Abstract
Ligand activation of peroxisome proliferator–activated receptor (PPAR)-β/δ and inhibition of cyclooxygenase-2 (COX-2) activity by nonsteroidal anti-inflammatory drugs can attenuate skin tumorigenesis. There is also evidence that attenuation of skin tumorigenesis by inhibition of COX-2 activity occurs through PPARβ/δ-independent mechanisms. The present study examined the hypothesis that combining ligand activation of PPARβ/δ with inhibition of COX-2 activity will cooperatively inhibit chemically induced skin tumor progression using both in vivo and ex vivo models. A two-stage chemical carcinogenesis bioassay was performed in wild-type and Pparβ/δ-null mice. After 22 weeks, cohorts of both mouse lines were divided into four experimental groups: (1) control, (2) topical application of the PPARβ/δ ligand GW0742, (3) dietary administration of the COX-2 inhibitor nimesulide, or (4) both GW0742 and nimesulide. Ligand activation of PPARβ/δ did not influence skin tumor progression, while a modest decrease in skin tumor multiplicity was observed with dietary nimesulide. Interestingly, the combined treatment of GW0742 and nimesulide increased the efficacy of the decrease in papilloma multiplicity for 6 weeks in wild-type mice, but this effect was not found at later time points and was not found in similarly treated Pparβ/δ-null mice. Neoplastic keratinocyte lines cultured with GW0742 and nimesulide also exhibited enhanced inhibition of cell proliferation coincident with increased expression of Keratin messenger RNAs. Results from these studies support the hypothesis that combining ligand activation of PPARβ/δ with inhibition of COX-2 activity can inhibit chemically induced skin tumor progression by modulating differentiation.
Keywords: peroxisome proliferator–activated receptor, nonsteroidal anti-inflammatory drugs, cyclooxygenase-2, differentiation
Peroxisome proliferator–activated receptor (PPAR)-β/δ is a member of the nuclear receptor superfamily related to PPARα and PPARγ. In response to ligand activation, PPARβ/δ can regulate physiological homeostasis by direct transcriptional upregulation of target genes that modulate biological functions ranging from fatty acid catabolism, glucose homeostasis, and inflammation (reviewed in Grimaldi 2007; Lee et al., 2003; Peters and Gonzalez, 2009; Peters et al., 2008). However, there is also evidence that PPARβ/δ can downregulate transcription of target genes, most notably those associated with inflammation (e.g., tumor necrosis factor-α, monocyte chemoattractant protein), and in doing so mediates anti-inflammatory activities via interfering with other transcription factors such as nuclear factor-κB (NF-κB) (Kilgore and Billin, 2008; Shan et al., 2008a,b). Because ligand activation of PPARβ/δ can modulate lipid and glucose homeostasis and inflammation, targeting PPARβ/δ for the treatment and prevention of diabetes, obesity, and dyslipidemias is of current interest.
There is also compelling evidence from many laboratories demonstrating that ligand activation of PPARβ/δ promotes terminal differentiation and is associated with inhibition of cell proliferation as assessed in a number of cell types including keratinocytes (reviewed in Bility et al., 2008; Borland et al., 2008; Burdick et al., 2006; Peters and Gonzalez, 2009; Peters et al., 2008). These collective observations support the hypothesis that PPARβ/δ can inhibit tumorigenesis by promoting terminal differentiation and/or inhibiting cell growth. Consistent with this idea, it was originally shown that disruption of PPARβ/δ in skin caused enhanced cell proliferation in response to tumor promotion using two different PPARβ/δ-null mouse models (Michalik et al., 2001; Peters et al., 2000), suggesting that PPARβ/δ attenuates cell proliferation in skin. Subsequent studies established that chemically induced skin cancer is exacerbated in the absence of PPARβ/δ expression (Kim et al., 2004) and that ligand activation of PPARβ/δ can attenuate chemically induced skin tumorigenesis (Bility et al., 2008). Inhibition of chemically induced skin tumorigenesis by ligand activation of PPARβ/δ is likely due to induction of terminal differentiation and associated inhibition of cell growth (Kim et al., 2006a) but could also be due in part to attenuation of kinase signaling (Kim et al., 2005) and/or inflammation. Combined, these findings suggest that ligand activation of PPARβ/δ may be suitable for chemoprevention and/or chemotherapy of skin tumorigenesis.
There is also a large body of evidence showing that inhibition of cyclooxygenase (COX) can prevent a number of cancers (reviewed in Mazhar et al., 2005). In particular, previous studies using genetic and pharmacological approaches suggest that inhibition of cyclooxygenase-2 (COX-2) activity can inhibit both chemically induced and ultraviolet (UV)-induced skin tumorigenesis (Fischer et al., 1999, 2007; Tiano et al., 2002; Wilgus et al., 2004). The mechanisms by which COX-derived prostaglandins exert their neoplastic effects are not completely understood but include both receptor-dependent and -independent activities. The mechanisms underlying the chemopreventive effects of COX inhibitors are largely due to downregulation of prostaglandin production by COX, which in turn leads to reduced activities of prostaglandins that promote cell growth and cell survival. While targeting COX for chemoprevention and chemotherapy using specific pharmacological inhibitors has recently been hampered due to significant toxicities associated with cardiovascular function, pharmacological inhibitors of COX and related signaling molecules (e.g., prostaglandin receptors) remain of interest (Hull et al., 2004).
Given the reported chemopreventive effect of PPARβ/δ agonists and COX-2 inhibitors and the recent finding that chemoprevention of chemically induced skin tumorigenesis by sulindac (a COX1 and COX-2 inhibitor) occurs through mechanisms that are independent of PPARβ/δ (Kim et al., 2006b), the present study examined the hypothesis that combining ligand activation of PPARβ/δ with inhibition of COX-2 activity will increase the efficacy of inhibition of chemically induced skin tumor progression.
MATERIALS AND METHODS
Chemicals.
