Abstract
Azaspiracids (AZAs) are a group of marine toxins recently described that currently includes 20 members. Not much is known about their mechanism of action, although the predominant analog in nature, AZA-1 targets several organs in vivo, including the central nervous system, and exhibits high neurotoxicity in vitro. AZA distribution is increasing globally with mussels being most widely implicated in AZA-related food poisoning events, with human poisoning by AZAs emerging as an increasing worldwide problem in recent years. We used pharmacological tools to inhibit the cytotoxic effect of the toxin in primary cultured neurons. Several targets for AZA-induced neurotoxicity were evaluated. AZA-1 elicited a concentration-dependent hyperpolarization in cerebellar granule cells of 2–3 days in vitro; however, it did not modify membrane potential in mature neurons. Furthermore, in immature cells, AZA-1 decreased the membrane depolarization evoked by exposure of the neurons to 50mM K+. Preincubation of the neurons with 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), 4-acetamido-4′-isothiocyanato-2,2′-stilbenedisulfonic acid (SITS), 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), amiloride, or ouabain before addition of AZA-1 decreased the AZA-1-induced neurotoxicity and the increase in phosphorylated c-Jun-N-terminal kinase (JNK) caused by the toxin, indicating that disruption in ion fluxes was involved in the neurotoxic effect of AZA-1. Furthermore, short exposures of cultured neurons to AZA-1 caused a significant decrease in neuronal volume that was reverted by preincubation of the neurons with DIDS or amiloride before addition of the toxin. The results presented here indicate that the JNK activation induced by AZA-1 is secondary to the decrease in cellular volume elicited by the toxin.
Keywords: azaspiracid, AZA-1, cytotoxicity, cerebellar granule cell, cell volume, c-Jun-N-terminal kinase
Human poisoning by azaspiracids (AZAs) has emerged as an increasing problem in Europe in the past years. The structure of the toxin was identified in Japan and given the name AZA, with reference to the existence within the molecule of a cyclic amine (aza group), a single tri-spiro assembly and one carboxylic acid group (James et al., 2003; Ofuji et al., 1999a). More than 20 compounds have been described in the AZA family (James et al., 2003; Ofuji et al., 1999a, 2001; Rehmann et al., 2008; Volmer et al., 2002). Two analogs of azaspiracid (AZA-1) were characterized, 8-methylazaspiracid (AZA-2) and 22-demethylazaspiracid (AZA-3). Other AZAs (i.e., AZA-4 through AZA-11) differ by the presence or lack of methyl and hydroxyl groups on the AZA structure (James et al., 2003; Ofuji et al., 1999a, 2001). The complete structures of AZA-1, -2, and -3 were recently revised (Nicolaou et al., 2003, 2006).
The toxic episodes caused by AZAs in humans show gastrointestinal illnesses, including nausea, vomiting, severe diarrhea, and stomach cramps (Ofuji et al., 1999b). Administration of AZA-1 to mice caused multiple organ injuries, including necroses in the lamina propria of the small intestine, thymus and spleen, lung tumors, and hyperplasia in the stomach, together with lymphocyte injury and fatty changes in the liver (Ito et al., 2000, 2002). Although these toxins have been associated with several human intoxications since 1995, so far there is no information about their mechanism of action or specific cellular targets.
It has recently been shown that AZA-1 is a potent neurotoxic agent in primary neuronal cultures showing an effect several orders of magnitude higher than the cytotoxic effects previously described in different cell lines (Twiner et al., 2005; Vale et al., 2007b). In neurons, the cytotoxic action of AZA-1 was independent of an alteration on the cytosolic calcium concentration or on F-actin cytoskeleton disruption and it needed the whole molecular structure of the toxin (Vale et al., 2007b). More recently, we found that c-Jun-N-terminal kinase (JNK) activation was associated with the neurotoxic effect of both AZA-1 and AZA-2 (Vale et al., 2007a, 2008b); however, the mechanism of AZAs action is yet unclear, as it remains to be determined whether JNK activation is an early or late event in the AZA-induced cytotoxicity. Recently, several membrane proteins involved in cell adhesion such as claudins and cadherins have been related with AZA cytotoxicity, and this effect was sensitive to extracellular signal regulated protein kinases (Twiner et al., 2008). Furthermore, AZA-1 exposure has been shown to elicit an unexpected complexity of cellular changes in human neuroblastoma cells including an increase in the level of several proteins involved in cellular metabolism and a pronounced but temporary depletion of ATP levels (Kellmann et al., 2009). In order to investigate the cellular mechanisms involved in the AZA-evoked JNK activation and cytotoxicity, we conducted a pharmacological approach to investigate modulatory activities in several systems involved in neuronal functioning as a way to investigate potential pharmacological targets of AZA-1 cytotoxicity in neurons.
MATERIALS AND METHODS
Chemicals and solutions.
Seven-day-old Swiss mice were obtained from the animal care facilities of the University of Santiago de Compostela. Plastic tissue culture dishes were purchased from Falcon (Madrid, Spain). Fetal calf serum was obtained from Gibco (Glasgow, UK), and Dulbecco’s modified Eagle’s medium (DMEM) was from Biochrom (Berlin, Germany). AZA-1 was synthesized by Nicolaou et al. (2003, 2006). The mouse monoclonal antibody against phospho-JNK (p-JNK, dually phosphorylated stress-activated protein kinase/c-Jun NH2 protein kinase [SAPK/JNK] [Thr183/Try185]) was purchased from Cell Signaling (Madrid, Spain). Bicuculline and γ-aminobutyric acid (GABA) were purchased from Tocris (Biogen Científica, Madrid, Spain). All other chemicals were purchased from Sigma-Aldrich (Madrid, Spain). Experimental solutions were based on Locke’s buffer containing (in millimolar) 154 NaCl, 5.6 KCl, 1.3 CaCl2, 1 MgCl2, 5.6 glucose, 3.6 NaHCO3, and 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4 adjusted with Tris. The pH of the buffer containing the different drugs used in this study was adjusted to 7.4 with Tris before addition to the cells.
Cell cultures.
