJ. Langmuir. 2008;24:7004–7010. doi: 10.1021/la8005597. [DOI] [PubMed] [Google Scholar]
- 28.Sung JH, Kim HS, Jin H, Choi HJ, Chin I. Macromolecules. 2004;37:9899–9902. [Google Scholar]
- 29.Wu Z, Chen Z, Du X, Logan JM, Sippel J, Nikolou M, Kamaras K, Reynolds JR, Tanner DB, Hebard AF, Rinzler AG. Science. 2004;305:1273–1277. doi: 10.1126/science.1101243. [DOI] [PubMed] [Google Scholar]
- 30.Hatton RA, Miller AJ, Silva SRP. J. Mater. Chem. 2008;18:1183–1192. [Google Scholar]
- 31.Podsiadlo P, Tang ZY, Shim BS, Kotov NA. Nano Letters. 2007;7(5):1224–1231. doi: 10.1021/nl0700649. [DOI] [PubMed] [Google Scholar]
- 32.Shim BS, Tang ZY, Morabito MP, Kotov NA. Chemistry of Materials. 2007;19(23):5467–5474. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Simple, Rapid, Sensitive, and Versatile SWNT-Paper Sensor for Environmental Toxin Detection Competitive with ELISA
Libing Wang,1# Wei Chen,1, 2# Dinghua Xu,1# Bong Sup Shim,2 Yingyue Zhu,1 Fengxia Sun,1 Liqiang Liu,1 Chifang Peng,1 Zhengyu Jin,1 Chuanlai Xu,1, 2* Nicholas A. Kotov2*
1School of Food Science and Technology, State Key Lab of the Food Science & Technology, Jiangnan University, Wuxi, Jiangsu Province, 214122, China;
2Department of Chemical Engineering, Department of Materials Science and Engineering, Department of Biomedical Engineering, University of Michigan, Ann Arbor, Michigan 48109, USA.
Figure S1. Optical photographs of the MWNT and SWNT coated paper electrodes.
*To Whom correspondence should be addressed. xcl@jiangnan.edu.cn; kotov@umich.edu
#These three authors contribute equally to this paper.
Figure S2. The conductivity of SWNT-impregnated filter paper
Figure S3. The SEM images of (a) the face and (b) the edge of the 13 deposition cycles paper electrode.
Figure S4. Amperometric i-t traces for sensing of the target samples of MC-LR
Figure S5. Amperometric i-t traces for sensing of the control samples of ochratoxin.
Figure S6. The sensing results for the control samples of ochratoxin.
The detailed process of the ELISA is as follows:
1. Coat each well in a 96-well plate (Costar #9018) with 100 μL of a coating antigen solution.
2. Cover and rock overnight in an incubator at 4°C.
3. Wash 3 times with PBS-Tween 20 in vacuum-apparatus and pat dry.
4. The plate was blocked with 100μL (0.5%, w/v) OVA solution in PBS solution for 2 h at 37 °C.
5. Wash 3times with PBS-Tween 20.
6. Add 100 μL/well MC-LR at different dilutions or samples with 100μL/well pAb then incubate for 0.5 hour at 37 °C.
7. Wash 3times with PBS-Tween 20 and pat dry.
8. Dilute horseradish peroxidase-conjugated goat anti-rabbit IgG 1 : 3000 in PBS-Tween 20 for 0.5 hour and incubate as before.
9. Wash 6times as before and pat dry.
10. Prepare color substrate (TMB) and add 100 μL/well for 15 min in dark at room temperature.
11. H2SO4 (2 mol/L) was added to stop the reaction and record the absorption in a micro plate reader at 450nm.