7,12-Dimethylbenz[a]anthracene (DMBA) and nimesulide were purchased from Sigma-Aldrich (St Louis, MO). GW0742 was provided as a gift from GlaxoSmithKline (Research Triangle Park, NC). 12-O-tetradecanoylphorbol-13-acetate (TPA) was provided by the National Cancer Institute's Chemical Carcinogen Reference Standard Repository operated under contract by Midwest Research Institute.
Two-stage chemical carcinogenesis bioassay.
Female wild-type and Pparβ/δ-null mice on a C57BL/6 genetic background (Peters et al., 2000) were initiated with 50 μg of DMBA dissolved in 200 μl acetone. One week after initiation, mice were treated topically with 5 μg of TPA, 3 days per week. After 22 weeks, the percentage of mice with papillomas was similar between genotypes. At this time, to determine the effect of combining ligand activation of PPARβ/δ and inhibition of COX-2 activity on skin tumor progression, four cohorts of mice from both genotypes were divided into one of the following groups: control diet with topical application of acetone, control diet with topical application of the PPARβ/δ ligand GW0742 (5μM), nimesulide diet (400 mg/kg) and topical application of acetone, or nimesulide diet (400 mg/kg) and topical application of GW0742 (5μM). The 22-week time point was chosen to eliminate bias due to differences in the percentage of mice with papillomas. The concentration of topical GW0742 was used because previous work has demonstrated that this is within the concentration range that will specifically activate PPARβ/δ in skin (Bility et al., 2008; Kim et al., 2006a). The concentration of nimesulide was used because previous work demonstrated that this concentration can inhibit chemically induced colon cancer (Hollingshead et al., 2008). After a total of 43 weeks, mice were euthanized by overexposure to carbon dioxide. Skin and tumor samples from each mouse were fixed in 10% neutral buffered formalin or 70% ethanol and then paraffin embedded, sectioned, and stained with hematoxylin and eosin stain (H&E). H&E-stained sections of suspected carcinomas were scored for benign or malignant pathology.
Quantification of prostaglandin E2 in mouse skin.
Female wild-type or Pparβ/δ-null mice were fed either a control diet or a nimesulide diet (400 mg/kg) and treated topically every other day for 7 days with TPA (5 μg/mouse; four times total) followed an hour later with either vehicle control (acetone) or GW0742 (5μM). Skin samples were collected 24 h after the last treatment. Prostaglandin E2 (PGE2) concentration was measured in skin samples using Prostaglandin E2 Correlate-EIA Kit (Assay Designs, Ann Arbor, MI) following the manufacturer’s recommended procedures. PGE2 concentration was normalized to total protein per sample.
Keratinocyte ex vivo cancer models.
To examine the hypothesis that combining ligand activation of PPARβ/δ with inhibition of COX-2 activity can inhibit cell proliferation of initiated or neoplastic keratinocytes, the following keratinocyte cell lines were used: (1) 308 keratinocyte cell line, derived from DMBA-treated mouse epidermis (Strickland et al., 1988; Yuspa and Morgan, 1981); (2) SP1 keratinocyte cell line, derived from a DMBA/TPA-induced papilloma (Strickland et al., 1988; Yuspa and Morgan, 1981); and (3) Pam212 keratinocyte cell line, derived from spontaneous transformation of neonatal keratinocytes (Yuspa et al., 1980).
Cell proliferation assay.
The 308, SP1, and Pam212 neoplastic keratinocytes were seeded at equal density and treated with either vehicle control, GW0742 (1μM), nimesulide (100μM), or GW0742 (1μM) and nimesulide (100μM) for up to 96 h. The concentration of GW0742 was used because previous work showed that this concentration is within the range that specifically activates PPARβ/δ (Kim et al., 2006a) and because modest inhibition of cell proliferation in 308 cells is observed with this concentration (Bility et al., 2008). Similarly, the concentration of nimesulide was used because previous work showed that this concentration is within the range for effectively inhibiting COX-2 activity, which was also confirmed biochemically (see below). Cell number was quantified over time in triplicate independent samples using a Z1 Coulter particle counter (Beckman Coulter, Fullerton, CA).
Flow cytometry.
Cells were seeded on six-well plates and treated with either vehicle control, GW0742 (1μM), nimesulide (100μM), or GW0742 (1μM) and nimesulide (100μM) for 72 h. Cell cycle progression was determined by flow cytometry as described previously (Burdick et al., 2007).
Cytotoxicity assay.
Cells were seeded in 96-well plates and cultured in phenol-free Dulbecco's Modified Eagle medium supplemented with 5% fetal bovine serum and treated with either vehicle control, GW0742 (1μM), nimesulide (100μM), or GW0742 (1μM) and nimesulide (100μM) for 96 h. The concentration of lactate dehydrogenase (LDH) was measured in the culture medium using a kit (Cayman Chemical Co., Ann Arbor, MI).
Measurement of PGE2 in keratinocytes.
Neoplastic keratinocyte cell lines were cultured with either GW0742, nimesulide, or both as described above. Twenty-four hours after treatment, the cellular concentration of PGE2 was determined using an enzyme-linked immunoassay (Assay Designs).
Western blot analysis.
Keratinocytes were lysed in 1× RIPA buffer (25mM Tris-HCl pH 7.6, 150mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) with supplemental protease and phosphatase inhibitors. Samples were centrifuged at 16,244 × g at 4°C for 30 min and the supernatant obtained. Twenty micrograms of protein from each sample was resolved using SDS-polyacrylamide gel electrophoresis. The samples were transferred onto a polyvinylidene difluoride membrane using an electroblotting method. Membranes were blocked in 5% milk in Tris-buffered saline-Tween-20 and incubated overnight at 4°C with the primary antibody. The following antibodies were used: anti-COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-PPARβ/δ (Girroir et al., 2008), or anti-PPARγ (Affinity BioReagents, Golden, CO). After washing, membranes were incubated with biotinylated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h. Immunoreactive proteins were detected by incubating membranes with [I125]-labeled streptavidin followed by exposure to phosphorimager plates and the level of radioactivity quantified with a Packard phosphorimager. Hybridization signals were normalized to the hybridization signals of LDH (Rockland, Gilbertsville, PA).