Primary cultures of cerebellar granule cells (CGCs) were obtained from cerebella of 7-day-old mice as previously described (Vale et al., 2007a,b, 2008b). In brief, cells were dissociated by mild trypsinization at 37°C, followed by trituration in a DNAse solution (0.004% wt/vol) containing a soybean trypsin inhibitor (0.05% wt/vol). The cells were suspended in DMEM containing 25mM KCl, 31mM glucose, and 0.2mM glutamine supplemented with p-amino benzoate, insulin, penicillin, and 10% fetal calf serum. The cell suspension was seeded in 22-mm glass coverslips precoated with poly-L-lysine and incubated in 6- or 96-multiwell plates for 8–11 days in a humidified 5% CO2/95% air atmosphere at 37°C. Cytosine arabinoside, 20μM, was added before 48 h in culture to prevent glial proliferation.
Cell viability assays.
The cytotoxic action of AZA-1 was studied in cultured CGC by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) test. The MTT assay was performed as described previously (Vale et al., 2007b, 2008b). This test, which measures mitochondrial function, was used to assess cell viability as it has been shown that in neuronal cells, there is a good correlation between a drug-induced decrease in mitochondrial activity and its cytotoxicity (Varming et al., 1996). Briefly, after 24 h of exposure to different concentrations of AZA-1 alone or in combination with the different drugs tested, cells were rinsed and incubated for 30 min with a solution of MTT (500 μg/ml) dissolved in Locke's buffer. After washing off excess MTT, the cells were disaggregated with 5% SDS and the colored formazan salt was measured at 590nM in a spectrophotometer plate reader. MTT data were normalized against the mean absorbance values of control cells (in the absence of drugs) under each experimental condition and expressed as percentage of control values.
Determination of plasma membrane potential (Em).
The coverslips plated with CGC were transferred to custom-made recording chambers, in which the cells were loaded with 20nM [DIBAC4(3)], to monitor Em (Rodriguez et al., 2008; Vale et al., 2008a). The DIBAC4(3) fluorescence was monitored using a Nikon C1 confocal microscope and Nikon Plan Apo Tirf 60X, NA 1.45 objective (Nikon, Melville, NY). The images of fluorescence emitted at 515 nm (after excitation at 488 nm) were collected every 5 s. The fluorescence intensities, measured in selected regions of interest, were analyzed off-line using the Nikon EZ-C1 viewer software.
Cell volume measurement.
Cell volume was determined from the maximum cross-sectional area of a cell, assuming that the cell soma swells and shrinks in a spherical manner. This assumption has been validated in neocortical cultures, where cell volume changes measured directly using optical sectioning techniques were not different from those calculated from the cross-sectional area (Churchwell et al., 1996). Measurement of cross-sectional areas was performed using the MetaMorph Imaging System (Universal Imaging Corporation, West Chester, PA). Area values were normalized to sham controls and expressed as relative cell volume changes.
Immunocytochemistry.
For immunocytochemistry, control and treated cells were fixed in 4% formaldehyde and subsequently labeled with a monoclonal antibody against dually phosphorylated SAPK/JNK (p-JNK and p-JNK, 1:250). Immunoreactivity was visualized using an Alexa 546–conjugated secondary antibody (1:1000 dilution; Molecular Probes, Barcelona, Spain). Cells were analyzed using a laser scanning confocal microscope (Nikon), with a Hamamatsu ORCA-ER camera (Hamamatsu Photonics KK, Hamamatsu City, Japan). Immunocytochemistry controls for the specificity of the detection methods were carried out by omitting the incubation step with the primary antibodies in one of each four consecutive coverslips. None of the coverslips run without primary antibody showed specific labeling comparable to that obtained with the primary antibodies.
Statistical method.
All data are expressed as means ± SEM of n experiments (each performed in duplicate). Statistical comparison was by nonpaired Student’s t-test. The p values < 0.05 were considered statistically significant.
RESULTS
In this study, we evaluated the contribution of several cellular pathways to the cytotoxic effects of AZA-1 in primary neuronal cultures and its relationship with the AZA-induced JNK activation in CGCs. We have recently shown that AZA-1 is a potent cytotoxic agent in this cellular model (Vale et al., 2007b), and this effect was related with the activation of the JNK kinase since the toxin increased the amount of phosphorylated JNK and caused the nuclear translocation of the phosphorylated protein (Vale et al., 2007a). The cellular routes examined in this study focused on cholinergic receptors, purinergic receptors, GABAA inhibitory receptors, the sodium-potassium pump, voltage-dependent sodium channels, and anion homeostasis. First, we examined the effect of the different drugs on the cytotoxic action of the toxin after 24 h of exposure of CGC to different concentrations of AZA-1. Since we have recently reported a higher cytotoxic effect of AZA-2 in young neurons that seemed to be related with a higher effect on JNK phosphorylation in younger cells (Vale et al., 2008b), all studies were routinely performed in young (2–3 days in vitro [div]) and mature (7–8 div) neurons.
Involvement of Neurotransmitters on AZA-1-Induced Neurotoxicity
In view of the neurological symptoms caused by ip injection of AZA-1 in mice, we tested the involvement of different neurotransmitter systems in the neurotoxic effect of the toxin. Initially, we evaluated the effect of exposure of cerebellar neurons to AZA-1 on glutamate release; however, we found that the toxin did not increase the release of glutamate (data not shown), therefore excluding the involvement of glutamate-induced neurotoxicity in the cytotoxic effect of AZA-1.
