Abstract
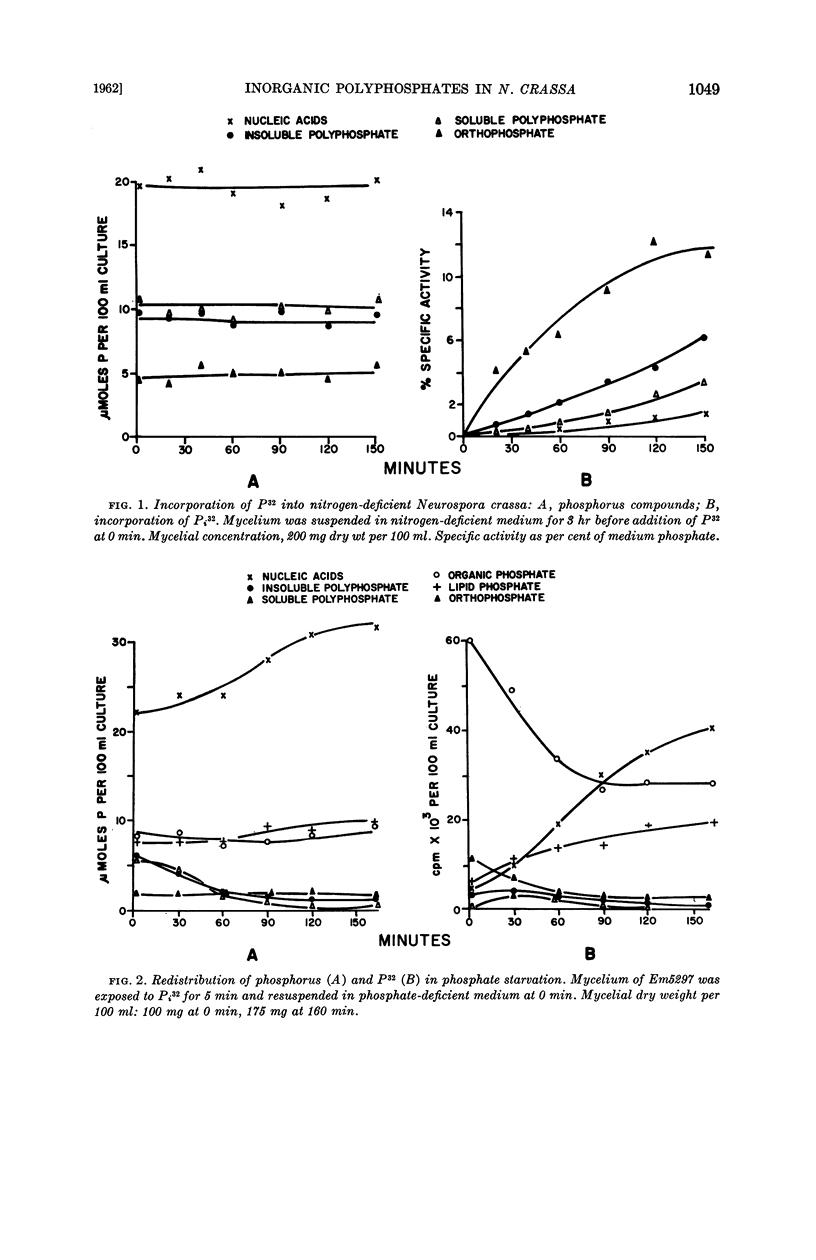
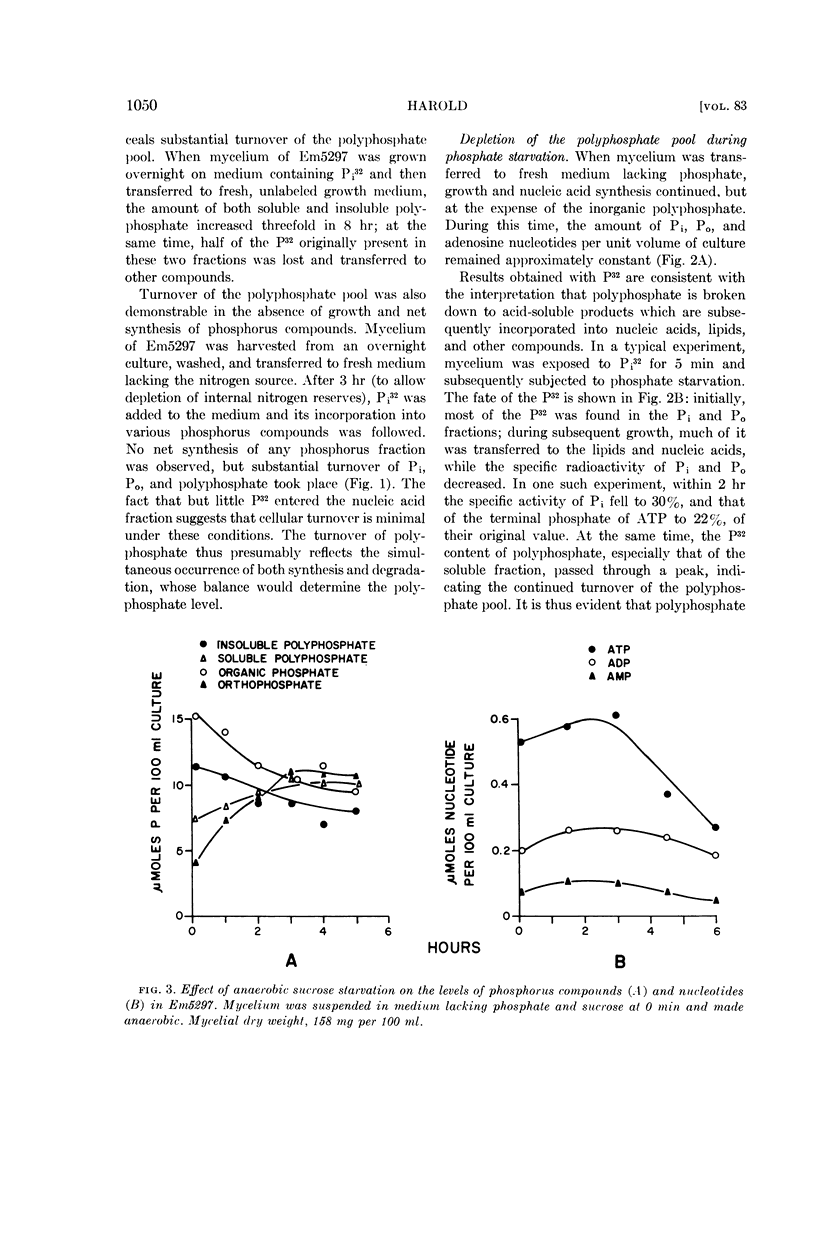
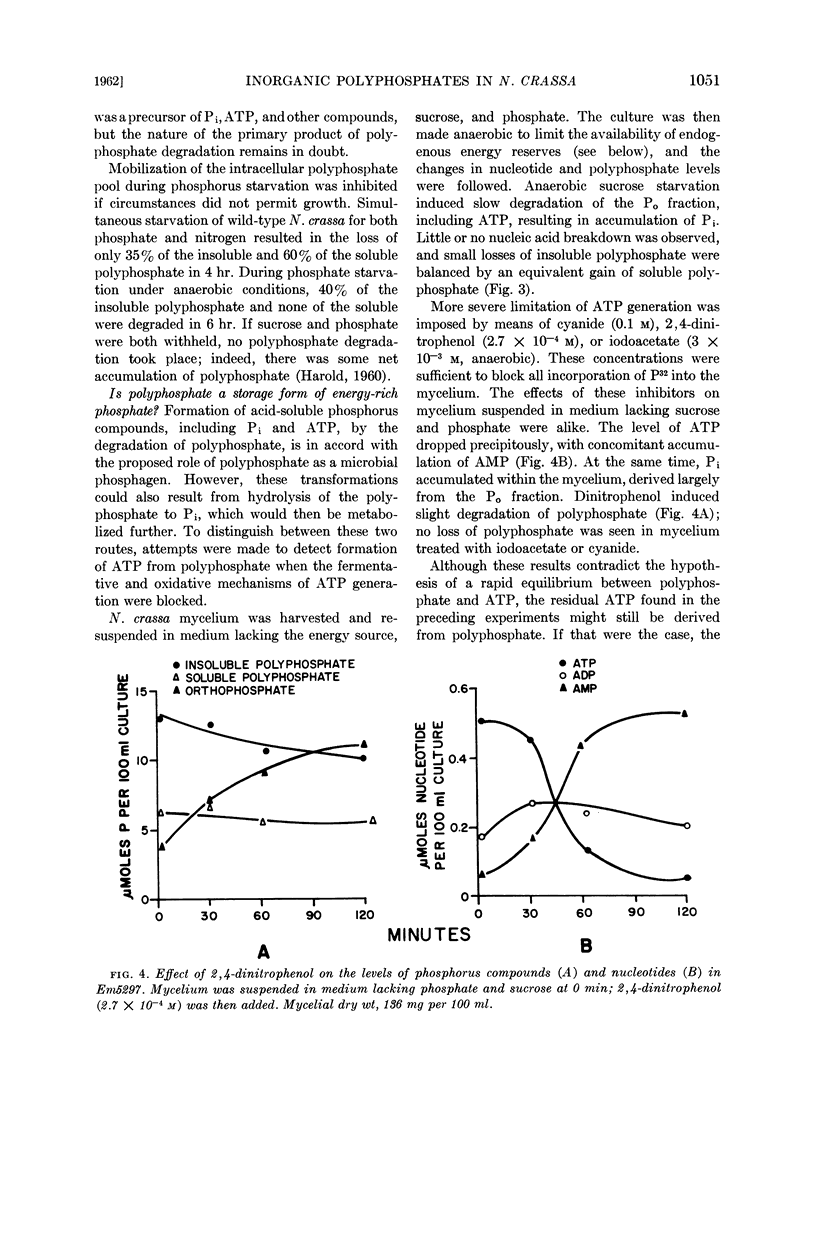
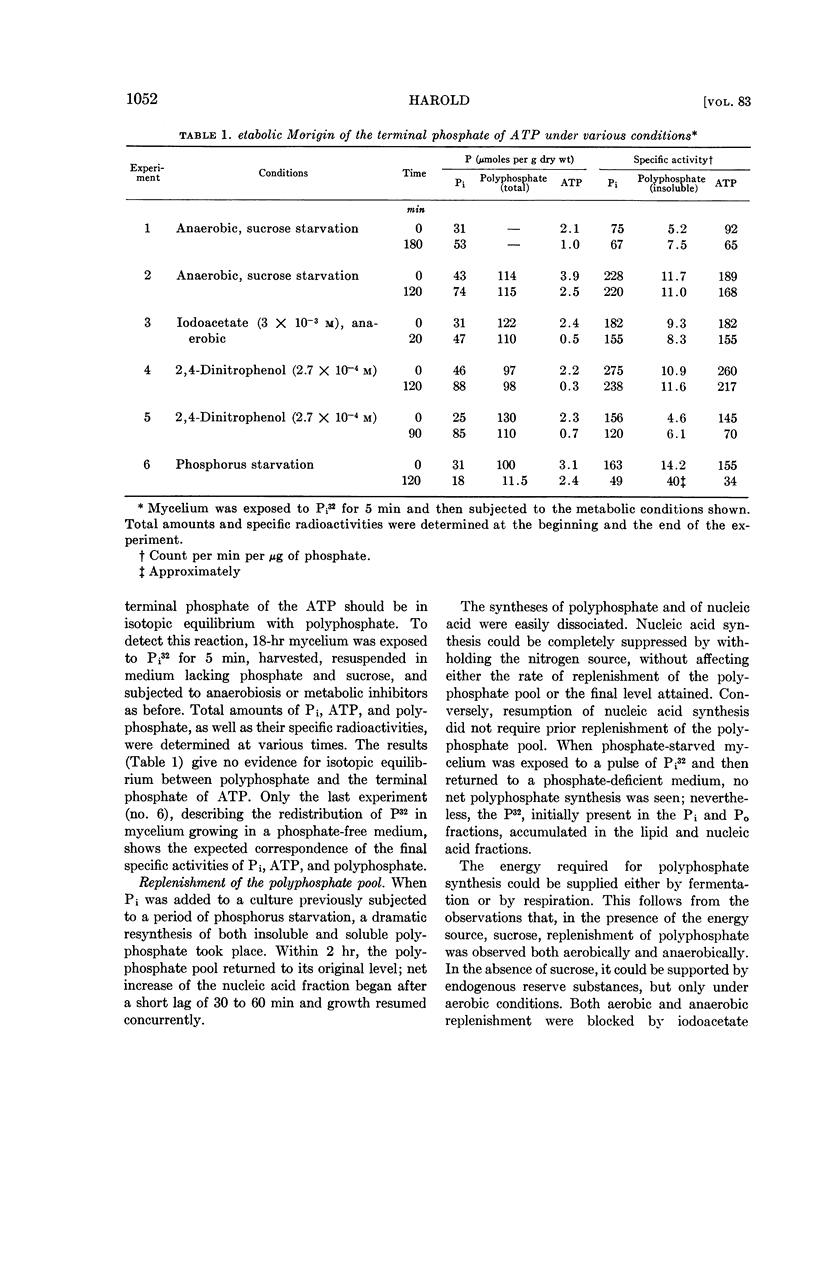
Harold, F. M. (National Jewish Hospital, Denver, Colo.). Depletion and replenishment of the inorganic polyphosphate pool in Neurospora crassa. J. Bacteriol. 83:1047–1057. 1962.—Turnover of the inorganic polyphosphate pool of Neurospora crassa was demonstrated in both growing and nongrowing mycelium. In nitrogen-deficient cultures, polyphosphate synthesis and degradation were in balance and no net changes occurred. When mycelium was suspended in a growth medium deficient in phosphate, polyphosphate was degraded to acid-soluble compounds, including adenosine triphosphate (ATP) and orthophosphate; net synthesis of nucleic acids and phospholipids occurred at the expense of polyphosphate. Various attempts were made to demonstrate direct formation of ATP from polyphosphate when oxidative and fermentative ATP generation were blocked. No evidence for this reaction could be obtained, suggesting that the primary product of polyphosphate degradation is not ATP but probably orthophosphate.