Examination of differentiation messenger RNA markers.
Keratins and adipose differentiation–related protein (Adrp) messenger RNA (mRNA) expression was examined using quantitative real-time PCR (qPCR) analysis. Total RNA was isolated from samples using TRIZOL reagent (Invitrogen, Carlsbad, CA). For qPCR analysis, complementary DNA was generated using 2.5 μg total RNA with M-MLV Reverse Transcriptase (Promega, Madison, WI). Primers were designed for qPCR using PrimerQuest software (Integrated DNA Technologies, Coralville, IA). qPCRs were performed using SYBR Green PCR Master Mix (Finnzymes, Espoo, Finland) in the iCycler and detected using the MyiQ Real-time PCR Detection System (Bio-Rad, Hercules, CA). The following conditions were used for PCR: 95°C for 15s, 94°C for 10 s, 60°C for 30 s, and 72°C for 30 s, and repeated for 45 cycles. The PCR included a no template control reaction to control for contamination and/or genomic amplification. All reactions had >90% efficiency. Relative expression levels of mRNA encoding Keratins and Adrp were normalized to glyceraldehyde 3-phosphate dehydrogenase (Gapdh) mRNA.
RESULTS
Combining Ligand Activation of PPARβ/δ with Inhibition of COX-2 Inhibits Chemically Induced Skin Tumor Progression
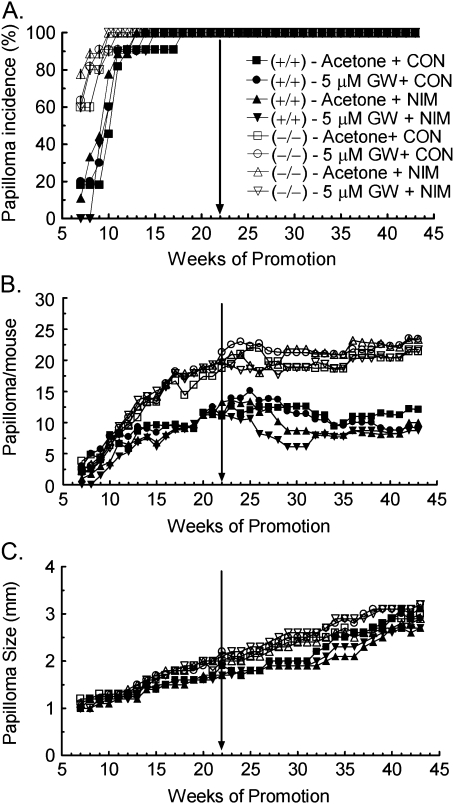
Consistent with previous reports (Bility et al., 2008; Kim et al., 2004), the onset of papilloma formation was earlier and tumor multiplicity greater in Pparβ/δ-null mice as compared to similarly treated wild-type mice (Fig. 1). To determine if combining ligand activation of PPARβ/δ with inhibition of COX-2 activity can inhibit skin tumor progression, wild-type and Pparβ/δ-null mice with a similar incidence of preexisting tumors were treated with the highly specific PPARβ/δ ligand GW0742 and the COX-2 inhibitor nimesulide, 22 weeks into the two-stage chemical carcinogenesis bioassay (Fig. 1). Topical application of the PPARβ/δ ligand GW0742 alone did not significantly affect skin tumor progression in either genotype as shown by a lack of change in tumor multiplicity and average tumor size (Fig. 1). Dietary nimesulide significantly decreased tumor multiplicity in wild-type mice between weeks 28 and 31, and this effect was not found in similarly treated Pparβ/δ-null mice (Fig. 1B). The combination of dietary nimesulide and topical application of GW0742 resulted in a decrease in tumor multiplicity between week 25 and 31 in wild-type mice, and this effect was greater as compared to that observed with dietary nimesulide alone (Fig. 1B). While tumor multiplicity was modestly lower in Pparβ/δ-null mice treated with the combination of dietary nimesulide and topical GW0742, this effect was not statistically significant (Fig. 1B). Average tumor size was modestly greater in the Pparβ/δ-null mice as compared to wild-type mice, but this difference was not statistically significant (Fig. 1C). None of the treatment regimes caused a significant change in average tumor size in either genotype (Fig. 1C).
FIG. 1.
Ligand activation of PPARβ/δ and inhibition of COX-2 inhibit chemically induced skin tumor progression. Wild-type (+/+) and Pparβ/δ-null (−/−) mice with skin tumors were treated with GW0742 (GW), nimesulide (NIM), or the combination of GW0742 and nimesulide beginning 22 weeks after initiating a two-stage chemical carcinogenesis bioassay (arrow), with continued treatment for 21 weeks. (A) The incidence and onset of tumor formation. (B) Tumor multiplicity. (C) Average tumor size per mouse.
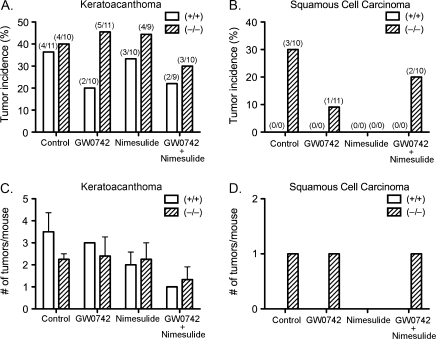
Topical application of GW0742 caused a decrease in the incidence of keratoacanthomas in wild-type mice that was not found in Pparβ/δ-null mice, but the average number of keratoacanthoma per mouse did not exhibit a PPARβ/δ-dependent decrease (Figs. 2A and 2C). The incidence of keratoacanthomas and the average number of keratoacanthoma per mouse were not influenced by dietary nimesulide (Figs. 2A and 2C). The lower incidence of keratoacanthomas as a result of combining GW0742 with nimesulide was similar as compared to GW0742 alone (Fig. 2A), and the average number of keratoacanthoma per mouse was lower in response to the combined treatment of both GW0742 with nimesulide in both wild-type and Pparβ/δ-null mice (Fig. 2C). Squamous cell carcinomas were only found in Pparβ/δ-null mice and not in wild-type mice (Figs. 2B and 2D).
