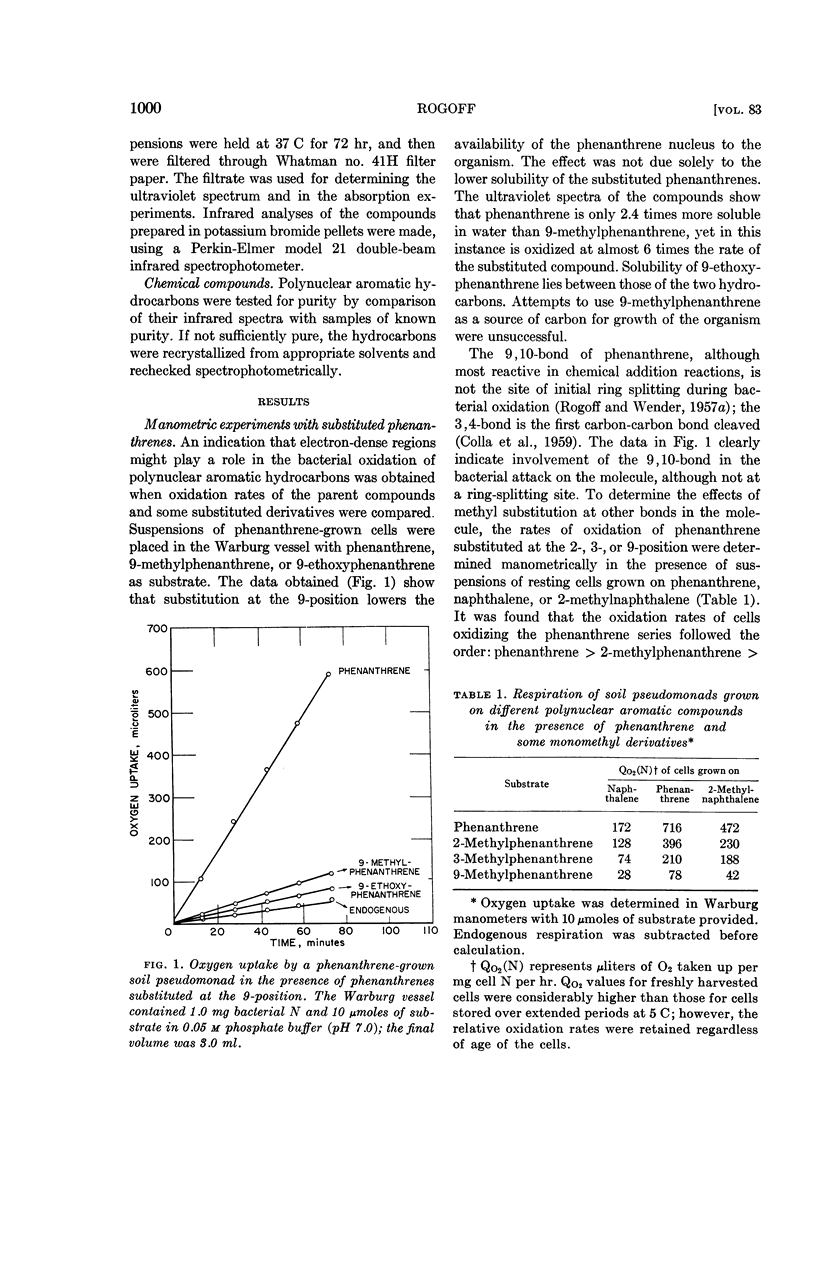
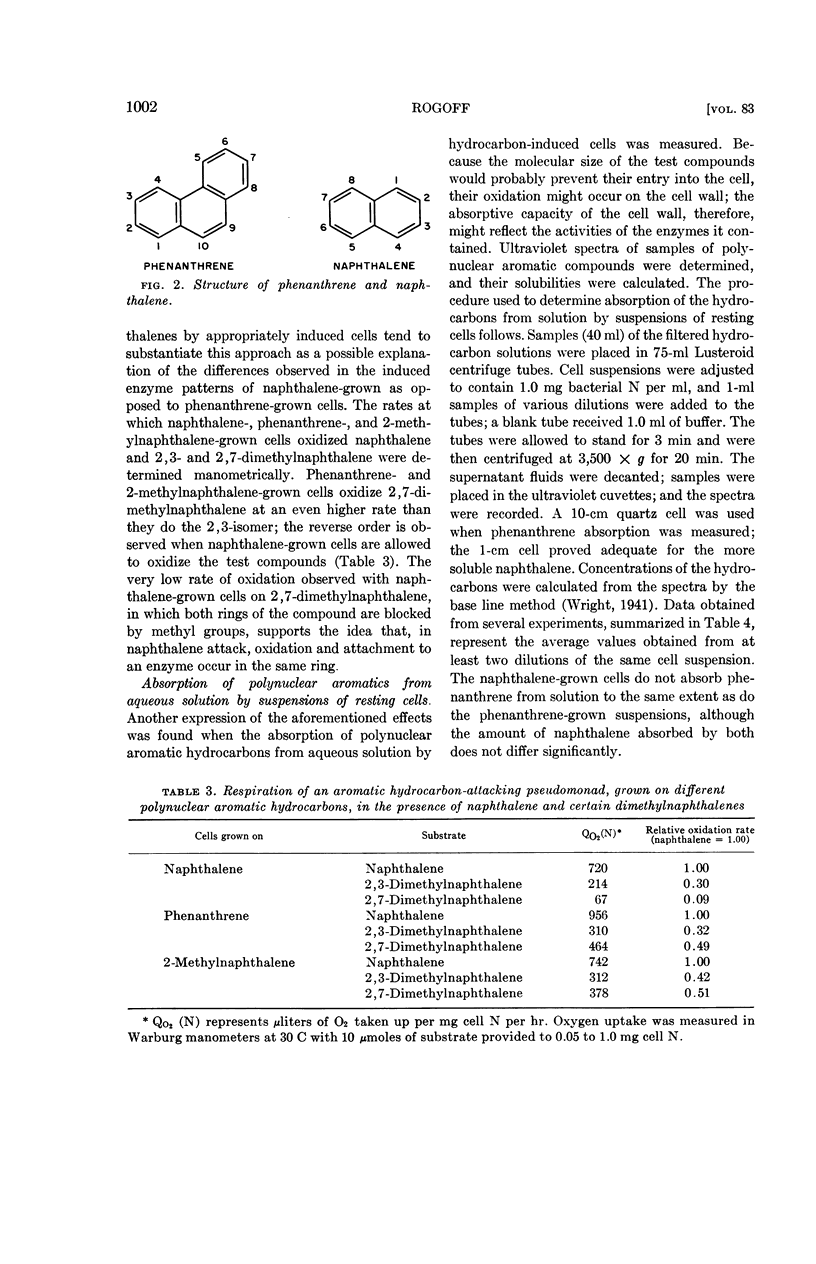
Abstract
Rogoff, Martin H. (U.S. Bureau of Mines, Pittsburgh, Pa.). Oxidation of polycyclic aromatic hydrocarbons by soil pseudomonads. J. Bacteriol. 83:998–1004. 1962.—Substitution of phenanthrene by a methyl group at the 9-carbon blocks oxidation of the compound by a resting-cell suspension of a phenanthrene-grown soil pseudomonad. When 2-methylphenanthrene is provided, the oxidation rate is considerably higher; 3-methylphenanthrene is oxidized at a rate intermediate between the other two, even though the methyl group is attached to a carbon directly involved in ring splitting. Cells grown on naphthalene or anthracene oxidize phenanthrene at a much lower rate than cells grown with phenanthrene or 2-methylnaphthalene as the source of carbon. Naphthalene-grown cells also absorb less phenanthrene from aqueous solution than do their phenanthrene-grown counterparts.
The data are in keeping with the hypothesis that polynuclear aromatic hydrocarbons attach to the relevant bacterial enzymes at carbon-carbon bonds of high electron density (K regions; localized double bonds), and that the ring-splitting reactions then occur at other bonds on the substrate molecule. The actual bond that undergoes fission is determined by the electronic and steric configurations of the enzyme-substrate complex. When linearly arranged aromatic compounds such as naphthalene or