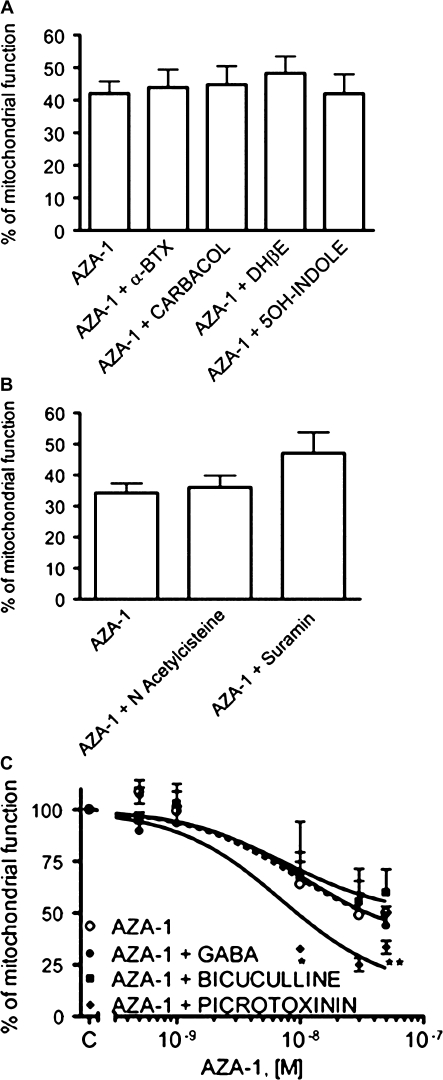
Since the neurological symptoms induced by AZA-1 in mice include paralysis, we evaluated the involvement of the cholinergic system in the AZA-1-induced cytotoxicity. With this purpose, neurons were exposed for 24 h to different concentrations of AZA-1 (0.5–50nM) in the presence of different modulators of the cholinergic function. Figure 1A shows that exposure of CGCs to 50nM AZA-1 for 24 h in the presence of the cholinergic agonists carbamylcholine (Carbachol, 1μM) or 5μM 5-hydroxyindole (5OH-indole) did not modify the cytotoxic effect of AZA-1. Similar results were found after treatment of the neurons with AZA-1 in the presence of the cholinergic antagonists α-bungarotoxin (α-BTX) at 100nM and dihydro-β-erythroidine (DHβE) at 10μM, therefore indicating that AZA-1-induced cytotoxicity is not related with the cholinergic neurotransmitter system.
FIG. 1.
Effect of different neurotransmitter modulators on the AZA-1-induced cytotoxicity as determined with the MTT assay. Neurons were exposed to 50nM AZA-1 for 24 h in the presence of cholinergic modulators (A) or purinergic agonists and antagonists (B). (C) Effect of GABAA receptors modulators on the cytotoxic effect caused after exposure of the cells to different AZA-1 concentrations (0.5–50nM). Values are means ± SEM of three independent experiments, each performed in triplicate. *p < 0.05 and **p < 0.01 versus control cells.
It has been documented that AZA-1 increases 3',S'-Cyclic Adenosine Monophosphate (cAMP) levels in human lymphocytes (Roman et al., 2002), although cAMP modulators did not modify the cytotoxic effect of AZA-1 in CGCs (Vale et al., 2007a). Since extracellular nucleotides regulate cellular function in many cell types via activation of multiple purinergic receptor subtypes, we also evaluated the involvement of purinergic receptors on AZA-1-induced cytotoxicity. Figure 1B shows that exposure of CGCs to 50nM AZA-1 in the presence of the purinergic agonist N-acetylcysteine (NAC) at 10μM or the purinergic antagonist suramin at 100μM did not modify the cytotoxic effect of AZA-1.
Since AZA-1 inhibits electrical activity in spinal cord neurons (Kulagina et al., 2006), we also evaluated the involvement of the GABAergic system on AZA-1-induced neurotoxicity. Figure 1C shows that 24-h treatment of CGCs with different concentrations of AZA-1 in the presence of 100μM GABA or 100μM bicuculline did not modify the effect of AZA-1 on cell viability. However, blockade of the chloride channel associated to the GABAA receptor by preincubation of the cells with 200μM picrotoxinin in the presence of AZA-1 increased AZA-1-induced cytotoxicity at the higher concentrations of the toxin evaluated. Because inhibitory GABAergic neurotransmission is influenced by the internal concentrations of chloride (Blaesse et al., 2009; Vale and Sanes, 2000), we also evaluated the effect of chloride cotransporters inhibition with 200μM furosemide or 200μM bumetanide on AZA-1-induced cytotoxicity, however, neither of these two treatments modified the effect of AZA-1 on cell viability (data not shown).
Effects of AZA-1 on Membrane Potential
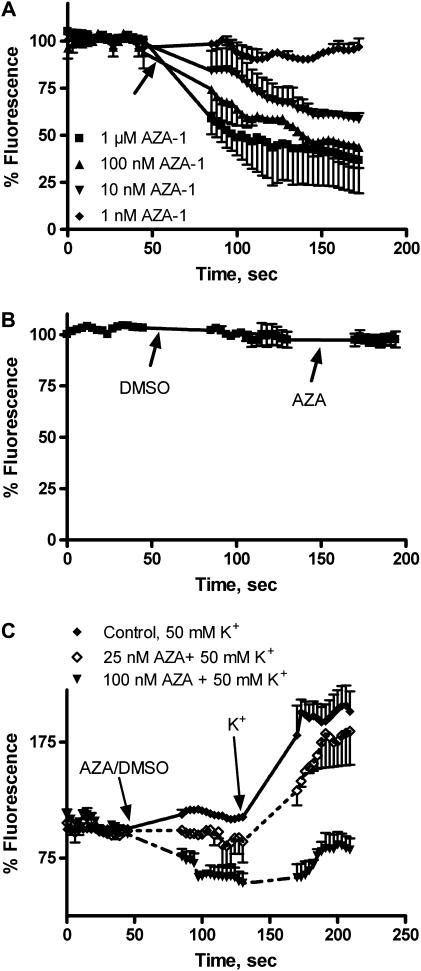
Although initial studies in our laboratory indicated no effect of AZA-1 on membrane potential in human neuroblastoma cells (Roman et al., 2002), an effect of AZA-1 on electrical activity has been recently reported in mouse spinal cord neurons (Kulagina et al., 2006). To further explore the possible action of AZA-1 in membrane potential, cultured neurons were loaded with the membrane potential–sensitive dye DIBAC4(3) (Vale et al., 2008a). In young neurons, AZA-1 (1–1000nM) caused an immediate and dose-dependent decrease in DIBAC4(3) fluorescence, indicating membrane hyperpolarization (Fig. 2A). Significant differences between control and AZA-1-treated cells were found at AZA-1 concentrations equal or higher than 10nM. However, in cultures of 7- to 8-div exposure of the cells to 1μM AZA-1 did not modify the membrane potential (Fig. 2B). In view of the effect of AZA-1 on membrane potential, we also explored the ability of AZA-1 to inhibit stimulus-evoked membrane depolarizations in CGCs of 2–3 div. As Figure 2C shows, 50mM K+ caused an increase in DIBAC4(3) fluorescence, indicating membrane depolarization of CGC, and the K+-evoked depolarization was almost completely blocked (p < 0.001) by preincubation of the cells with 100nM AZA-1 before addition of K+.
