Abstract
Many insect-borne pathogens have complex life histories because they must colonize both hosts and vectors for successful dissemination. In addition, the transition from host to vector environments may require changes in gene expression before the pathogen's departure from the host. Xylella fastidiosa is a xylem-limited plant-pathogenic bacterium transmitted by leafhopper vectors that causes diseases in a number of economically important plants. We hypothesized that factors of host origin, such as plant structural polysaccharides, are important in regulating X. fastidiosa gene expression and mediating vector transmission of this pathogen. The addition of pectin and glucan to a simple defined medium resulted in dramatic changes in X. fastidiosa's phenotype and gene-expression profile. Cells grown in the presence of pectin became more adhesive than in other media tested. In addition, the presence of pectin and glucan in media resulted in significant changes in the expression of several genes previously identified as important for X. fastidiosa's pathogenicity in plants. Furthermore, vector transmission of X. fastidiosa was induced in the presence of both polysaccharides. Our data show that host structural polysaccharides mediate gene regulation in X. fastidiosa, which results in phenotypic changes required for vector transmission. A better understanding of how vector-borne pathogens transition from host to vector, and vice versa, may lead to previously undiscovered disease-control strategies.
Keywords: biofilm, disease, host transition, vector-borne, Xylella fastidiosa
Insect vectors are responsible for transmitting a variety of pathogens to a range of animal and plant hosts. Interactions between vector and pathogen vary from simple mechanical transmission to intricate interactions where the pathogen must develop and multiply within vectors. In addition, pathogens may only be transmissible, or present in transmissible tissue, at certain times within an infected host; the concentrations of some human microfilariae in the peripheral circulatory system are highest during times of day that coincide with vector biting activity (1). It is also plausible that factors, such as host-stress responses because of infection (2) or molecules of host origin that mediate the expression of genes (3), are required for pathogen transmission or increase disease spread rates, as vector competence is frequently context-dependent (4–6).
A wide range of signal molecules, environmental stresses, and other factors trigger changes in gene expression in human, animal, and plant pathogens, resulting in new phenotypes that increase the fitness of individuals (7, 8). Phenotypic changes are especially important for pathogens with complex life histories, which must survive in multiple environments under different habitat-dependent stresses. The etiological agent of Lyme disease, Borrelia burgdorferi, for example, colonizes hosts as diverse as mice, birds, and ticks, which represent distinct habitats to which B. burgdorferi must appropriately respond (9). The facultative human pathogen Vibrio cholerae, which occurs in aquatic environments and the human gastrointestinal tract, modifies its transcription profile in late stages of human infection (before leaving the host) to enhance its chances of survival in its aquatic habitat (8, 10). Therefore, many pathogens have phenotypic plasticity that permits the exploration of multiple environments, which is likely regulated by signals or cues provided by their habitat.
In many cases, pathogens may interact with host surfaces without live cells. V. cholerae colonizes the exoskeleton of copepods where it is maintained in the environment. It has a regulon for chitin utilization (11), which, among other things, facilitates V. cholerae colonization of zooplankton and the formation of biofilms (12). In this case, a structural compound (chitin) of V. cholerae's aquatic hosts function as an environmental cue to which the bacterium responds. Pathogens may also respond to contact with host cells. The pathogen Ralstonia solanacearum requires a type-III secretion system to cause disease in host plants. Brito et al. (13) showed that triggering of genes associated with R. solanacearum pathogenicity occurs in a plant cell contact-dependent manner after an outer-membrane receptor contacts a host-plant cell. Therefore, the regulation of colonization and pathogenicity genes is also dependent on a wide range of signals and cues received by bacteria.
Xylella fastidiosa is a vector-borne bacterial pathogen that colonizes a large number of plants, including crops of economic importance, such as grape, citrus, coffee, and almond (14). One of X. fastidiosa's unique characteristics is that it interacts with polysaccharide-coated surfaces in both host plant and vector (15). In plants, as a xylem-limited organism, X. fastidiosa is confined in vessels composed of polysaccharides, such as cellulose and pectin. Within its leafhopper vectors, this bacterium colonizes the foregut surface (16), which is part of the insect's exoskeleton and is composed primarily of chitin and chitin-like polysaccharides. X. fastidiosa transmission is dependent on cell-cell signaling (i.e., quorum sensing), as a signal-production mutant is not transmissible by vectors and does not colonize vectors (17). In addition, this bacterium requires afimbrial adhesins to colonize leafhoppers (18), which are under control of a cell-cell signaling regulatory system (19). Therefore, it is expected that cells grown in vitro to high densities should be transmitted by vectors if provided to insects through an artificial diet system. However, X. fastidiosa grown in commonly used media are not transmissible by vectors, despite the fact that they reach high cell densities in culture and are acquired by vectors in large numbers through artificial diet systems (20). These observations suggest that biotic interactions impacting gene regulation in plant hosts, but not in vitro, or an unidentified plant factor, are required for X. fastidiosa to transition into a vector-transmissible phenotype.
