Abstract
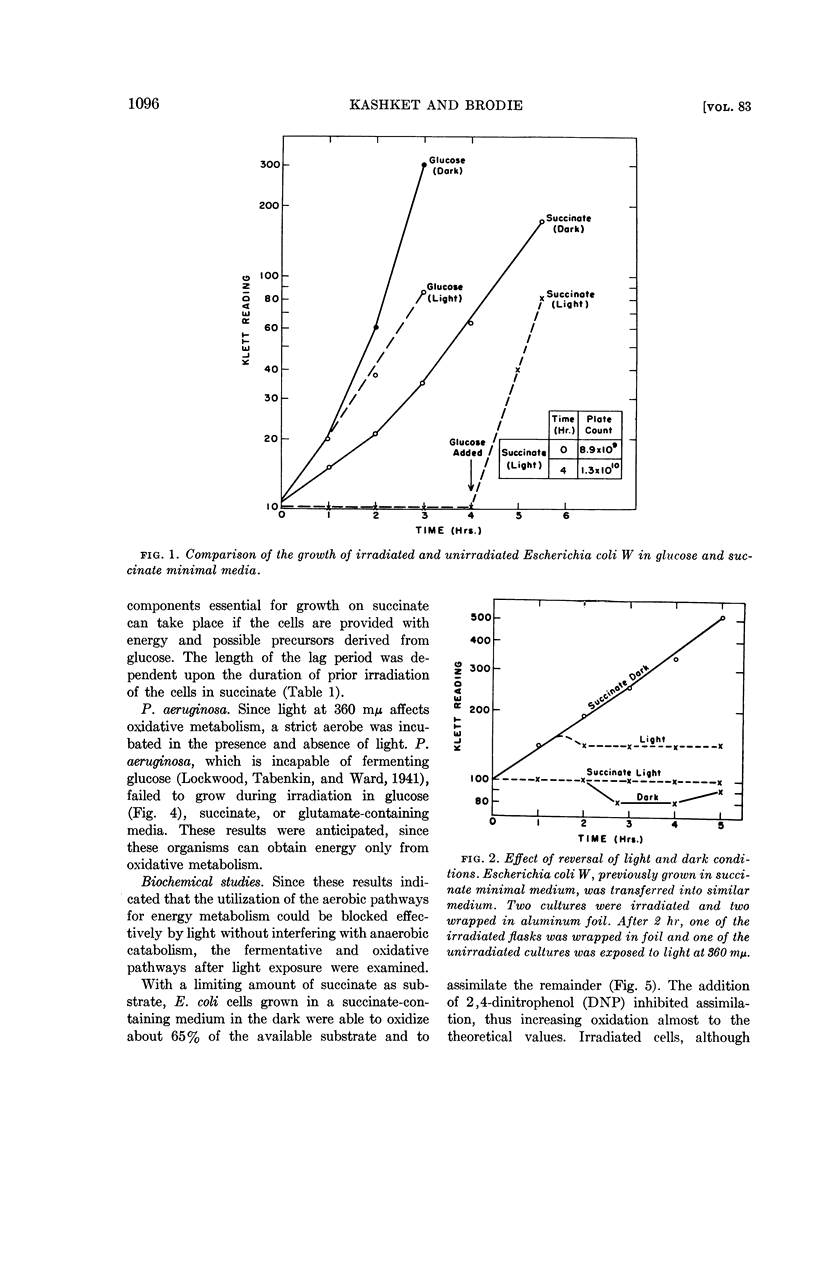
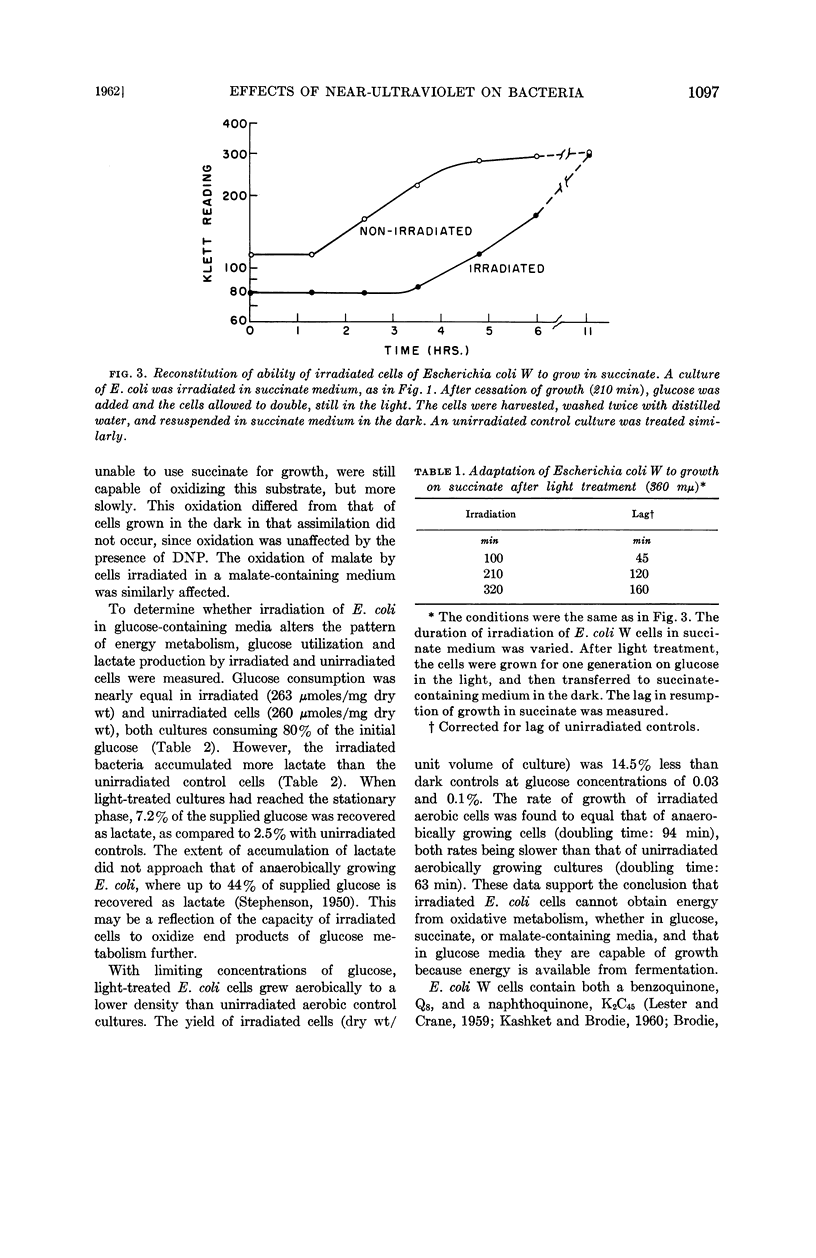
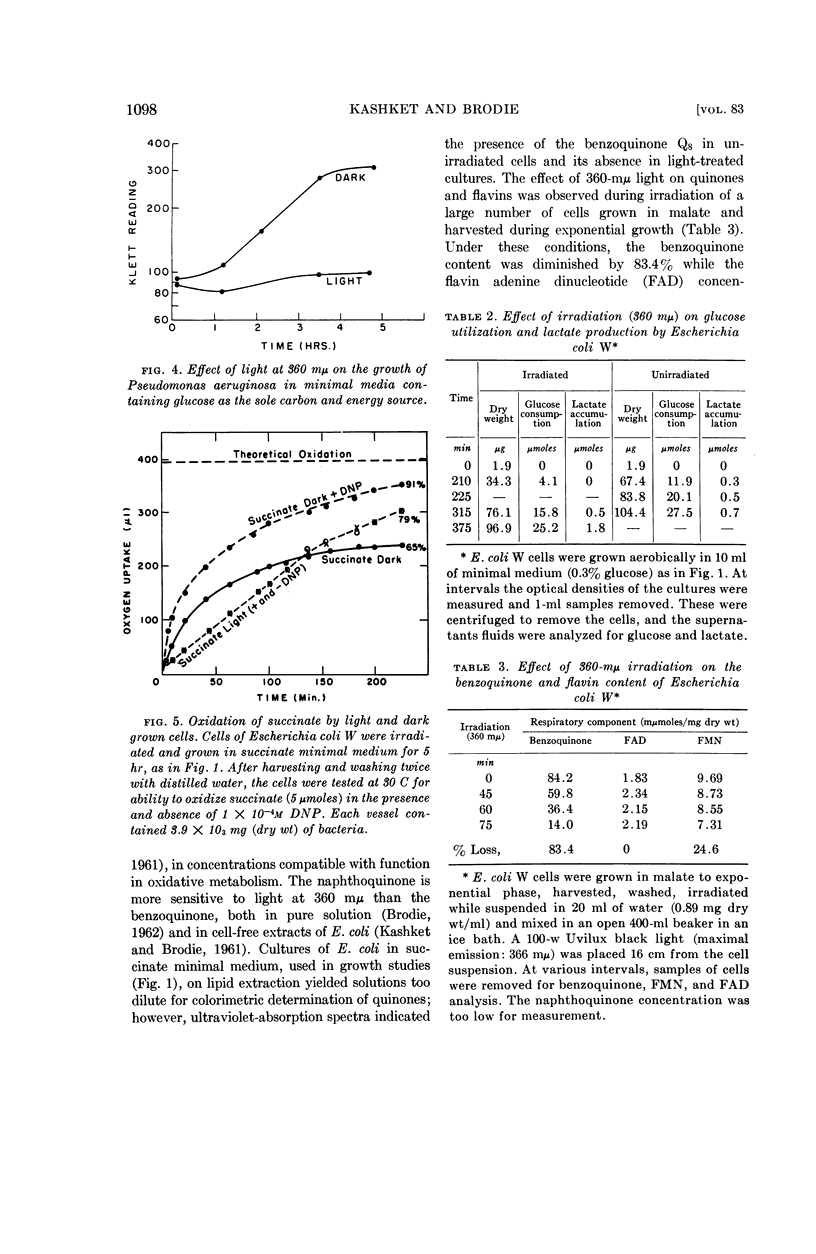
Kashket, E. R. (Harvard Medical School, Boston, Mass.) and A. F. Brodie. Effects of near-ultraviolet irradiation on growth and oxidative metabolism of bacteria. J. Bacteriol. 83:1094–1100. 1962.—The effects of irradiation with near-ultraviolet light (360 mμ) have been studied with Escherichia coli W and a strain of Pseudomonas aeruginosa. The growth of the aerobe P. aeruginosa was inhibited by light on minimal salts media containing succinate, glutamate, or glucose as sole carbon sources. The facultative anaerobe E. coli was capable of growth under irradiation on a fermentable carbon source, such as glucose, but with a smaller yield of cells on limiting substrate, as compared to unirradiated control cultures. The rate of growth of aerobic irradiated cells on glucose was equal to that of anaerobic growth on that carbon source, and there was a greater accumulation of end products of glucose catabolism aerobically in the light as compared to dark controls. When irradiated in media containing carbon sources from which energy was obtainable only by oxidative phosphorylation, such as succinate or malate, E. coli cells were still capable of oxidizing these substrates but could not grow on them. This bacteriostatic effect of 360-mμ light could be reversed by the addition of glucose, which resulted in the growth of irradiated cells. Visible (400 to 600 