FIG. 2.
Effect of AZA-1 on DIBAC4(3) fluorescence in primary cultures of CGCs. In young neurons, AZA-1 (2–3 div) caused a concentration-dependent decrease on DIBAC4(3) fluorescence (A), whereas at the higher concentration employed (1μM), it did not affect DIBAC4(3) fluorescence in cerebellar neurons of 7–8 div (B). (C) At 2–3 div, addition of K+ 50mM caused a dose-dependent increase in DIBAC4(3) fluorescence reflecting membrane depolarization and the effect of 50mM K+ was decreased by preincubation of the cells with AZA-1 25 or 100nM. Data are means ± SEM of three independent experiments, each performed in duplicate. Addition of drugs is indicated by the arrows.
Effect of Anion Channel Blockers on the Neurotoxic Effect of AZA-1 in Primary Cultures of CGCs
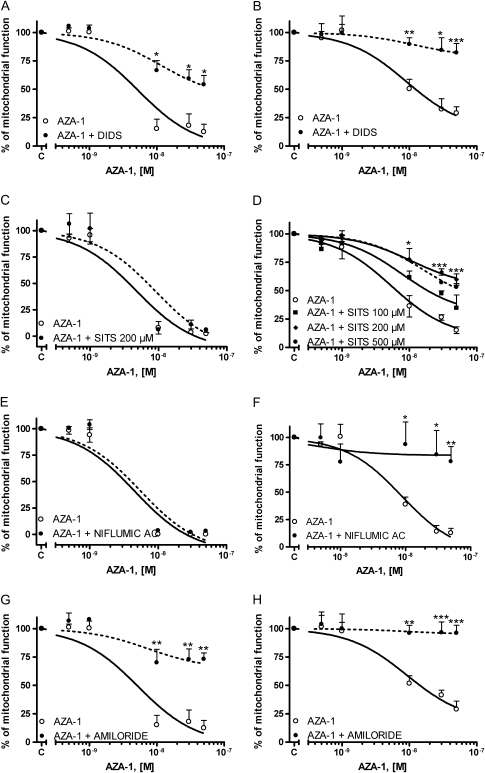
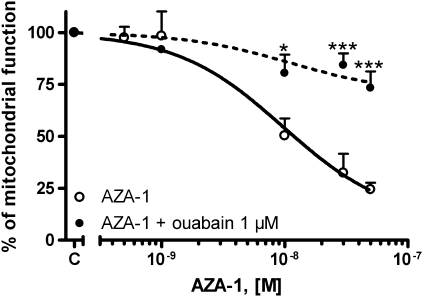
Cellular membrane hyperpolarization is mainly carried by chloride and K+ ions. Therefore, in view of the effect of AZA-1 on membrane potential in CGCs, we further explored the involvement of cellular mechanisms that participate in the maintenance of membrane hyperpolarization, such as the effect of drugs implicated in anion homeostasis, on AZA-1-induced cytotoxicity. With this purpose, we analyzed the effect of several anion channel blockers on the AZA-1-induced neurotoxicity in primary cultures of CGC. First, the effect of AZA-1 on cell viability in CGCs was investigated in the presence of the chloride channel blocker 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS). As shown in Figure 3, treatment of the neurons with 100μM DIDS before addition of AZA-1 significantly decreased the neurotoxic effect of AZA-1 in CGC. However, the neuroprotective effect of DIDS on AZA-induced cytotoxicity was different in cultures of 2–3 div and in 7–8 div neurons. Whereas in young neurons, DIDS only partially, but significantly (p < 0.01), attenuated the AZA-induced cytotoxicity, the neurotoxic effect of AZA was completely reverted at 7–8 div. This apparent difference in the protective effect of DIDS in immature and mature neurons is due to the higher cytotoxicity of AZA-1 in younger cells, as previously observed with AZA-2 (Vale et al., 2008b). In fact, in young cultures, AZA-1 caused an average decrease in cell viability of 90% with an half maximal effective concentration (EC50) of 5.2 × 10−9M (95% confidence intervals from 2.7 × 10−9M to 10.1 × 10−8M). However, under the same conditions, AZA-1 decreased cell viability by 65% at 7–8 div, with an EC50 of 9.2 × 10−9M (95% confidence intervals from 5.9 × 10−9M to 14.3 × 10−8M). Under these conditions, the cytoprotective effect of DIDS was about 42% in younger neurons and 54% in older cultures. In contrast, SITS (4-acetamido-4′-isothiocyanato-2,2′-stilbene-disulfonic acid) another broad-spectrum inhibitor of anion channels did not revert the AZA-1-induced cytotoxicity in 2–3 div CGCs, whereas it partially protected CGC of 7–8 div against AZA-1-induced cytotoxicity. In a similar way, the broad-spectrum chloride channel blocker niflumic acid at 100μM completely reverted the cytotoxic effect of AZA-1 in mature neurons, however, it did not affect the AZA-1-induced cytotoxicity in 2–3 div CGCs (Fig. 3). To further explore the role of anion homeostasis on the AZA-1-induced cytotoxicity, we evaluated the effect of amiloride as an antagonist of Na+/H+ exchangers. Pretreatment of the cells with 100μM amiloride before exposure to different concentrations of AZA-1 (0.5–50nM) during 24 h completely eliminated the cytotoxic effect of AZA-1 in older neurons and greatly attenuated the cytotoxic effect of AZA-1 in younger cultures (Fig. 3). Again, the apparent difference in the neuroprotective effect of amiloride in young and mature neurons is due to the higher cytotoxicity of AZA-1 in immature neurons. In fact, amiloride decreased the cytotoxic effect of AZA-1 by 61% in young neurons and 68% in older cultures. Altogether, these data indicate that AZA-1 neurotoxicity was related with changes in ion homeostasis. Therefore, to gain more insight on the role of altered ionic homeostasis on AZA-1-induced cytotoxicity, we evaluated the possible involvement of intracellular K+ on the cytotoxic effect of AZA-1. With this purpose, the Na+-K+ pump was blocked with a low concentration of ouabain thus preventing K+ efflux through the Na+-K+ pump. CGC were exposed to ouabain 1μM before treatment with different concentrations of AZA-1 during 24 h. Figure 4 shows that ouabain significantly reverted the cytotoxic effect of AZA-1 in cultures of 7–8 div, thus indicating that K+ efflux is involved in the neurotoxic effect of AZA-1. The effect of ouabain on AZA-1 cytotoxicity in young cultures could not be evaluated because even low concentrations of ouabain decreased cell viability at 2–3 div (data not shown). In view of the protective effect of DIDS and amiloride against AZA-1-induced neurotoxicity, we evaluated whether long-term (4 h) exposure of the neurons to the toxin (50nM) caused changes in intracellular pH; however, we did not observe any modification in intracellular pH even after 4 h of treatment (data not shown).