Pectin degradation is required for X. fastidiosa pathogenicity and movement within plants because it is the major component of pit membranes between xylem vessels (21). Pit membrane degradation may also expose cell wall polysaccharides that could then be digested by other extracellular enzymes, such as glucanases (15). Thus, it is possible that such compounds of host origin, specifically the polysaccharides pectin and glucan, induce a state change in X. fastidiosa gene expression that leads to vector colonization and transmission. We tested the hypothesis that X. fastidiosa responds to environmental cues in a context-dependent fashion so that the balance between host plant colonization and dispersal can be optimized through phenotypic transitions. We show that host plant structural carbohydrates, especially pectin, induce regulons that are required for plant pathogen vector transmission.
Results
Carbohydrate-Driven Phenotypic Changes in X. fastidiosa.
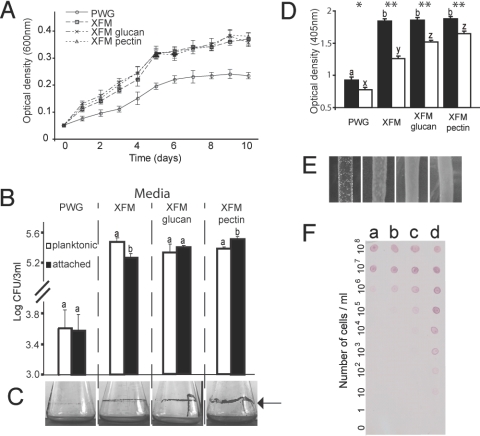
The growth of X. fastidiosa was compared in four different media, a rich complex medium (periwinkle wilt GelRite or PWG) (22), a simple defined medium (X. fastidiosa medium or XFM), and XFM supplemented with either glucan or pectin. Cell growth was similar in XFM and the supplemented media but lower in PWG when compared to all other media (P < 0.0001) (Fig. 1A). However, the ratio of planktonic/attached cells found in the various media was variable. In XFM we found more planktonic than attached cells, whereas that proportion was equal for XFM-glucan; in XFM-pectin, more cells were attached to the surface of vials than in suspension (Fig. 1B). These results were visually confirmed by staining cells forming biofilms in the air/broth interface of flasks maintained in a shaker (Fig. 1C). We used an immunological assay to relatively quantify the amount of exopolysaccharides (EPS, gum) produced by cells grown in the different media and found that all XFM-based media had approximately twice as much EPS present on cells compared to PWG (Fig. 1D). Bacterial lawns grown on solid XFM and XFM-polysaccharides had a glossy appearance when compared to PWG (Fig. 1E). Afimbrial adhesins (Hxfs) have been shown to be important for X. fastidiosa adhesion to insect vectors (18) and pathogenicity to plants (24). We used a dot-blot assay based on polyclonal antibodies produced against a domain of X. fastidiosa's Hxfs to show that cells in XFM-pectin produced more Hxfs than in other media (Fig. 1F), indicating that expression of these adhesins are regulated by polysaccharides and associated with the bacterial-adhesion phenotypes observed. All together, our results show several phenotypic changes in X. fastidiosa based on media composition; pectin and, to a lesser extent glucan, induced phenotypic changes in X. fastidiosa.
Fig. 1.
Xylella fastidiosa phenotypic differences in four different media. (A) Bacterial growth curves in liquid media. (B) Populations of planktonic versus glass-attached cells grown in vitro; bars with the same letter are not different from each other within media treatments (t test, P < 0.05). (C) Biofilm formation at air/broth interface in different media (indicated by arrow). (D) EPS production quantified immunologically in four media (filled bars, unwashed cells; empty bars, washed cells), asterisks (P < 0.05 for one, P < 0.001 for two) indicate within-media differences; bars with the same letter are not different from each other within wash treatments. (E) Visual aspect of bacterial lawns on solid media; glossy phenotype likely associated with EPS production. (F) Immunological detection of Hxfs in 10-fold dilution series of cells grown in the four different media (a, PWG; b, XFM; c, XFM-glucan; d, XFM-pectin).