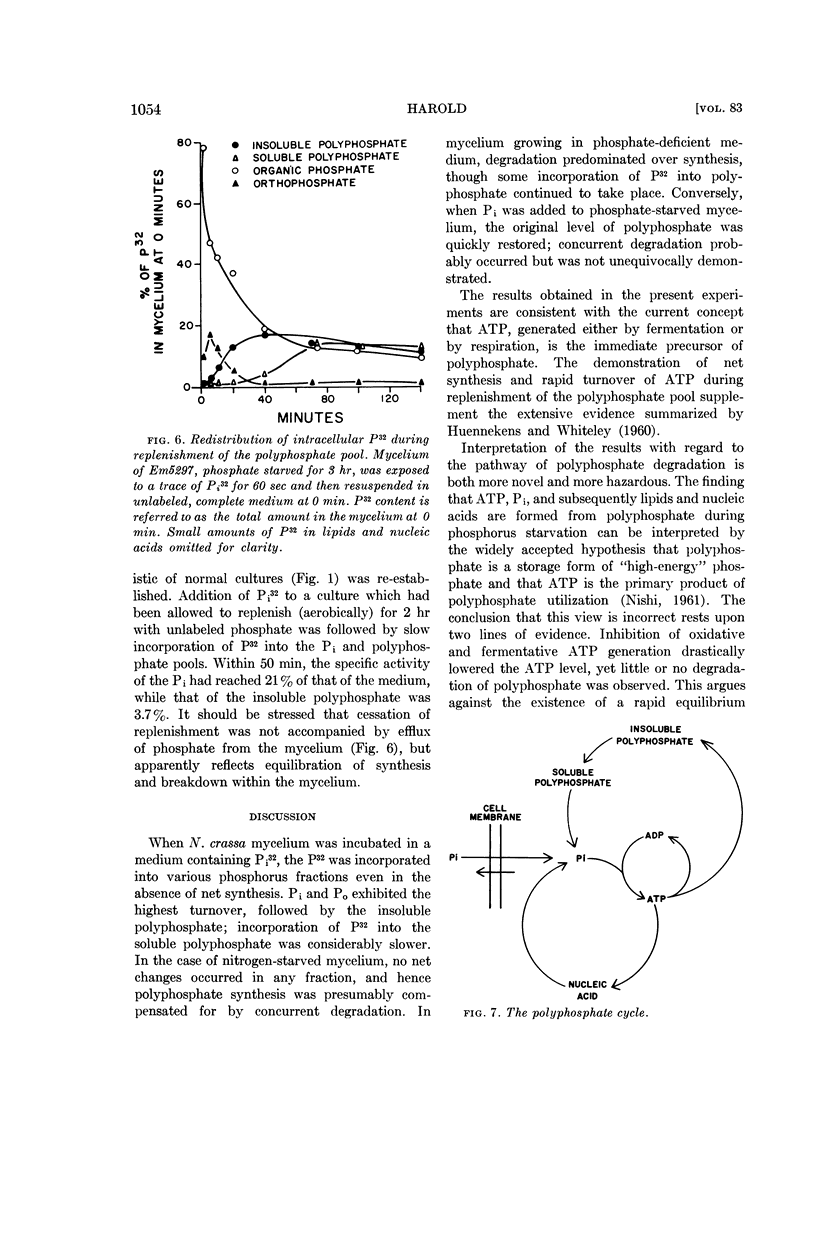
Addition of phosphate to phosphate-starved mycelium induced rapid replenishment of the polyphosphate pool; polyphosphate accumulation ceased abruptly when the original level had been restored. The finding that ATP accumulation within the mycelium preceded polyphosphate synthesis, together with the rapid turnover of this compound, supports the view that ATP is the metabolic precursor of polyphosphate.