mμ) light was found to have no effect. Irradiated E. coli cells in succinate were found to contain no naphtho- or benzoquinones, compounds which are more sensitive to 360-mμ irradiation than other components of the respiratory chain. It is suggested that the effect of 360-mμ light on whole cells is the destruction of light-sensitive components, such as the benzoquinone Q8 and naphthoquinone K2C45 of E. coli W, which are essential for obtaining energy from oxidative metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE A. F., BALLANTINE J. Oxidative phosphorylation in fractionated bacterial systems. II. The role of vitamin K. J Biol Chem. 1960 Jan;235:226–231. [PubMed] [Google Scholar]
- BRODIE A. F. Vitamin K and other quinones as coenzymes in oxidative phosphorylation in bacterial systems. Fed Proc. 1961 Dec;20:995–1004. [PubMed] [Google Scholar]
- BRODIE A. F., WEBER M. M., GRAY C. T. The role of vitamin K1 in coupled oxidative phosphorylation. Biochim Biophys Acta. 1957 Aug;25(2):448–449. doi: 10.1016/0006-3002(57)90510-3. [DOI] [PubMed] [Google Scholar]
- Hollaender A. Effect of long ultraviolet and short visible radiation (3500 to 4900A) on escherichia coli. J Bacteriol. 1943 Dec;46(6):531–541. doi: 10.1128/jb.46.6.531-541.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROGMANN D. W. A requirement for plastoquinone in photosynthetic phosphorylation. Biochem Biophys Res Commun. 1961 Mar 24;4:275–277. doi: 10.1016/0006-291x(61)90233-9. [DOI] [PubMed] [Google Scholar]