Host Polysaccharides Induce Changes in Gene Expression Profiles.
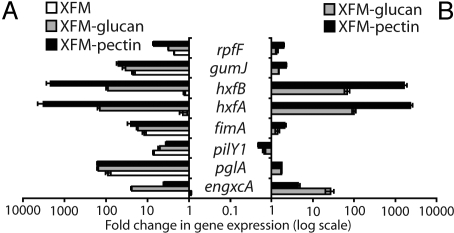
To better explore the phenotypic changes observed, we compared transcription levels of 2,036 X. fastidiosa genes through gene-expression microarrays. Gene-expression profiles were different among cells grown in the four media tested (Table S1), with XFM-pectin inducing a larger number of changes to gene transcription levels (Fig. S1). We divided the microarray data into six clusters of genes according to their expression in the different media (Table S2). A large number of genes (133) were differentially regulated only in the presence of pectin. Those up-regulated included type-I fimbriae (fimA), afimbrial adhesins (hxfs), EPS (gumJ), and phage-related genes, in addition to a considerable number of hypothetical proteins. On the other hand, down-regulated genes were more diverse in function. We used real-time quantitative PCR to estimate the level of gene expression of several genes previously shown to affect X. fastidiosa's pathogenicity and adhesion to surfaces (Fig. 2 and Table S3). All genes tested had higher transcription levels on XFM, XFM-glucan, and XFM-pectin when compared with the rich-medium PWG (see Fig. 2A). To analyze the role of polysaccharides on gene regulation, gene transcription rate on XFM-glucan and XFM-pectin in relation to XFM alone was compared (see Fig. 2B). The cell-cell signaling molecule synthase gene (rpfF) was up-regulated by pectin and glucan. This may explain results observed for some of the genes tested, as it would affect cell-cell signaling-dependent genes; exceptions are the afimbrial adhesins hxfA and hxfB that were up-regulated over three orders of magnitude, and the induction of engxcA (an endoglucanase) in the presence of its substrate (glucan). Accordingly, one gene associated with pathogen movement within plants (pilY1, a component of type-IV pili) was down-regulated. These results suggest that polysaccharides differentially mediate gene expression in X. fastidiosa by (i) affecting rpfF expression levels and, thus, cell−cell-signaling dependent genes; (ii) substrate-dependent induction of enzymes; and (iii) possibly directly inducing genes associated with pathogenicity (i.e., afimbrial adhesins). These results are consistent with our phenotypic observations (see Fig. 1).
Fig. 2.
Quantification of transcription level for genes implicated in X. fastidiosa pathogenicity and transmission. (A) Fold-changes in gene expression of XFM-based media in relation to medium PWG; value of 1 indicates no difference in relation to PWG. (B) Polysaccharide-induced changes in X. fastidiosa gene expression; value of 1 indicates no difference in relation to basal XFM medium.
Vector Transmission of Cells Grown in Pectin-Supplemented Medium.
Artificial diet systems are useful to study how insect-borne pathogens interact with their respective vectors. Through these systems, pathogen cells (or virus particles) are delivered to vectors under controlled conditions that allow for experimental manipulation in the absence of host plants and host plant-vector interactions. We tested whether cells grown in each of our four different media were transmissible by leafhopper vectors when an artificial diet system was used. Cells in PWG were not transmissible (Fig. 3). However, cells in the basal medium XFM, XFM-glucan, and XFM-pectin were transmissible by vectors, albeit with different efficiencies (see Fig. 3). It is notable that genes with high transcription levels (>1,000-fold) in the presence of pectin include hxfA and hxfB (see Fig. 2), which were directly implicated in the initial colonization of vectors' mouthparts (18).
Fig. 3.
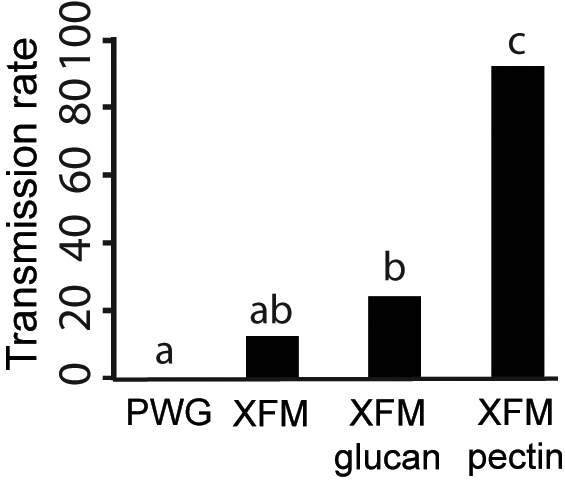
Pectin induction of X. fastidiosa vector transmission. Cells grown on different media were suspended in feeding medium and provided to 50 leafhoppers individually per treatment. Media with different letters on bars are statistically different (P < 0.05).