FIG. 2.
Incidence and average number of keratoacanthoma and squamous cell carcinomas in wild-type (+/+) and Pparβ/δ-null (−/−) mice in response to ligand activation of PPARβ/δ and inhibition of COX-2. Suspected keratoacanthomas and squamous cell carcinomas were examined microscopically and classified as either keratoacanthomas or squamous cell carcinomas by an expert pathologist. Incidence of (A) keratoacanthomas or (B) squamous cell carcinomas. Values represent the percentage of mice with lesion. Actual number of mice with lesion within each group of mice is shown in parentheses. Multiplicity of (C) keratoacanthoma or (D) squamous cell carcinomas. Values represent the average number of lesions per mouse with lesion.
Combining Ligand Activation of PPARβ/δ with Inhibition of COX-2 Inhibits Cell Proliferation of Keratinocyte Cancer Cell Lines
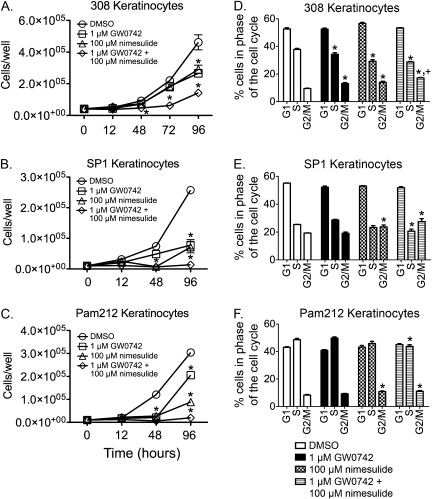
The observed inhibition of chemically induced skin tumor progression by ligand activation of GW0742 and inhibition of COX-2 activity suggests that this combined treatment paradigm could inhibit proliferation during later stages of tumor progression. To examine this idea in greater detail, the effect of combining GW0742 with nimesulide on neoplastic keratinocyte proliferation was evaluated in cell lines representing varying stages of skin carcinogenesis (e.g., initiated cells, benign papillomas, and carcinomas). The 308 keratinocyte cell line has a Ras mutation and was derived from mouse skin following initiation with DMBA (Strickland et al., 1988; Yuspa and Morgan, 1981). The 308 cells can form papillomas with and without tumor promotion when grafted onto mouse skin in vivo (Strickland et al., 1988; Yuspa and Morgan, 1981). The SP1 keratinocyte cell line is a papilloma-like cell line derived from DMBA/TPA-treated animals with a Ras mutation and produces papillomas in vivo when grafted onto mouse skin (Strickland et al., 1988; Yuspa and Morgan, 1981). The Pam212 keratinocyte cell line is a carcinoma-like cell line derived from spontaneous transformation of neonatal keratinocytes in culture condition and produces squamous cell carcinoma in vivo when grafted onto mouse skin (Yuspa et al., 1980). These three keratinocyte cancer lines are resistant to calcium-induced terminal differentiation (Hennings et al., 1990). In the 308 keratinocyte cell line, inhibition of cell proliferation was observed after 48 h of culture in response to GW0742, nimesulide, and GW0742 and nimesulide as compared to control (Fig. 3A). This inhibition of cell proliferation was greater in response to combined GW0742 and nimesulide as compared to the other groups after 72 h of culture (Fig. 3A). Similar results were observed with both the SP1 and the Pam212 cell lines with inhibition of cell proliferation being observed with GW0742, nimesulide, and GW0742 and nimesulide as compared to control and significantly greater inhibition of cell proliferation after 96 h of culture by combining GW0742 with nimesulide (Fig. 3). Flow cytometry was performed 72 h after treatments to examine cell cycle progression. In 308 cells, GW0742 and nimesulide each caused a decrease in the percentage of cells in the S phase and an increase in the percentage of cells in the G2/M phase (Fig. 3D). An increase in the percentage of cells in the G2/M phase and a decrease in the percentage of cells in the S phase were also found with the combined treatment of nimesulide and GW0742 in 308 cells, but the change in G2/M phase was greater as compared to either treatment alone (Fig. 3D). In both SP1 and Pam212 cells, no significant changes in cell cycle progression were found with GW0742, while an increase in the percentage of cells in the G2/M phase was observed by treatment with nimesulide (Figs. 3E and 3F). The combined treatment of GW0742 and nimesulide caused an increase in the percentage of cells in the G2/M phase and a decrease in the percentage of cells in the S phase, with the change in S phase being greater as compared to either treatment alone in both SP1 and Pam 212 cells (Figs. 3E and 3F). In 308 cells, no change in cytotoxicity was found following treatment with either GW0742 or nimesulide, but a modest fourfold increase in LDH was found in response to the combined treatment of GW0742 and nimesulide (data not shown). Similarly, in SP1 and Pam212 cells, no change in cytotoxicity was found following treatment with either GW0742 or nimesulide and only a modest less than or equal to twofold increase in LDH was found in response to the combined treatment of GW0742 and nimesulide (data not shown).
FIG. 3.
Ligand activation of PPARβ/δ and inhibition of COX-2 inhibits cell proliferation in keratinocyte cancer lines. The 308, SP1, and Pam212 keratinocyte cancer lines were cultured in low (0.05mM) calcium keratinocyte culture medium containing GW0742 (1μM), nimesulide (100μM), or the combination of both (n = 3 replicates per treatment group). Average cell number for (A) 308 keratinocytes, (B) SP1 keratinocytes, and (C) Pam212 keratinocytes were quantified using a Coulter counter. Values represent mean ± SEM. *Significantly different as determined by ANOVA and post hoc testing, p ≤ 0.05. Cell cycle progression was determined by flow cytometry after 72 h with the indicated treatment in (D) 308 keratinocytes, (E) SP1 keratinocytes, and (F) Pam212 keratinocytes. Values are the percentage of cells within a specific phase of the cell cycle and represent the mean ± SEM from three independent replicates. *Significantly different, p ≤ 0.05. *,+Significantly different than control and individual treatments, p ≤ 0.05.