FIG. 3.
Effect of blockers of anion channels on the AZA-1-induced cytotoxicity as determined with the MTT assay in young and mature neurons. Neurons of 2–3 div (left) or 7–8 div (right) were exposed to different AZA-1 concentrations (0.5–50nM) for 24 h in the presence of 100μM DIDS (A and B), SITS (C and D), 100μM niflumic acid (E and F), or 100μM amiloride (G and H). Values are means ± SEM of three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.005 versus control cells.
FIG. 4.
Effect of blockade of the Na+-K+-ATPase on the AZA-1-induced cytotoxicity as determined with the MTT assay neurons of 7–8 div. Cerebellar neurons were exposed to different AZA-1 concentrations (0.5–50nM) for 24 h in the presence of 1μM ouabain. Values are means ± SEM of three independent experiments. *p < 0.05 and ***p < 0.005 versus control cells.
Effect of Anion Channel Blockers on the AZA-1-Induced JNK Activation
We have recently reported that AZA-1-induced cytotoxicity is associated with activation of p-JNK in cultured neurons (Vale et al., 2007a) and that selective blockade of JNK also blocks the AZA-1-induced neurotoxicity. Therefore, we examined the effect of the different anion blockers that showed protection against AZA-1-induced toxicity on the AZA-1-induced JNK activation. With this aim, cerebellar cultures were treated either with 50nM AZA-1 for 4 h or with AZA-1 in combination with the different anion blockers. Figures 5 and 6 show that the AZA-1-induced increase in p-JNK and the nuclear translocation of the phosphorylated protein were ameliorated in the presence of DIDS or amiloride both at 3 (Fig. 5) and at 7 div (Fig. 6).
FIG. 5.
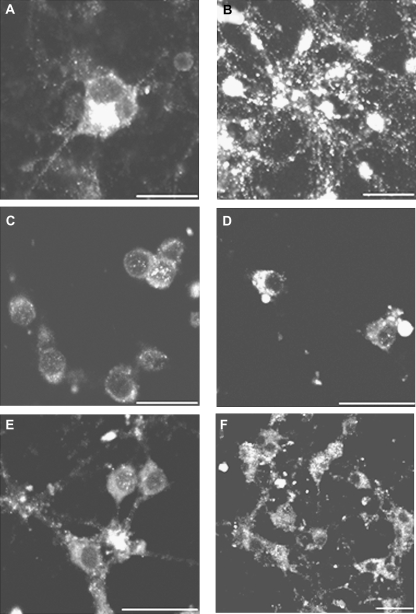
Exposure of CGCs of 3 div to 50nM AZA-1 during 4 h alters p-JNK staining and the cellular localization of active-JNK. (A) Control neurons showed moderate p-JNK immunoreactivity localized to processes and cell bodies. (B) Cells treated with 50nM AZA-1 showed intense p-JNK staining covering the cell soma and nuclei. Treatment of the neurons with 100μM DIDS (C) or 100μM amiloride (E) did not modify p-JNK immunoreactivity. However, pretreatment of the cells with DIDS (D) or amiloride (F) before addition of AZA-1 decreased the nuclear translocation of p-JNK caused by AZA-1. Scale bar is 20 μm.
FIG. 6.
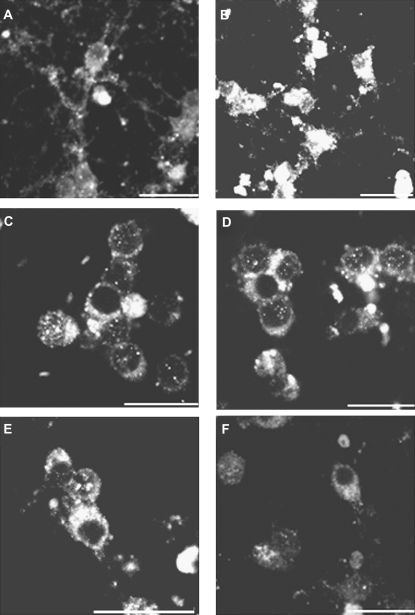
Chloride channel blockers prevented the increase in p-JNK staining caused by AZA-1 in neurons of 7 div. (A) Control neurons showed moderate p-JNK immunoreactivity localized to processes and cell bodies. (B) Cells treated with 50nM AZA-1 showed intense p-JNK staining covering the cell soma and nuclei. Treatment of the neurons with DIDS (C) or amiloride (E) did not modify p-JNK immunoreactivity. Pretreatment of the cells with 100μM DIDS (D) or 100μM amiloride (F) before addition of AZA-1 decreased the nuclear translocation of p-JNK caused by AZA-1. Scale bar is 20 μm.