Pectin Is Not Directly Responsible for Gene Regulation.
Because pectin is a large molecule, we tested whether it was directly involved in the phenotype observed in our bioassays by using a polygalacturonase (pglA) mutant, which is the only X. fastidiosa gene expected to degrade pectin. We found that the phenotypic changes observed required pectin degradation, as the pglA mutant had the same phenotype on XFM-pectin as on XFM (Fig. S2). The pglA bacterial lawns grown on solid XFM-polysaccharides had a glossy appearance when compared to PWG (see Fig. S2C) and an immunological assay showed that the amount of EPS produced by the pglA mutant was similar in both the XFM-pectin medium and XFM (see Fig. S2D). These data show that pectin degradation into its subunits is required for the phenotypic changes observed.
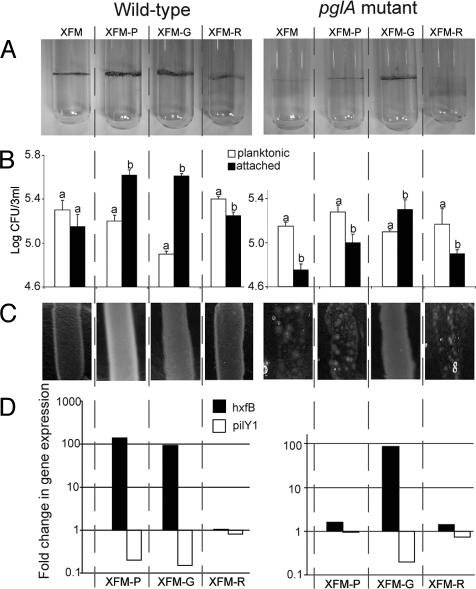
Pectin is primarily composed of galacturonic acid with rhamnose side chains; the ratio of these sugars is host-plant species-dependent (25, 26). We compared the phenotype and gene expression level of wild-type cells and a pglA mutant grown in XFM, and XFM-pectin, XFM-Na-galacturonate (in place of galacturonic acid), and XFM-rhamnose (Fig. 4). Biofilm formation on glass vials was more pronounced for wild-type cells in XFM-pectin and XFM-Na-galacturonate. A similar biofilm ring was observed in XFM-Na-galacturonate but not XFM-pectin for the pglA mutant, suggesting that pectin is indirectly mediating gene expression of adhesins (see Fig. 4A). These results were confirmed by comparing the ratio of planktonic cells to those adhered to glass under the same conditions (see Fig. 4B). As EPS production and glossy colonies were correlated to growth conditions (see Fig. 1), a comparison was made between bacterial lawns formed by cells grown in these different media. Again, glossy colonies were only observed for the pglA mutant grown in XFM supplemented with Na-galacturonate, indicating that pectin degradation is required for the phenotypic changes observed (see Fig. 4C). We also determined transcription levels of one gene up-regulated in the presence of pectin (hxfB) and another down regulated (pilY1). Expression patterns support the phenotype-based findings that Na-galacturonate, not pectin, was responsible for gene induction and subsequent phenotypic changes in X. fastidiosa (see Fig. 4D). Finally, the presence of pectin or Na-galacturonate in XFM induced the formation of small wild-type colonies on solid medium rather than a diffuse lawn; however, this was only observed for the pglA mutant when Na-galacturonate was added to XFM (Fig. S3). This phenotype may be a consequence of increased cell-cell adhesion in the presence of pectin, as it up-regulates the afimbrial adhesins Hxfs.
Fig. 4.
The main subunit of pectin is responsible for gene induction in X. fastidiosa. (A) Biofilm formation at air/broth interface for the X. fastidiosa wild-type and polygalacturonase (pglA) mutant in four media; XFM, and XFM supplemented with -P(ectin), -G(alacturonate-Na), and -R(hamnose). (B) Populations of planktonic versus glass-attached cells grown in four media; bars with the same letter are not different from each other within media treatments (t test, P < 0.05). (C) Visual aspect of bacterial lawns on solid media. (D) Quantification of hxfB and pilY1 transcription under the same conditions for the wild-type and mutant; value of 1 indicates transcription level equal to cells grown in the basal medium XFM.