anthracene are attacked, attachment to an enzyme and ring splitting may take place on the same ring; angular aromatic compounds such as phenanthrene afford attachment to an enzyme at a bond in a ring other than the one containing the ring-splitting site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- PULLMAN A., PULLMAN B. Electronic structure and carcinogenic activity of aromatic molecules: new developments. Acta Unio Int Contra Cancrum. 1954;10(2):153–154. [PubMed] [Google Scholar]
- PULLMAN B., BAUDET J. Sur le métabolisme des hydrocarbures cancérogènes. C R Hebd Seances Acad Sci. 1954 Feb 22;238(8):964–966. [PubMed] [Google Scholar]
- ROELS O. A. Correlation between the fat and the protein content of human milk. Nature. 1958 Sep 6;182(4636):673–673. doi: 10.1038/182673a0. [DOI] [PubMed] [Google Scholar]
- ROGOFF M. H., WENDER I. 3-Hydroxy-2-naphthoic acid as an intermediate in bacterial dissimilation of anthracene. J Bacteriol. 1957 Jul;74(1):108–109. doi: 10.1128/jb.74.1.108-109.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOFF M. H., WENDER I. Methylnaphthalene oxidations by pseudomonads. J Bacteriol. 1959 Jun;77(6):783–788. doi: 10.1128/jb.77.6.783-788.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOFF M. H., WENDER I. The microbiology of coal. I. Bacterial oxidation of phenanthrene. J Bacteriol. 1957 Feb;73(2):264–268. doi: 10.1128/jb.73.2.264-268.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


