Abstract
Eagon, R. G. (University of Georgia, Athens) and C. H. Wang. Dissimilation of glucose and gluconic acid by Pseudomonas natriegens. J. Bacteriol. 83:879–886. 1962—When glucose dissimilation of a marine pseudomonad, Pseudomonas natriegens, was studied, enzymes of both the glycolytic pathway and of the hexose monophosphate pathway were detected in extracts of glucose-grown cells. Enzymes of the Entner-Doudoroff pathway and phosphoketolase were not detected. Data from radiorespirometric experiments indicated that approximately 92 and 8% of glucose actually catabolized were routed via the glycolytic and the hexose monophosphate pathways, respectively.
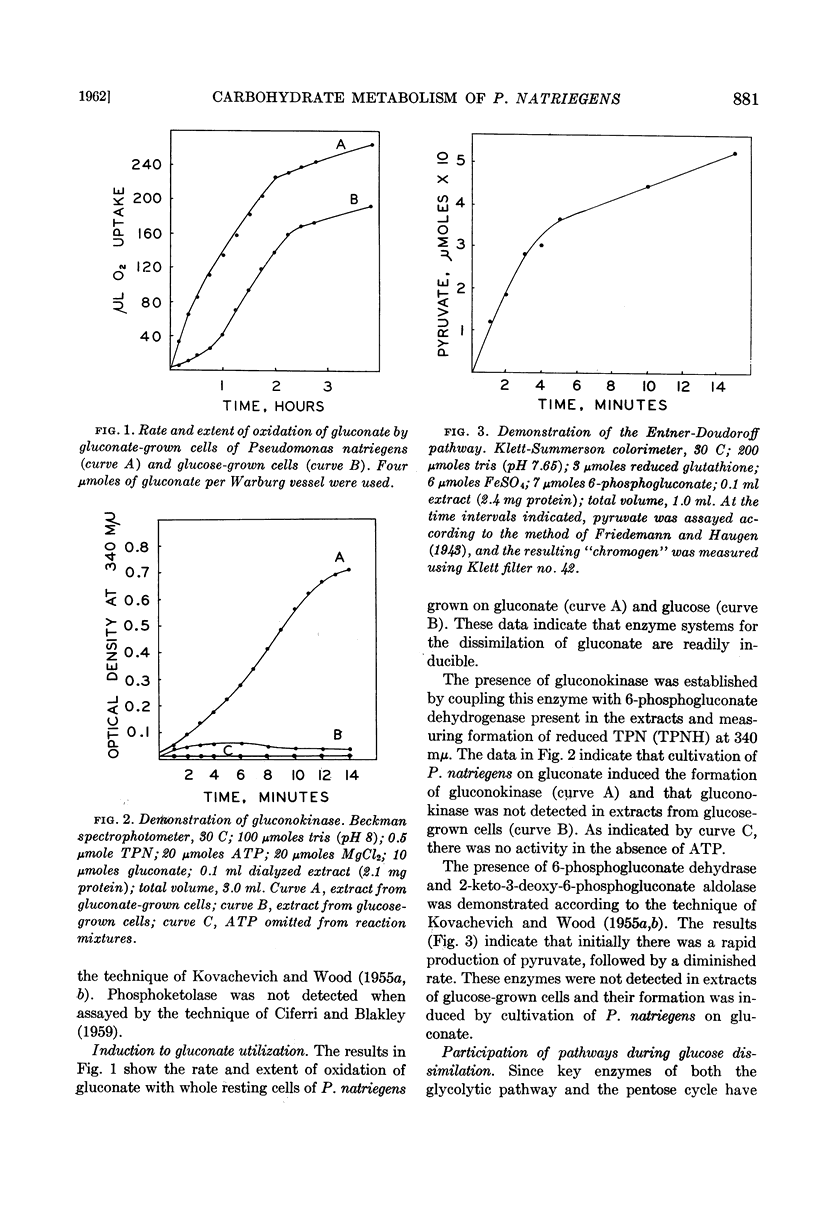
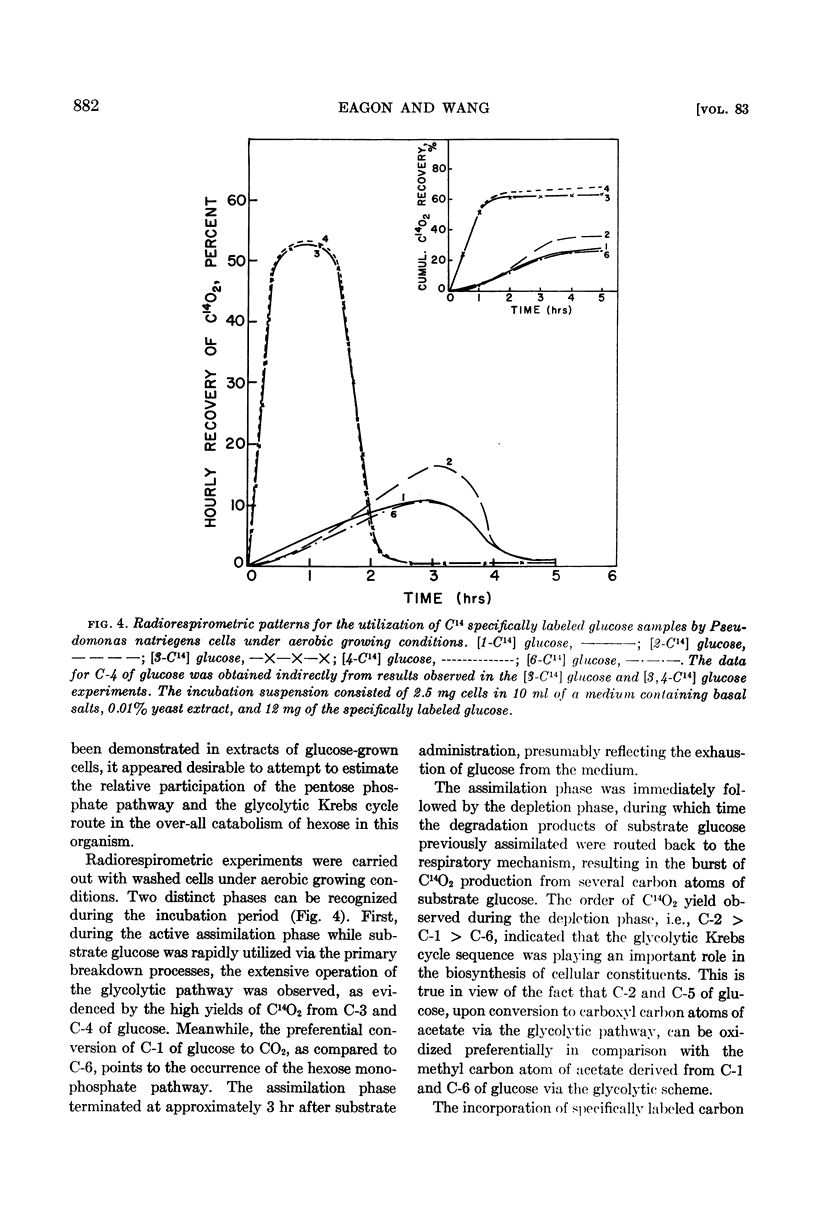