Discussion
The transmission of X. fastidiosa was initially thought to be the consequence of simple insect-pathogen interactions because of the fact that a large group of insects (sharpshooter leafhoppers and spittlebugs) are vectors of this pathogen, apparently without any specificity (20). However, the finding that transmission is regulated by cell-cell signaling (17) indicated that X. fastidiosa colonization of vectors was not a trivial mechanical process. Our results show that two host structural polysaccharides, pectin and glucan, induced regulons that affect the phenotype of this pathogen. The role of pectin in up-regulating genes associated with cell adhesion to surfaces was of particular significance, as observed through in vitro phenotypic results and the fact that cells grown in XFM-pectin were transmissible by vectors more than in any other treatment tested here. Because pectin is a complex and large polysaccharide, we hypothesized that one of its building blocks was responsible for gene induction. We found that Na-galacturonate, not pectin itself or another one of its subunits, rhamnose, was in fact responsible for the molecular, biochemical, and biological results obtained.
Chatterjee et al. (15) proposed that X. fastidiosa has a dual lifestyle controlled by cell-cell signaling, particularly during plant colonization and initial attachment to insects. In low cell densities, X. fastidiosa moves within the xylem network of plants by up-regulating genes required for degradation of pit membranes and movement, such as type-IV pilus. At higher densities cells have adhesins induced, some of which have been associated with reduced movement within plants and insect colonization (18, 24, 27). Our quantitative PCR results showed that the signal synthase gene (rpfF) is up-regulated in the presence of pectin. Although cell density reached similar levels in all XFM media tested, induction of genes such as adhesins was only observed in XFM supplemented with polysaccharides. It is possible that alternative regulatory pathways independent of, or functioning in tandem with, the cell-cell signaling cascade exist in X. fastidiosa. Future work with knockout mutants and different media conditions are needed to explore this possibility. It is also possible that carbohydrate utilization pathways are linked to virulence in X. fastidiosa, as has been observed in Group A Streptococcus (28). Although mechanistically unclear, our results support the general hypothesis of Chatterjee et al. (15), but we provide evidence that environmental cues affect gene regulation of X. fastidiosa, apparently inducing an overdrive of the expected high cell density-derived phenotype.
The observed phenotypic transition from host colonization to dispersal-prone or environment-resistant cells has been observed in other pathogens, such as V. cholerae and Citrobacter rodentium, although in different contexts. A hyperinfectious C. rodentium state is observed after this enteric mouse pathogen leaves the host, but the phenotype is transient and lost after culturing on rich medium (29). Phenotypic transitions in V. cholerae are better explored and involve transitions that occur in response to cues from a new host (chitin regulon) or within the same host (early and late infections in humans) (8, 10, 11, 30, 31). In the case of X. fastidiosa, changes to expression that permit vector acquisition occurred within the host plant the pathogen already colonized and not as a result of its encounter with an insect vector. Because of the differences between the plant and insect environments, and observed differences in colonization patterns in these habitats (16, 17, 32), it is possible that X. fastidiosa also responds to cues in the vector. Those cues may induce a regulon required for the colonization of the foregut's chitinous surface, similarly to the chitin regulon in V. cholerae.
Induction of the dispersal state in X. fastidiosa is controlled by cell-cell signaling (17), but it is also regulated by the presence of host structural carbohydrates. In this context, cells prepare for dispersal once they reach high densities within a vessel, which only occurs after other vessels in the xylem network have already been colonized (32). Because X. fastidiosa colonization of plants is heterogeneous (32), some vessels may harbor cells in a nontransmissible state, while others that are fully colonized have transitioned into a vector-transmissible phenotype. This dichotomy might be a mechanism used to optimize dispersal opportunities and increase fitness within plants, as both occur in the presence of host structural carbohydrates. At low cell densities the probability of being acquired by vectors is likely lower than at high cell densities, and within-plant movement would increase the probability of eventually being acquired by vectors; at high cell densities colonies confined to vessels may eventually die and not result in new vessel infections (15). Therefore, X. fastidiosa cells capable of colonizing vectors might originate primarily from microcolonies permanently restricted to individual xylem vessels and no longer involved in plant colonization.