Effect of GW0742 and Nimesulide on PGE2 Concentration
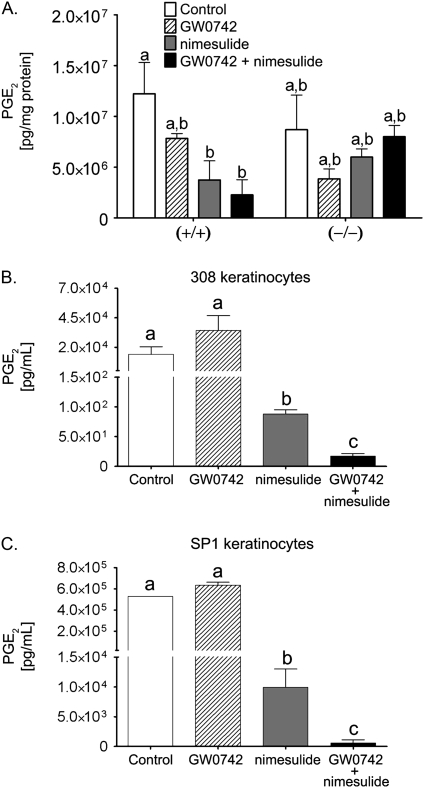
To begin to determine the mechanisms underlying the inhibition of cell proliferation observed by combining ligand activation of PPARβ/δ with inhibition of COX-2 activity, the concentration of prostaglandin was measured in both the in vivo and the in vitro models. The concentration PGE2 was significantly reduced in wild-type mouse skin by dietary nimesulide (with or without supplemental GW0742) as compared to control, but GW0742 had no effect on the concentration of PGE2 in mouse skin (Fig. 4A). Dietary nimesulide did not cause a similar reduction in the concentration of PGE2 in Pparβ/δ-null mouse skin (Fig. 4A). The intracellular concentration of PGE2 was significantly suppressed by nimesulide in both 308 cells and SP1 cells (Fig. 4). While culturing the cells in GW0742 did not modulate the intracellular concentration of PGE2, the concentration of PGE2 was significantly lower in 308 cells and SP1 cells cotreated with nimesulide and GW0742 as compared to all other treatments (Fig. 4).
FIG. 4.
PGE2 concentration in (A) wild-type (+/+) and Pparβ/δ-null (–/–) mouse skin, or (B) 308, or (C) SP1 neoplastic keratinocyte cell lines. Cells were cultured with either GW0742 (1μM), nimesulide (100μM), or the combination of both GW0742 and nimesulide. PGE2 concentration was determined as described in “Materials and Methods” section. Values represent the mean ± SEM. Values with different letters are significantly different, p ≤ 0.05.
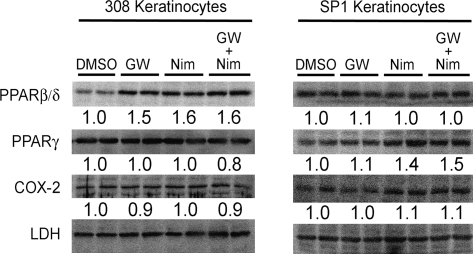
Others have suggested that one mechanism by which nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit tumor growth is by downregulating PPARβ/δ expression, although this idea is not consistently observed in all models (reviewed in Peters et al., 2008). Quantitative Western blotting was performed to examine the notion that nimesulide modulates cell proliferation by altering expression of PPARβ/δ. Expression of PPARβ/δ, PPARγ, or COX-2 was not influenced by GW0742, nimesulide, or cotreating nimesulide with GW0742 in either the 308 or the SP1 cell lines (Fig. 5).
FIG. 5.
The effect of ligand activation of PPARβ/δ and inhibition of COX-2 activity on COX-2, PPARβ/δ, and PPARγ expression in neoplastic keratinocytes. Representative Western blot showing the protein levels of COX-2, PPARβ/δ, and PPARγ quantified in 308 and SP1 keratinocyte cancer lines following treatment with GW0742 (1μM), nimesulide (100μM), or the combination of both GW0742 and nimesulide. Values represent the fold change from control and are the mean based on analysis of four independent samples.
Effect of GW0742 and Nimesulide on Terminal Differentiation Markers
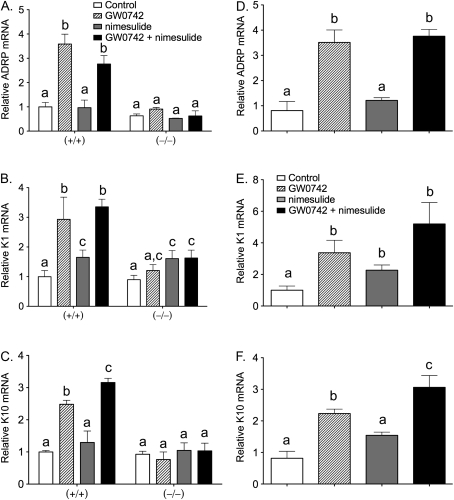
There is evidence that terminal differentiation of keratinocytes can be induced by ligand activation of PPARβ/δ (Kim et al., 2006a; Schmuth et al., 2004; Westergaard et al., 2001) and/or inhibiting COX activity (Akunda et al., 2004; Tiano et al., 2002). Expression of keratinocyte differentiation markers was examined in skin and skin cancer cell lines to determine if modulation of terminal differentiation could underlie the observed inhibition of epithelial tumorigenesis and/or cell proliferation. In response to topical application of GW0742 (with or without cotreatment with dietary nimesulide), expression of the known PPARβ/δ target gene Adrp was increased in wild-type mouse skin and this was not found in similarly treated Pparβ/δ-null mouse skin (Fig. 6A). Similarly, topical application of GW0742 (with or without cotreatment with dietary nimesulide) caused an increase in expression of Keratin 1 (K1) and Keratin 10 (K10) in wild-type mouse skin, and this was not found in similarly treated Pparβ/δ-null mouse skin (Fig. 6). Interestingly, expression of K10 was higher in response to cotreatment with GW0742 and nimesulide as compared to GW0742 alone (Fig. 6C). Similar results were also observed in 308 cells where treatment with GW0742 (with or without cotreatment with nimesulide) caused an increase in Adrp, K1, and K10 (Fig. 6), with higher expression of K10 noted in response to cotreatment with GW0742 and nimesulide (Fig. 6F).