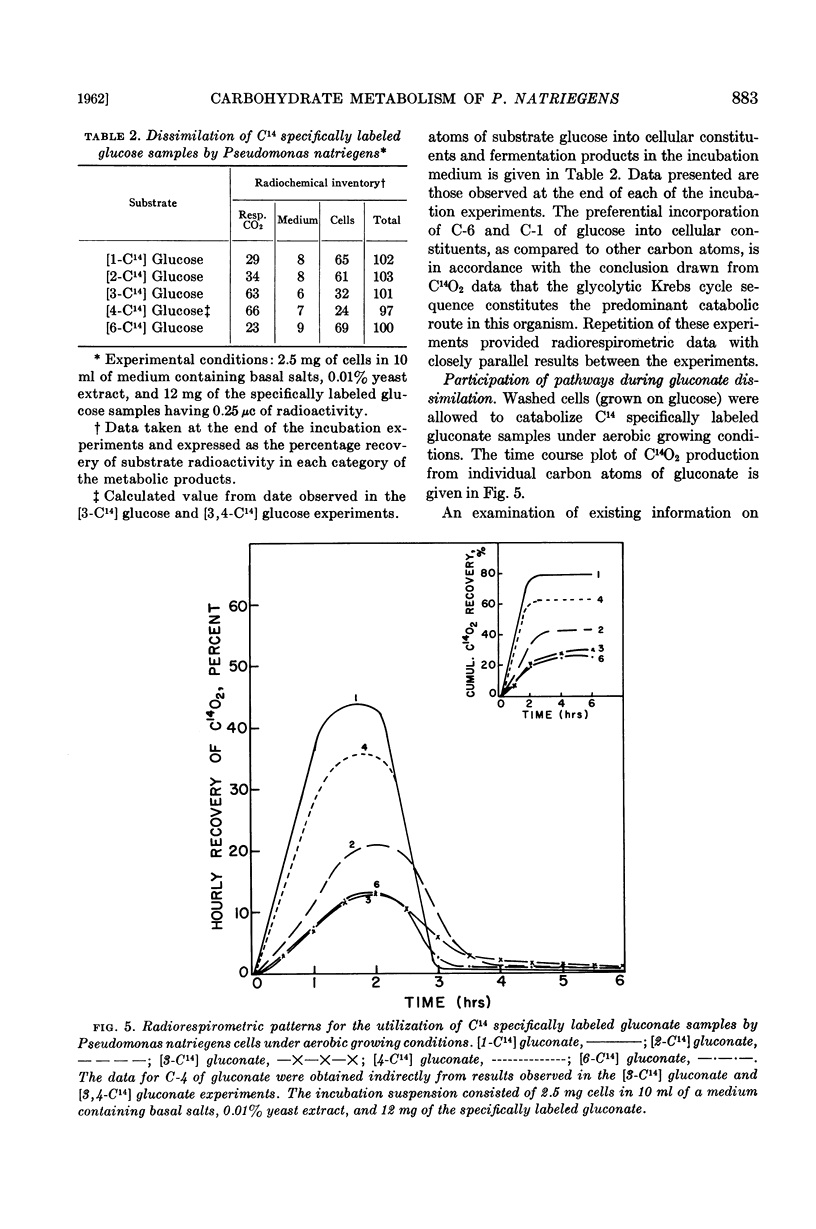
When P. natriegens was induced to utilize gluconate, it was demonstrated that gluconokinase and enzymes of the Entner-Doudoroff pathway were induced. Radiorespirometric experiments with cells under growing conditions revealed that gluconate was dissimilated predominantly (80%) via the Entner-Doudoroff pathway. This observation was in contrast to the observation that the glycolytic pathway is practically the exclusive catabolic pathway for glucose dissimilation. A minor portion of substrate gluconate was also catabolized by this organism via the hexosemonophosphate pathway. However, the pentose phosphate derived from substrate gluconate is believed not to be catabolized extensively.