It is possible that phenotypic transitions associated with dispersal or increased fitness in new environments, regulated in a host-factor and cell density-dependent fashion, are common for bacterial pathogens that explore multiple environments. Investigation of such potential state switches may yield valuable information on the biology of pathogens with a complex life history. Similar processes, but in a different context, may also be relevant to other plant pathogens, such as Xanthomonas spp., which have a distinct biology such as vector-independent dispersal, but have a regulatory system dependent on cell-cell signaling homologous to X. fastidiosa.
Materials and Methods
Bacterial Strains and Growth Conditions.
We used the fully sequenced X. fastidiosa grape strain Temecula (33) and site-specific knockout mutant of pglA generated by Roper et al. (21). Strains were grown in vitro at 28 °C for 7 days on modified PWG solid medium (22) with or without kanamycin at 5 μg/mL. Cells were suspended in buffer and concentrations were adjusted to OD600 0.05; then 20-μL drops of cell suspensions were striped onto four different solid-media plates. The media were PWG and a defined medium XFM (described below) in the absence or presence of 0.01% pectin (P8471 Sigma-Aldrich) or β-glucan (G6513 Sigma-Aldrich). For other experiments, we used liquid media and did not incorporate the gelling agent into medium. Solid and liquid (at 200 rpm) media cultures were incubated up to 10 days at 28 °C.
XFM Medium.
We modified the medium XFD2 (23) to generate a unique medium, XFM. We autoclaved: 800 mL of deionized water, 1.5 g of K2HPO4·3H2O, 1 g of KH2PO4, 1 g of MgSO4·7H2O, 1.5 g of disodium succinate, 1.5 g of trisodium citrate, 10 mL of hemin chloride (0.05% in NaOH 0.5 μM) and 10 g of GelRite. Afterward, the following filter-sterilized components were incorporated, diluted in 100 mL of water: l-asparagine (1 g), l-cysteine (0.5 g), l-glutamine (3 g), and bovine serum albumin (3 g). If added into media, 0.1 g of pectin was slowly added to 100 mL of water while stirring rapidly at 75 °C (≈30 min) on a heat-stir plate, then gravity-filtered through Whatman #1 paper three times. When cold, the suspension was filtered to 0.22 μm (up to a couple of hours under vacuum). Pectin was added to a final concentration of 0.01% (wt/vol) of the medium; for XFM-glucan, 0.1 g of glucan was added to 100 mL of water while stirring at 50 °C, then filtered through 0.22 μm and added to the medium. For XFM without polysaccharides, 100 mL of sterilized water was added to complete 1 L of medium. For the liquid media, GelRite was excluded. All media components were purchased from Sigma-Aldrich.
Growth Curve and Adhesion Assays.
Cells of X. fastidiosa were incubated in liquid media at 28 °C with agitation at 200 rpm for up to 10 days. To quantify cell growth, daily samples were taken from five independent replicates for each medium and measured cultures optical density at 600 nm. We performed ANOVA, with media treatment and day as factors, to compare growth curves among the four media across 10 consecutive days. A further post hoc test (Tukey) was performed to conduct pairwise comparisons of media types. Log-transformation of ODs provided the closest fit to the normality assumption. For the attachment assays, the formed ring of cells adhered to the glass surface at the air–medium interface for 10-day-old cultures was stained with 0.1% (wt/vol) crystal violet. We followed Chatterjee et al. (19) to determine the total number of planktonic and attached cells in each tissue-culture glass tube. To compare the number of planktonic and attached cells, paired t tests were used.
Immunoquantification of EPS and Hfxs-Like Proteins.
We quantified EPS by using a protein-A double-antibody sandwich ELISA as described in ref. 23, with minor modifications. For Hxfs, a dot-blot immunodetection assay was carried out with polyclonal antibodies produced against those proteins with a shared putative adhesion domain (provided by B. Kirkpatrick, University of California, Davis). We used two-way ANOVA followed by multiple comparisons of means (Tukey test, ∞ = 0.05) to analyze the results obtained on EPS quantification. For details, see SI Materials and Methods.
Transmission Tests with Artificial Diet System.