FIG. 6.
Expression of differentiation marker mRNAs in (A–C) wild-type (+/+) and Pparβ/δ-null (−/−) mouse skin or (D–F) 308 neoplastic keratinocyte cell line. Total RNA was isolated from mouse skin or cells following treatment with either GW0742, nimesulide, or GW0742 and nimesulide as described in “Materials and Methods” section. mRNA encoding Adrp (A and D), K1 (B and E), or K10 (C and F) was quantified using qPCR. Values represent the mean ± SEM. Values with different letters are significantly different, p ≤ 0.05.
DISCUSSION
Previous work showed that chemically induced skin tumorigenesis is exacerbated in the absence of PPARβ/δ expression (Kim et al., 2004) and that ligand activation of PPARβ/δ is chemopreventive against chemically induced skin tumorigenesis (Bility et al., 2008). Furthermore, dietary administration of sulindac (a drug that inhibits COX1 and COX-2) is chemopreventive against chemically induced skin tumorigenesis in both wild-type and Pparβ/δ-null mice (Kim et al., 2006b), suggesting that the mechanisms underlying inhibition of chemically induced skin tumorigenesis are independent of PPARβ/δ. The present studies were designed to extend the previous studies by testing the hypothesis that targeting ligand activation of PPARβ/δ combined with specific inhibition of COX-2 will additively or synergistically inhibit skin tumor progression. Results from this study are consistent with past reports showing enhanced chemically induced tumorigenicity in Pparβ/δ-null mice as compared to wild type, including earlier onset of tumor formation, increased tumor multiplicity, and the presence of squamous cell carcinomas (Bility et al., 2008; Kim et al., 2004). In contrast to previous work showing the chemopreventive effects of topical application of the PPARβ/δ ligand GW0742 (Bility et al., 2008), ligand activation of PPARβ/δ did not suppress preexisting skin papilloma multiplicity or growth. However, inhibiting COX-2 with nimesulide suppressed papilloma multiplicity for 4 weeks, and greater efficacy of this inhibition was observed by combining nimesulide with ligand activation of PPARβ/δ. This effect requires a functional PPARβ/δ as revealed by a lack of inhibition in Pparβ/δ-null mice. These findings are in contrast to the study showing that sulindac was chemopreventive in both wild-type and Pparβ/δ-null mice (Kim et al., 2006b). There are a number of variables that could contribute to this difference, including the timing of COX-2 inhibitors (administration before or after tumor formation) and differences in the particular NSAID administered (sulindac vs. nimesulide). The latter possibility is supported by recent studies showing that nimesulide, but not sulindac, increases expression and function of PPARβ/δ in colon cancer cell lines (Foreman et al., 2009). Thus, it is possible that nimesulide causes similar changes in skin tumors that reflect the observed PPARβ/δ-dependent phenotype. Further studies are necessary to examine this possibility.
Since no squamous cell carcinomas were found in wild-type mice, these studies cannot determine whether malignant conversion was influenced by either ligand activation of PPARβ/δ and/or inhibition of COX-2. As a surrogate approach, cell lines representing different stages of skin tumorigenesis were examined. Indeed, the PPARβ/δ ligand GW0742 or the COX-2 inhibitor nimesulide inhibited cell growth of 308 keratinocytes (initiated keratinocyte model), SP1 keratinocytes (papilloma model), and Pam212 (carcinoma model). Furthermore, inhibition of proliferation was enhanced by combining GW0742 and nimesulide in all three cell lines. The reason why greater efficacy was observed with the in vitro findings as compared to in vivo findings could be due to differences in the relative bioavailability of compound that may be influenced by differences in pharmacokinetic metabolism or disposition of either GW0742 and/or nimesulide. For example, although plasma nimesulide was not measured in the present study, previous work by others showed that administration of dietary nimesulide (500 mg/kg diet) to mice resulted in an average plasma concentration of ∼4μM nimesulide (Shaik et al., 2004), which is considerably lower than the 100μM concentration used for in vitro analysis in the present study.
These studies provide some evidence for the mechanisms underlying the inhibitory effects observed in vivo and in vitro. Nimesulide inhibited COX-2 activity as shown by decreased concentration of PGE2 in 308 and SP1 keratinocytes and in wild-type mouse skin. Thus, inhibition of prostaglandin-dependent signaling through prostaglandin receptors, which is known to promote cell proliferation and oncogenesis, is likely central to the observed inhibition of skin tumor multiplicity and inhibition of cell proliferation in the skin cancer cell lines. The reasons for the lack of a decrease in PGE2 concentrations in Pparβ/δ-null mouse skin following nimesulide cannot be determined from the present studies, but this difference could also contribute to the PPARβ/δ-dependent effect observed in vivo. It remains possible that PPARβ/δ-dependent interference with NF-κB signaling (Kilgore and Billin, 2008; Shan et al., 2008a,b), which could also influence COX-2-dependent signaling, could explain this difference. It has also been suggested that NSAIDs can inhibit tumor growth by suppressing PPARβ/δ expression, although this literature is controversial since this effect is not consistently observed (Foreman et al., 2009; Peters and Gonzalez, 2009; Peters et al., 2008). Results from the present study do not support this idea since no reduction in expression of PPARβ/δ was noted in response to nimesulide.