Effect of AZA-1 on Cell Volume
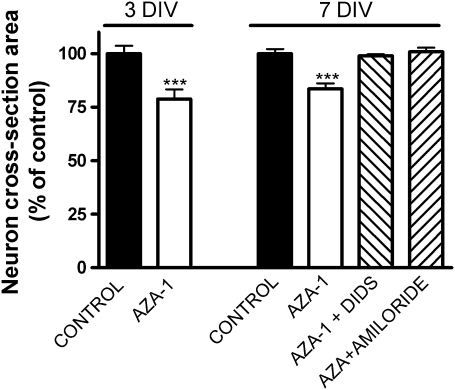
Nowadays, it is still unclear whether the AZA-1-induced cell damage is a consequence of necrotic or apoptotic mechanisms, or both (Kellmann et al., 2009). Although caspase activation by AZA has been recently reported in neuroblastoma cells, the cytoskeletal changes induced by AZA-1 preceded caspase activation (Vilarino et al., 2007). It is now known that anion channel blockers attenuate delayed neuronal death in a variety of cell types, and it has recently become evident that one of the early events in apoptosis is the normotonic shrinkage of cells mediated by the efflux of K+ and Cl− ions that produce apoptotic volume decrease (AVD) of the cell (Maeno et al., 2000). In view of the protective effects of anion blockers against AZA-1-induced cytotoxicity, we explored whether cell volume was altered after AZA treatment. In order to do this, CGCs were exposed for 1 h to AZA-1 10nM either in the presence or absence of anion channel blockers. Figure 7 shows that short exposure times (1 h) to 10nM AZA significantly decreased the neuron cross-sectional area by 22% at 3 div and 17% at 7 div. Furthermore, pretreatment of the neurons with DIDS or amiloride before addition of AZA-1 prevented the cell shrinkage induced by AZA-1. Similar results were found with lower toxin concentrations. Exposure of CGCs to 1nM AZA-1 for 1 h decreased the neuron cross-sectional area by 19% at 3 div and 14% at 7 div. Furthermore, the effect of AZA-1 on cell volume seemed to be very fast since cell volume changes were already evident after 20 min of exposure of the cells to 10nM AZA-1.
FIG. 7.
Exposure of CGCs to 10nM AZA-1 for 1 h significantly decreased the cellular volume measured as the neuron cross-sectional area, both at 3 div and at 7 div. At 7 div, the decrease in cell volume after exposure of CGCs to AZA-1 was reverted by preincubation of the cells with either 100μM DIDS or 100μM amiloride before addition of AZA-1. Values are means ± SEM of three independent experiments. ***p < 0.001 versus control neurons.
DISCUSSION
The aim of this work was to investigate the mechanisms involved in AZA-induced activation of JNK, recently reported in cerebellar neurons (Vale et al., 2007a, 2008b). Several potential targets for AZA have been evaluated, involving cholinergic and purinergic receptors and inhibitory GABAA receptors. We failed to observe a neuroprotective effect of cholinergic, purinergic, and GABAA modulators against AZA-1-induced cytotoxicity in cultured neurons. Similar results were obtained with the blockers of chloride cotransport bumetanide and furosemide. Surprisingly, the evaluation of AZA effect on membrane potential indicated that AZA-1 caused membrane hyperpolarization in young neurons, but it did not affect membrane potential in adult neurons. The inhibitory effect of AZA-1 on membrane potential in young neurons is in agreement with previous reports, indicating an inhibitory effect of AZA on spike rate in spinal neurons (Kulagina et al., 2006), although the mechanism for this inhibitory action of AZA-1 is not fully elucidated yet. However, the different effect of AZA-1 on membrane potential in young and adult cultured neurons could be attributable to the developmental properties of this neuronal model. In fact, in many neuronal cells, the resting membrane potential shifts from a relatively depolarized state to a more depolarized one during development (Nakanishi and Okazawa, 2006). The granule cell in the cerebellum is one such neuronal cell that changes its resting membrane potential and intrinsic membrane properties during development (Cathala et al., 2003).
In neurons, inhibitory neurotransmission is mainly carried out by chloride entry through chloride channels into the cells. Chloride influx can be mediated by neurotransmitters, voltage-gated chloride channels, or electroneutral chloride cotransporters. Cytotoxicity experiments indicated that GABAergic inhibitors and chloride cotransporters were not involved in the cytotoxic effect of AZA-1. Interestingly, in this neuronal system, anion channel blockers and ouabain greatly ameliorated the cytotoxic effect of AZA-1 in immature neurons and completely eliminated it in older cultures. This difference in the neuroprotection elicited by anion channel blockers is due to the higher cytotoxic effect of AZA-1 in younger cells, as previously described for AZA-2 in the same neuronal model (Vale et al., 2008b).
In recent years, it has become evident that efflux of K+ and Cl− leads to AVD of the cell. Both intrinsic and extrinsic apoptotic stimuli have been reported to rapidly activate Cl− conductances in a large variety of cell types (Okada et al., 2006). The central hypothesis is that several apoptotic events such as cell body shrinkage, caspase-3 cleavage, cytochrome c release, endonuclease activation, and, finally, apoptotic death are mediated by a dramatic disruption of ion homeostasis, mainly due to loss of intracellular K+ via potassium permeable channels and/or a failure of the Na+-K+-ATPase (Yu, 2003). Moreover, emerging evidence suggests that the cell volume regulatory mechanisms and the activity of Cl− channels, in conjunction with K+ movement, may also play important roles in apoptosis (Maeno et al., 2000; Wei et al., 2004), and chloride and potassium channel blockers are known to inhibit apoptosis in cortical neurons (Wei et al., 2004). Since AZAs have been recently shown to produce delayed caspase activation after cytoskeletal disruption (Vilarino et al., 2007), we hypothesized that the cytoprotective effect of anion channel blockers against AZA-induced cytotoxicity could be related to the apoptotic effect of these toxins. In fact, the results presented here indicated that the cytoprotective effects of anion channel blockers against AZA-1 cytotoxicity are presumably due to inhibition of AZA-1-induced apoptosis by these compounds. On the basis of early occurrence of cell shrinkage and its relationship to other apoptotic events, it has become evident that cell volume decrease mediated by K+ and Cl− efflux may be a prerequisite for apoptosis (Maeno et al., 2000, 2006). Indeed, short exposures of the neurons to nontoxic concentrations of AZA (1 and 10nM, during 30 or 60 min) significantly decreased the neuron cross-sectional area, an effect similar to the effect of the apoptosis inducers staurosporine or ceramide in cortical cells (Wei et al., 2004). Furthermore, the cell shrinkage caused by AZAs was completely reverted in the presence of DIDS or amiloride, thus indicating that the neuroprotective action of anion channel blockers against AZA-1-induced cytotoxicity was mediated by the effect of the toxin on cell volume.