X. fastidiosa cells were grown for 7 days on PWG, XFM, XFM-pectin, and XFM-glucan, after which cells were suspended in a diet solution (L-glutamine, 0.7 mM; l-asparagine, 0.1 mM; sodium citrate, 1 mM; pH 6.4). The final cell suspensions were adjusted to 108 CFU/mL (OD600 0.25). Each replicate consisted of 50-μL cell suspensions loaded on a layer of stretched Parafilm on top of a 1-cm diameter plastic vial opened at both ends, with the drop being later covered by another layer of Parafilm. One insect was inserted in the tube from the remaining open side, which was then sealed with a cork (Fig. S4). We replicated the test 50 times for each medium. After a 6-h X. fastidiosa acquisition access period at 20 °C, insects were transferred to grape plants for a 4-day inoculation access period. Three months later, X. fastidiosa was cultured from plants following standard protocols (22). The blue-green sharpshooter, Graphocephala atropunctata (Signoret) (Hemiptera: Cicadellidae) was used for these tests. For details, see SI Materials and Methods. A two by four contingency table was used to analyze data obtained, followed by pairwise comparisons using Fisher's exact test (∞ = 0.05) with Bonferroni's correction to account for multiple comparisons.
Microarray Experiments.
Total RNA was isolated and purified by using TRIzol Plus RNA Purification Kit (Invitrogen). Synthesis and labeling of the cDNA and microarray hybridization were performed as described by the arrays manufacturer (NimbleGen-Roche). Four-plex arrays with 192,000 features covering 2,036 genes, 18 probes of 60mer per gene, and 5 replicates each probe were used in this study. Experiments with different media were repeated four times. Microarrays were scanned with a GenePix 4000B Scanner (Axon Instruments) using the associated software. Data were extracted by using Roche NimbleGen NimbleScan. ArrayStar software from DNAStar was used to analyze the data and to produce the hierarchical clustering. Data are available at National Center for Biotechnology Information's Gene Expression Omnibus under GEO series accession no. GSE18369.
Quantitative Real-Time RT-PCR.
Reactions were conducted with SYBER Green mix and 7500 Fast Real-Time PCR System (Applied Biosystems). For details, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Sandy Purcell, Steve Lindow, Subhadeep Chatterjee, and our laboratory colleagues for helpful suggestions and discussion. We thank Bruce Kirkpatrick for the Hxf and Gum antibodies and polygalacturonase mutant, and Matt Daugherty and Arash Rashed for their assistance with statistical analyses. This work was supported with funding from the California Department of Food and Agriculture.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE18369).
This article contains supporting information online at www.pnas.org/cgi/content/full/0908562106/DCSupplemental.
References
- 1.Shriram A, Ramaiah K, Krishnamoorthy K, Sehgal S. Diurnal pattern of human-biting activity and transmission of subperiodic Wuchereria bancrofti (Filariidea: Dipetalonematidae) by Ochlerotatus niveus (Diptera: Culicidae) on the Andaman and Nicobar Islands of India. Am J Trop Med Hyg. 2005;72:273–277. [PubMed] [Google Scholar]
- 2.Blua MJ, Perring TM. Alatae production and population increase of aphid vectors on virus-infected host plants. Oecologia. 1992;92:65–70. doi: 10.1007/BF00317263. [DOI] [PubMed] [Google Scholar]
- 3.Grant MR, Jones JDG. Hormone (dis)harmony moulds plant health and disease. Science. 2009;324:750–752. doi: 10.1126/science.1173771. [DOI] [PubMed] [Google Scholar]
- 4.Power AG. Competition between viruses in a complex plant-pathogen. Ecology. 1996;77:1004–1010. [Google Scholar]
- 5.Brisson D, Dykhuizen DE, Ostfeld RS. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc R Soc London Ser B. 2008;275:227–235. doi: 10.1098/rspb.2007.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopes JRS, Daugherty MP, Almeida RPP. Context-dependent transmission of a generalist plant pathogen: host species and pathogen strain mediate insect vector competence. Entomol Exp Appl. 2009;131:216–224. [Google Scholar]