Results from the present study also support the hypothesis that ligand activation of PPARβ/δ and/or nimesulide may act similarly by inducing terminal differentiation. Ligand activation of PPARβ/δ is known to induce terminal differentiation of keratinocytes as shown by multiple laboratories (Borland et al., 2008; Burdick et al., 2007; Kim et al., 2006a; Schmuth et al., 2004; Westergaard et al., 2001). Recent evidence also shows that the induction of terminal differentiation may be a key mechanism by which ligand activation of PPARβ/δ attenuates chemically induced skin tumorigenesis (Bility et al., 2008). The observed increase in expression of the terminal differentiation markers (e.g., Adrp, K1, and K10) is consistent with the idea that ligand activation of PPARβ/δ inhibits cell proliferation by modulating terminal differentiation. However, it is also of interest to note that inhibiting COX has also been shown to induce terminal differentiation of keratinocytes (Akunda et al., 2004; Tiano et al., 2002). Results from the present study suggests that combining ligand activation with COX-2 inhibition may increase signaling leading to terminal differentiation as revealed by induction of K10, which was greater in the presence of GW0742 and nimesulide as compared to that observed with GW0742 alone. This is of interest because increased expression of K10 inhibits cell proliferation of human HaCaT keratinocytes through a retinoblastoma-dependent mechanism involving the nonhelical terminal domain of K10 (Paramio et al., 1999). Since the induction of terminal differentiation is known to be associated with withdrawal from the cell cycle, it is possible that induction of K10 in keratinocytes may be a key determinant for the enhanced inhibition of cell proliferation observed in the present study by combining ligand activation of PPARβ/δ with inhibition of COX-2 activity. This idea is also supported by the flow cytometry analysis where greater changes indicative of inhibited cell cycle progression were noted in the keratinocyte cell lines when GW0742 and nimesulide treatments were combined. Future studies examining the effects of direct PPARβ/δ target genes will be required to fully elucidate the mechanisms by which PPARβ/δ inhibits cell proliferation in keratinocytes.
In summary, results from this study show that combining ligand activation of PPARβ/δ with inhibition of COX-2 activity can modestly improve the efficacy of inhibition of chemically induced skin tumor progression found by nimesulide treatment alone. Whether increasing the concentration of either GW0742 and/or nimesulide could improve the efficacy of this transient effect should be examined in greater detail. Additionally, based on these results, it will be of interest to determine if these agents can be used to inhibit UV-induced skin tumor progression.
FUNDING
National Institutes of Health (CA124533 to J.M.P.).
Acknowledgments
We gratefully acknowledge Drs Andrew Billin and Timothy Willson for providing the GW0742 used for these studies; Drs Adam Glick, Stuart Yuspa, Henry Hennings, and Ulrike Lichti for providing the keratinocyte cell lines; and Elaine Kunze, Susan Magargee, and Nicole Bem from the Center for Quantitative Cell Analysis at the Huck Institutes of Life Sciences of The Pennsylvania State University for their technical support with flow cytometry and data analysis.
References
- Akunda JK, Lao HC, Lee CA, Sessoms AR, Slade RM, Langenbach R. Genetic deficiency or pharmacological inhibition of cyclooxygenase-1 or -2 induces mouse keratinocyte differentiation in vitro and in vivo. FASEB J. 2004;18:185–187. doi: 10.1096/fj.02-1192fje. [DOI] [PubMed] [Google Scholar]
- Bility MT, Devlin-Durante MK, Blazanin N, Glick AB, Ward JM, Kang BH, Kennett MJ, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits chemically-induced skin tumorigenesis. Carcinogenesis. 2008;29:2406–2414. doi: 10.1093/carcin/bgn219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland MG, Foreman JE, Girroir EE, Zolfaghari R, Sharma AK, Amin SM, Gonzalez FJ, Ross AC, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits cell proliferation in human HaCaT keratinocytes. Mol. Pharmacol. 2008;74:1429–1442. doi: 10.1124/mol.108.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick AD, Bility MT, Girroir EE, Billin AN, Willson TM, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits cell growth of human N/TERT-1 keratinocytes. Cell. Signal. 2007;19:1163–1171. doi: 10.1016/j.cellsig.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM. The role of peroxisome proliferator-activated receptor-β/δ in epithelial cell growth and differentiation. Cell. Signal. 2006;18:9–20. doi: 10.1016/j.cellsig.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, Conti CJ. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol. Carcinog. 1999;25:231–240. [PubMed] [Google Scholar]
- Fischer SM, Pavone A, Mikulec C, Langenbach R, Rundhaug JE. Cyclooxygenase-2 expression is critical for chronic UV-induced murine skin carcinogenesis. Mol. Carcinog. 2007;46:363–371. doi: 10.1002/mc.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman JE, Sorg JM, McGinnis KS, Rigas B, Williams JL, Clapper ML, Gonzales FJ, Peters JM. Regulation of peroxisome proliferator-activated receptor-β/δ by the APC/β-CATENIN pathway and nonsteroidal anti-inflammatory drugs. Mol. Carcinog. 2009 doi: 10.1002/mc.20546. doi: 10.1002/mc.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girroir EE, Hollingshead HE, He P, Zhu B, Perdew GH, Peters JM. Quantitative expression patterns of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) protein in mice. Biochem. Biophys. Res. Commun. 2008;371:456–461. doi: 10.1016/j.bbrc.2008.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi PA. Regulatory functions of PPARβ in metabolism: implications for the treatment of metabolic syndrome. Biochim. Biophys. Acta. 2007;1771:983–990. doi: 10.1016/j.bbalip.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Hennings H, Robinson VA, Michael DM, Pettit GR, Jung R, Yuspa SH. Development of an in vitro analogue of initiated mouse epidermis to study tumor promoters and antipromoters. Cancer Res. 1990;50:4794–4800. [PubMed] [Google Scholar]