We have recently reported that inhibition of the JNK activation prevents the cytotoxic effect of AZA and exposure of the neurons to AZA causes JNK activation in this same neuronal system (Vale et al., 2007a, 2008b), and hypothesized that this kinase was involved in the neurotoxic effect of AZAs. Moreover, JNK activation was also observed previously, after exposure of CGCs to AZA-2, and was higher in 2–3 div neurons than in 7–8 div cultures correlating well with the cytotoxicity of this AZA analog in cerebellar neurons (Vale et al., 2008b). In light of the results presented here, we evaluated the effect of DIDS and amiloride on AZA-1-induced JNK activation. Interestingly, we found that preincubation of the cells with DIDS or amiloride before addition of AZA completely eliminated the AZA-induced JNK activation and nuclear translocation of the protein. The JNK kinase is activated in response to a variety of stimulus, including apoptosis in CGCs (Mei et al., 2008; Mundy and Freudenrich, 2006). Although we could suggest that AZA-1 could interact with transmembrane proteins and disturb the ion concentration inside the cell, it seems most likely that the JNK activation caused by AZA-1 is an intermediate event in the apoptotic process caused by the toxin. Another possibility that remains to be explored is the interaction of AZA-1 with volume-sensitive chloride channels (VSOR). Although activation of the VSOR Cl− channel is induced by cell swelling, activation of the channel takes place during the AVD process in nonswollen or even shrunken apoptotic cells (Okada et al., 2006). Since this apoptosis-associated activity of the VSOR Cl− channel subsides upon more drastic cell shrinkage induced by hypertonicity, the threshold cell volume at which channel opening is triggered must be shifted to a lower level in apoptotic cells (Cannon et al., 1998; Shimizu et al., 2004). Reactive oxygen species are known to be involved in the activation of the VSOR Cl− channel (Okada et al., 2006); however, we have recently shown that AZA-1, even at concentrations of 250nM, did not modify the formation of peroxides in CGCs (Vale et al., 2007a). A direct activation of VSOR Cl− channels by AZA-1 could be supported by the fact that the cytotoxic effect of AZA-1 was blocked by VSOR Cl− channel blockers but not by blockers of GABAA receptors or chloride cotransporters, as previously observed after activation of VSOR Cl− channels in the presence of N-methyl-D-aspartic acid (Okada et al., 2006). However, the cytoprotective effect of ouabain against AZA-1-induced cytotoxicity suggests that the cytoprotective effect of anion channel blockers against AZA-induced cytotoxicity is mainly due to the preventive effect of these compounds against apoptosis (Gulbins et al., 2000; Heimlich and Cidlowski, 2006). With regard to the neuroprotective effect of amiloride against AZA-1-induced cytotoxicity, it should be pointed out that activation of the Na+/H+ exchanger is dependent on intracellular chloride (Aharonovitz et al., 2001; Goss et al., 2001; Hogan et al., 1997) and requires JNK activation (Goss et al., 2001). Altogether, the results presented here indicate that AZA-1-induced JNK activation is secondary to the decrease in cell volume, presumably during an apoptotic insult, initiated by the toxin. Notwithstanding, several intra and extracellular events can be responsible for JNK activation in neuronal cells (Davis, 2000), and our data indicate that AZA-1 cytotoxicity is linked with anion channel disturbances.
FUNDING
The Skaggs Institute for Chemical Biology; National Institutes of Health (USA); a predoctoral fellowship from the National Science Foundation to M.O.F.; Amgen and Merck grants to K.C.N.; Ministerio de Ciencia y Tecnología, Spain (AGL2006-08439/ALI, AGL2007-60946/ALI); Xunta de Galicia, Spain (GRC 30/2006, PGIDIT 07MMA006261PR, PGIDT07CSA012261PR); and 2009/XA044 from Conselleria de Educacion e Ordenación Universitaria, 2008/CP389 (EPITOX, Consellería de Innovación e Industria, programa IN.CI.TE.); EU VIth Frame Program: IP FOOD-CT-2004-06988 (BIOCOP) and CRP 030270-2 (SPIES-DETOX); EU VIIth Frame Program: 211326—CP (CONffIDENCE); and STC-CP2008-1-555612 (Atlantox) to Spanish work.
References
- Aharonovitz O, Kapus A, Szaszi K, Coady-Osberg N, Jancelewicz T, Orlowski J, Grinstein S. Modulation of Na+/H+ exchange activity by Cl. Am. J. Physiol. Cell Physiol. 2001;281:C133–C141. doi: 10.1152/ajpcell.2001.281.1.C133. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Cannon CL, Basavappa S, Strange K. Intracellular ionic strength regulates the volume sensitivity of a swelling-activated anion channel. Am. J. Physiol. 1998;275:C416–C422. doi: 10.1152/ajpcell.1998.275.2.C416. [DOI] [PubMed] [Google Scholar]
- Cathala L, Brickley S, Cull-Candy S, Farrant M. Maturation of EPSCs and intrinsic membrane properties enhances precision at a cerebellar synapse. J. Neurosci. 2003;23:6074–6085. doi: 10.1523/JNEUROSCI.23-14-06074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell KB, Wright SH, Emma F, Rosenberg PA, Strange K. NMDA receptor activation inhibits neuronal volume regulation after swelling induced by veratridine-stimulated Na+ influx in rat cortical cultures. J. Neurosci. 1996;16:7447–7457. doi: 10.1523/JNEUROSCI.16-23-07447.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Goss GG, Jiang L, Vandorpe DH, Kieller D, Chernova MN, Robertson M, Alper SL. Role of JNK in hypertonic activation of Cl(-)-dependent Na(+)/H(+) exchange in Xenopus oocytes. Am. J. Physiol. Cell Physiol. 2001;281:C1978–C1990. doi: 10.1152/ajpcell.2001.281.6.C1978. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Jekle A, Ferlinz K, Grassme H, Lang F. Physiology of apoptosis. Am. J. Physiol. Renal Physiol. 2000;279:F605–F615. doi: 10.1152/ajprenal.2000.279.4.F605. [DOI] [PubMed] [Google Scholar]