- 7.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schild S, et al. Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe. 2007;2:264–277. doi: 10.1016/j.chom.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson EJ, et al. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009;7:693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meibom KL, et al. The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci USA. 2004;101:2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruzzo C, Vezzulli L, Colwell RR. Global impact of Vibrio cholerae interactions with chitin. Environ Microbiol. 2008;10:1400–1410. doi: 10.1111/j.1462-2920.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- 13.Brito B, et al. A signal transfer system through three compartments transduces the plant cell contact-dependent signal controlling Ralstonia solanacearum hrp genes. Mol Plant-Microbe Interact. 2002;15:109–119. doi: 10.1094/MPMI.2002.15.2.109. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins DL, Purcell AH. Xylella fastidiosa: Cause of Pierce's disease of grapevine and other emergent diseases. Plant Disease. 2002;86:1056–1066. doi: 10.1094/PDIS.2002.86.10.1056. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S, Almeida RPP, Lindow S. Living in two worlds: The plant and insect lifestyles of Xylella fastidiosa. Annu Rev Phytopathol. 2008;46:243–271. doi: 10.1146/annurev.phyto.45.062806.094342. [DOI] [PubMed] [Google Scholar]
- 16.Almeida RPP, Purcell AH. Patterns of Xylella fastidiosa colonization on the precibarium of sharpshooter vectors relative to transmission to plants. Ann Entomol Soc Am. 2006;99:884–890. [Google Scholar]
- 17.Newman KL, Almeida RPP, Purcell AH, Lindow SE. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc Natl Acad Sci USA. 2004;101:1737–1742. doi: 10.1073/pnas.0308399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killiny N, Almeida RPP. Xylella fastidiosa afimbrial adhesins mediate cell transmission to plants by leafhopper vectors. Appl Environ Microbiol. 2009;75:521–528. doi: 10.1128/AEM.01921-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee S, Wistrom C, Lindow SE. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc Natl Acad Sci USA. 2008;105:2670–2675. doi: 10.1073/pnas.0712236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida RPP, Blua MJ, Lopes JRS, Purcell AH. Vector transmission of Xylella fastidiosa: Applying fundamental knowledge to generate disease management strategies. Ann Entomol Soc Am. 2005;98:775–786. [Google Scholar]
- 21.Roper MC, et al. Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Mol Plant-Microbe Interact. 2007;20:411–419. doi: 10.1094/MPMI-20-4-0411. [DOI] [PubMed] [Google Scholar]
- 22.Hill BL, Purcell AH. Multiplication and movement of Xylella fastidiosa within grapevine and four other plants. Phytopathology. 1995;85:1368–1372. [Google Scholar]
- 23.Roper MC, Greve LC, Labavitch JA, Kirkpatrick BC. Detection and visualization of an exopolysaccharide produced by Xylella fastidiosa in vitro and in planta. Appl Environ Microbiol. 2007;73:7252–7258. doi: 10.1128/AEM.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilhabert MR, Kirkpatrick BC. Identification of Xylella fastidiosa antivirulence genes: Hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Mol Plant-Microbe Interact. 2005;18:856–868. doi: 10.1094/MPMI-18-0856. [DOI] [PubMed] [Google Scholar]
- 25.Cho SW, Lee S, Shin W. The X-ray structure of Aspergillus aculeatus polygalacturonase and a modeled structure of the polygalacturonase-octagalacturonate complex. J Mol Biol. 2001;311:863–878. doi: 10.1006/jmbi.2001.4919. [DOI] [PubMed] [Google Scholar]
- 26.Coutinho PM, Henrissat B. Carbohydrate-active enzymes: an integrated database approach. In: Gilbert HJ, Davies GJ, Henrissat B, Svensson B, editors. Recent Advances in Carbohydrate Engineering. Cambridge, UK: Royal Soc Chemistry; 1999. pp. 3–12. [Google Scholar]
- 27.Meng Y, et al. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J Bacteriol. 2005;187:5560–5567. doi: 10.1128/JB.187.16.5560-5567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shelburne SA, et al. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci USA. 2008;105:1698–1703. doi: 10.1073/pnas.0711767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiles S, Dougan G, Frankel G. Emergence of a ‘hyperinfectious’ bacterial state after passage of Citrobacter rodentium through the host gastrointestinal tract. Cell Microbiol. 2005;7:1163–1172. doi: 10.1111/j.1462-5822.2005.00544.x. [DOI] [PubMed] [Google Scholar]
- 30.Merrell DS, et al. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417:642–645. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson EJ, et al. Complexity of rice-water stool from patients with Vibrio cholerae plays a role in the transmission of infectious diarrhea. Proc Natl Acad Sci USA. 2007;104:19091–19096. doi: 10.1073/pnas.0706352104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman KL, Almeida RPP, Purcell AH, Lindow SE. Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Appl Environ Microbiol. 2003;69:7319–7327. doi: 10.1128/AEM.69.12.7319-7327.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Sluys MA, et al. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J Bacteriol. 2003;185:1018–1026. doi: 10.1128/JB.185.3.1018-1026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.