- Hollingshead HE, Borland MG, Billin AN, Willson TM, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) and inhibition of cyclooxygenase 2 (COX2) attenuate colon carcinogenesis through independent signaling mechanisms. Carcinogenesis. 2008;29:169–176. doi: 10.1093/carcin/bgm209. [DOI] [PubMed] [Google Scholar]
- Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Mol. Cancer Ther. 2004;3:1031–1039. [PubMed] [Google Scholar]
- Kilgore KS, Billin AN. PPARβ/δ ligands as modulators of the inflammatory response. Curr. Opin. Investig. Drugs. 2008;9:463–469. [PubMed] [Google Scholar]
- Kim DJ, Akiyama TE, Harman FS, Burns AM, Shan W, Ward JM, Kennett MJ, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor beta (delta)-dependent regulation of ubiquitin C expression contributes to attenuation of skin carcinogenesis. J. Biol. Chem. 2004;279:23719–23727. doi: 10.1074/jbc.M312063200. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, Peters JM. PPARβ/δ selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006a;13:53–60. doi: 10.1038/sj.cdd.4401713. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Murray IA, Burns AM, Gonzalez FJ, Perdew GH, Peters JM. Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits epidermal cell proliferation by down-regulation of kinase activity. J. Biol. Chem. 2005;280:9519–9527. doi: 10.1074/jbc.M413808200. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Prabhu KS, Gonzalez FJ, Peters JM. Inhibition of chemically-induced skin carcinogenicity by sulindac is independent of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) Carcinogenesis. 2006b;27:1105–1112. doi: 10.1093/carcin/bgi346. [DOI] [PubMed] [Google Scholar]
- Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- Mazhar D, Gillmore R, Waxman J. COX and cancer. QJM. 2005;98:711–718. doi: 10.1093/qjmed/hci119. [DOI] [PubMed] [Google Scholar]
- Michalik L, Desvergne B, Tan NS, Basu-Modak S, Escher P, Rieusset J, Peters JM, Kaya G, Gonzalez FJ, Zakany J, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)α and PPARβ mutant mice. J. Cell Biol. 2001;154:799–814. doi: 10.1083/jcb.200011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramio JM, Casanova ML, Segrelles C, Mittnacht S, Lane EB, Jorcano JL. Modulation of cell proliferation by cytokeratins K10 and K16. Mol. Cell. Biol. 1999;19:3086–3094. doi: 10.1128/mcb.19.4.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim. Biophys. Acta. 2009;1796:230–241. doi: 10.1016/j.bbcan.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Hollingshead HE, Gonzalez FJ. Role of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) in gastrointestinal tract function and disease. Clin. Sci. (Lond.) 2008;115:107–127. doi: 10.1042/CS20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Lee SST, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ. Growth, adipose, brain and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β/δ. Mol. Cell. Biol. 2000;20:5119–5128. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Haqq CM, Cairns WJ, Holder JC, Dorsam S, Chang S, Lau P, Fowler AJ, Chuang G, Moser AH, et al. Peroxisome proliferator-activated receptor (PPAR)-β/δ stimulates differentiation and lipid accumulation in keratinocytes. J. Invest. Dermatol. 2004;122:971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- Shaik MS, Chatterjee A, Singh M. Effect of a selective cyclooxygenase-2 inhibitor, nimesulide, on the growth of lung tumors and their expression of cyclooxygenase-2 and peroxisome proliferator- activated receptor-gamma. Clin. Cancer Res. 2004;10:1521–1529. doi: 10.1158/1078-0432.ccr-0902-03. [DOI] [PubMed] [Google Scholar]
- Shan W, Nicol CJ, Ito S, Bility MT, Kennett MJ, Ward JM, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-β/δ protects against chemically induced liver toxicity in mice. Hepatology. 2008a;47:225–235. doi: 10.1002/hep.21925. [DOI] [PubMed] [Google Scholar]
- Shan W, Palkar PS, Murray IA, McDevitt EI, Kennett MJ, Kang BH, Isom HC, Perdew GH, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor β/δ (PPARβ/δ) attenuates carbon tetrachloride hepatotoxicity by downregulating proinflammatory gene expression. Toxicol. Sci. 2008b;105:418–428. doi: 10.1093/toxsci/kfn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JE, Greenhalgh DA, Koceva-Chyla A, Hennings H, Restrepo C, Balaschak M, Yuspa SH. Development of murine epidermal cell lines which contain an activated rasHa oncogene and form papillomas in skin grafts on athymic nude mouse hosts. Cancer Res. 1988;48:165–169. [PubMed] [Google Scholar]
- Tiano HF, Loftin CD, Akunda J, Lee CA, Spalding J, Sessoms A, Dunson DB, Rogan EG, Morham SG, Smart RC, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–3401. [PubMed] [Google Scholar]
- Westergaard M, Henningsen J, Svendsen ML, Johansen C, Jensen UB, Schroder HD, Kratchmarova I, Berge RK, Iversen L, Bolund L, et al. Modulation of keratinocyte gene expression and differentiation by PPAR-selective ligands and tetradecylthioacetic acid. J. Invest. Dermatol. 2001;116:702–712. doi: 10.1046/j.1523-1747.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Breza TS, Jr, Tober KL, Oberyszyn TM. Treatment with 5-fluorouracil and celecoxib displays synergistic regression of ultraviolet light B-induced skin tumors. J. Invest. Dermatol. 2004;122:1488–1494. doi: 10.1111/j.0022-202X.2004.22606.x. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, Hawley-Nelson P, Koehler B, Stanley JR. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980;40:4694–4703. [PubMed] [Google Scholar]
- Yuspa SH, Morgan DL. Mouse skin cells resistant to terminal differentiation associated with initiation of carcinogenesis. Nature. 1981;293:72–74. doi: 10.1038/293072a0. [DOI] [PubMed] [Google Scholar]