- Kelner A. PHOTOREACTIVATION OF ULTRAVIOLET-IRRADIATED ESCHERICHIA COLI, WITH SPECIAL REFERENCE TO THE DOSE-REDUCTION PRINCIPLE AND TO ULTRAVIOLET-INDUCED MUTATION. J Bacteriol. 1949 Oct;58(4):511–522. doi: 10.1128/jb.58.4.511-522.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- Lockwood L. B., Tabenkin B., Ward G. E. The Production of Gluconic Acid and 2-Keto-Gluconic Acid from Glucose by Species of Pseudomonas and Phytomonas. J Bacteriol. 1941 Jul;42(1):51–61. doi: 10.1128/jb.42.1.51-61.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- NEWCOMBE H. B., WHITEHEAD H. A. Photoreversal of ultraviolet-induced mutagenic and lethal effects in Escherichia coli. J Bacteriol. 1951 Mar;61(3):243–251. doi: 10.1128/jb.61.3.243-251.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE A. C., Jr, GALE P., WALLICK H., WALTON R. B., McDANIEL L. E., WOODRUFF H. B., FOLKERS K. Coenzyme Q. 17. Isolation of coenzyme Q10 from bacterial fermentation. Arch Biochem Biophys. 1960 Aug;89:318–321. doi: 10.1016/0003-9861(60)90062-x. [DOI] [PubMed] [Google Scholar]
- PUMPHREY A. M., REDFEARN E. R. A method for determining the concentration of ubiquinone in mitochondrial preparations. Biochem J. 1960 Jul;76:61–64. doi: 10.1042/bj0760061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER M. M., BRODIE A. F., MERSELIS J. E. Possible role for vitamin K in electron transport. Science. 1958 Oct 17;128(3329):896–898. doi: 10.1126/science.128.3329.896-a. [DOI] [PubMed] [Google Scholar]