The important facet uncovered by these experiments was the extensive operation of the glycolytic route of glucose dissimilation. This is in contrast to other pseudomonads studied to date, which have been reported to dissimilate glucose predominantly via the Entner-Doudoroff pathway and which do not utilize the glycolytic pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., JANG R. Purification and properties of phosphoribo-isomerase from alfalfa. J Biol Chem. 1954 Aug;209(2):847–855. [PubMed] [Google Scholar]
- BECK W. S. Determination of triose phosphates and proposed modifications in the aldolase method of Sibley and Lehninger. J Biol Chem. 1955 Feb;212(2):847–857. [PubMed] [Google Scholar]
- CIFERRI O., BLAKLEY E. R. The metabolism of 2-keto-D-gluconate by cell-free extracts of Leuconostoc mesenteroides. Can J Microbiol. 1959 Oct;5:547–560. doi: 10.1139/m59-065. [DOI] [PubMed] [Google Scholar]
- KITOS P. A., WANG C. H., MOHLER B. A., KING T. E., CHELDELIN V. H. Glucose and gluconate dissimilation in Acetobacter suboxydans. J Biol Chem. 1958 Dec;233(6):1295–1298. [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. III. Purification and properties of a 6-phosphogluconate dehydrase. J Biol Chem. 1955 Apr;213(2):745–756. [PubMed] [Google Scholar]
- KOVACHEVICH R., WOOD W. A. Carbohydrate metabolism by Pseudomonas fluorescens. IV. Purification and properties of 2-keto-3-deoxy-6-phosphogluconate aldolase. J Biol Chem. 1955 Apr;213(2):757–767. [PubMed] [Google Scholar]
- McDonald J. K., Cheldelin V. H., King T. E. GLUCOSE CATABOLISM IN THE ERGOT FUNGUS, CLAVICEPS PURPUREA. J Bacteriol. 1960 Jul;80(1):61–71. doi: 10.1128/jb.80.1.61-71.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRORIE R. A., WILLIAMS A. K., PAYNE W. J. Alduronic acid metabolism by bacteria. J Bacteriol. 1959 Feb;77(2):212–216. doi: 10.1128/jb.77.2.212-216.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE W. J., EAGON R. G., WILLIAMS A. K. Some observations on the physiology of Pseudomonas natriegens nov. spec. Antonie Van Leeuwenhoek. 1961;27:121–128. doi: 10.1007/BF02538432. [DOI] [PubMed] [Google Scholar]
- PAYNE W. J. Effects of sodium and potassium ions on growth and substrate penetration of a marine pseudomonad. J Bacteriol. 1960 Nov;80:696–700. doi: 10.1128/jb.80.5.696-700.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE W. J. Studies on bacterial utilization of uronic acids. III. Induction of oxidative enzymes in a marine isolate. J Bacteriol. 1958 Sep;76(3):301–307. doi: 10.1128/jb.76.3.301-307.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN I. J., WANG C. H., GILMOUR C. M. Comparative catabolism of carbohydrates in Pseudomonas species. J Bacteriol. 1960 Apr;79:601–611. doi: 10.1128/jb.79.4.601-611.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C. H., STERN I., GILMOUR C. M., KLUNGSOYR S., REED D. J., BIALY J. J., CHRISTENSEN B. E., CHELDELIN V. H. Comparative study of glucose catabolism by the radiorespirometric method. J Bacteriol. 1958 Aug;76(2):207–216. doi: 10.1128/jb.76.2.207-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]