The results suggest a cyclic pattern of polyphosphate metabolism. In N. crassa at least, polyphosphate does not appear to function as a reservoir of “high-energy” phosphate, but the polyphosphate cycle may be involved in the dissipation of excess ATP.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRENBERG M. [Phosphorus metabolism by Saccharomyces cerevisiae in relation to intracellular and extracellular phosphate concentration]. Arch Mikrobiol. 1961;40:126–152. [PubMed] [Google Scholar]
- HAROLD F. M. Accumulation of inorganic polyphosphate in mutants of Neurospora crassa. Biochim Biophys Acta. 1960 Dec 4;45:172–188. doi: 10.1016/0006-3002(60)91438-4. [DOI] [PubMed] [Google Scholar]
- KATCHMAN B. J., FETTY W. O. Phosphorus metabolism in growing cultures of Saccharomyces cerevisiae. J Bacteriol. 1955 Jun;69(6):607–615. doi: 10.1128/jb.69.6.607-615.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG A., KORNBERG S. R., SIMMS E. S. Metaphosphate synthesis by an enzyme from Escherichia coli. Biochim Biophys Acta. 1956 Apr;20(1):215–227. doi: 10.1016/0006-3002(56)90280-3. [DOI] [PubMed] [Google Scholar]
- KORNBERG S. R. Adenosine triphosphate synthesis from polyphosphate by an enzyme from Escherichia coli. Biochim Biophys Acta. 1957 Nov;26(2):294–300. doi: 10.1016/0006-3002(57)90008-2. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- LANGEN P., LISS E. Uber Bildung und Umsatz der Polyphosphate der Hefe. Biochem Z. 1958;330(6):455–466. [PubMed] [Google Scholar]
- LEE K. H., EILER J. J. Temperature dependent characteristics of an adenylpyrophosphatase preparation from potatoes. Science. 1951 Oct 12;114(2963):393–395. doi: 10.1126/science.114.2963.393-a. [DOI] [PubMed] [Google Scholar]
- MATTENHEIMER H. Die Substratspezifität anorganischer Poly- und Metaphosphatasen. III. Papierchromatographische Untersuchungen beim enzymatischen Abbau von anorganischen Poly- und Metaphosphaten. Hoppe Seylers Z Physiol Chem. 1956 Mar 17;303(3-6):125–139. [PubMed] [Google Scholar]
- NISHI A. Role of polyphosphate and phospholipid in germinating spores of Aspergillus niger. J Bacteriol. 1961 Jan;81:10–19. doi: 10.1128/jb.81.1.10-19.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER V. H. The effect of 3:5-dinitroortho-cresol on phosphocreatine and the adenosine phosphate compounds of rat tissues. Biochem J. 1954 Jul;57(3):381–386. doi: 10.1042/bj0570381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH I. W., WILKINSON J. F., DUGUID J. P. Volutin production in Aerobacter aerogenes due to nutrient imbalance. J Bacteriol. 1954 Oct;68(4):450–463. doi: 10.1128/jb.68.4.450-463.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORN W., PFLEIDERER G., FROWEIN R. A., ROSS I. Stoffwechselvorgänge im Gehirn bei akuter Anoxie, akuter Ischämie und in der Erholung. Pflugers Arch. 1955;261(4):334–360. doi: 10.1007/BF00364124. [DOI] [PubMed] [Google Scholar]
- THRELFALL C. J. An analytical procedure for the acid-soluble phosphorus compounds in rat-skeletal muscle. Biochem J. 1957 Apr;65(4):694–699. doi: 10.1042/bj0650694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUBOI K. K., PRICE T. D. Isolation, detection and measure of microgram quantities of labeled tissue nucleotides. Arch Biochem Biophys. 1959 Mar;81(1):223–237. doi: 10.1016/0003-9861(59)90192-4. [DOI] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R., BARTLEY W., WEBER G. The kinetics of the exchange of the phosphate groups of adenosine triphosphate during oxidative phosphorylation. Biochem J. 1955 Apr;59(4):590–599. doi: 10.1042/bj0590590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDRA A. Metachromatic granules of microorganisms. J Bacteriol. 1959 Nov;78:664–670. doi: 10.1128/jb.78.5.664-670.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDER F., DENNENY J. M. Utilization of metaphosphate for phosphorylation by cell-free extracts of Mycobacterium smegmatis. Nature. 1955 Apr 9;175(4458):636–636. doi: 10.1038/175636a0. [DOI] [PubMed] [Google Scholar]