- Heimlich G, Cidlowski JA. Selective role of intracellular chloride in the regulation of the intrinsic but not extrinsic pathway of apoptosis in Jurkat T-cells. J. Biol. Chem. 2006;281:2232–2241. doi: 10.1074/jbc.M507367200. [DOI] [PubMed] [Google Scholar]
- Hogan EM, Davis BA, Boron WF. Intracellular Cl-dependence of Na-H exchange in barnacle muscle fibers under normotonic and hypertonic conditions. J. Gen. Physiol. 1997;110:629–639. doi: 10.1085/jgp.110.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E, Satake M, Ofuji K, Higashi M, Harigaya K, McMahon T, Yasumoto T. Chronic effects in mice caused by oral administration of sublethal doses of azaspiracid, a new marine toxin isolated from mussels. Toxicon. 2002;40:193–203. doi: 10.1016/s0041-0101(01)00226-4. [DOI] [PubMed] [Google Scholar]
- Ito E, Satake M, Ofuji K, Kurita N, McMahon T, James K, Yasumoto T. Multiple organ damage caused by a new toxin azaspiracid, isolated from mussels produced in Ireland. Toxicon. 2000;38:917–930. doi: 10.1016/s0041-0101(99)00203-2. [DOI] [PubMed] [Google Scholar]
- James KJ, Sierra MD, Lehane M, Brana Magdalena A, Furey A. Detection of five new hydroxyl analogues of azaspiracids in shellfish using multiple tandem mass spectrometry. Toxicon. 2003;41:277–283. doi: 10.1016/s0041-0101(02)00288-x. [DOI] [PubMed] [Google Scholar]
- Kellmann R, Schaffner CA, Gronset TA, Satake M, Ziegler M, Fladmark KE. Proteomic response of human neuroblastoma cells to azaspiracid-1. J. Proteomics. 2009;72:695–707. doi: 10.1016/j.jprot.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Twiner MJ, Hess P, McMahon T, Satake M, Yasumoto T, Ramsdell JS, Doucette GJ, Ma W, O’Shaughnessy TJ. Azaspiracid-1 inhibits bioelectrical activity of spinal cord neuronal networks. Toxicon. 2006;47:766–773. doi: 10.1016/j.toxicon.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno E, Shimizu T, Okada Y. Normotonic cell shrinkage induces apoptosis under extracellular low Cl conditions in human lymphoid and epithelial cells. Acta Physiol. (Oxf.) 2006;187:217–222. doi: 10.1111/j.1748-1716.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- Mei Y, Yuan Z, Song B, Li D, Ma C, Hu C, Ching YP, Li M. Activating transcription factor 3 up-regulated by c-Jun NH(2)-terminal kinase/c-Jun contributes to apoptosis induced by potassium deprivation in cerebellar granule neurons. Neuroscience. 2008;151:771–779. doi: 10.1016/j.neuroscience.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM. Apoptosis of cerebellar granule cells induced by organotin compounds found in drinking water: involvement of MAP kinases. Neurotoxicology. 2006;27:71–81. doi: 10.1016/j.neuro.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Okazawa M. Membrane potential-regulated Ca2+ signalling in development and maturation of mammalian cerebellar granule cells. J. Physiol. 2006;575:389–395. doi: 10.1113/jphysiol.2006.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou KC, Chen DY, Li Y, Qian W, Ling T, Vyskocil S, Koftis TV, Govindasamy M, Uesaka N. Total synthesis of the proposed azaspiracid-1 structure, part 2: coupling of the C1-C20, C21-C27, and C28-C40 fragments and completion of the synthesis. Angew. Chem. Int. Ed. Engl. 2003;42:3649–3653. doi: 10.1002/anie.200351826. [DOI] [PubMed] [Google Scholar]
- Nicolaou KC, Koftis TV, Vyskocil S, Petrovic G, Tang W, Frederick MO, Chen DY, Li Y, Ling T, Yamada YM. Total synthesis and structural elucidation of azaspiracid-1. Final assignment and total synthesis of the correct structure of azaspiracid-1. J. Am. Chem. Soc. 2006;128:2859–2872. doi: 10.1021/ja054750q. [DOI] [PubMed] [Google Scholar]
- Ofuji K, Satake M, McMahon T, James KJ, Naoki H, Oshima Y, Yasumoto T. Structures of azaspiracid analogs, azaspiracid-4 and azaspiracid-5, causative toxins of azaspiracid poisoning in Europe. Biosci. Biotechnol. Biochem. 2001;65:740–742. doi: 10.1271/bbb.65.740. [DOI] [PubMed] [Google Scholar]
- Ofuji K, Satake M, McMahon T, Silke J, James KJ, Naoki H, Oshima Y, Yasumoto T. Two analogs of azaspiracid isolated from mussels, Mytilus edulis, involved in human intoxication in Ireland. Nat. Toxins. 1999a;7:99–102. doi: 10.1002/(sici)1522-7189(199905/06)7:3<99::aid-nt46>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Ofuji K, Satake M, Oshima Y, McMahon T, James KJ, Yasumoto T. A sensitive and specific determination method for azaspiracids by liquid chromatography mass spectrometry. Nat. Toxins. 1999b;7:247–250. doi: 10.1002/1522-7189(199911/12)7:6<247::aid-nt68>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Okada Y, Shimizu T, Maeno E, Tanabe S, Wang X, Takahashi N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J. Membr. Biol. 2006;209:21–29. doi: 10.1007/s00232-005-0836-6. [DOI] [PubMed] [Google Scholar]
- Rehmann N, Hess P, Quilliam MA. Discovery of new analogs of the marine biotoxin azaspiracid in blue mussels (Mytilus edulis) by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:549–558. doi: 10.1002/rcm.3385. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Alfonso A, Vale C, Alfonso C, Vale P, Tellez A, Botana LM. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008;80:5622–5629. doi: 10.1021/ac800769e. [DOI] [PubMed] [Google Scholar]
- Roman Y, Alfonso A, Louzao MC, de la Rosa LA, Leira F, Vieites JM, Vieytes MR, Ofuji K, Satake M, Yasumoto T, et al. Azaspiracid-1, a potent, nonapoptotic new phycotoxin with several cell targets. Cell. Signal. 2002;14:703–716. doi: 10.1016/s0898-6568(02)00015-3. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl(-) channel. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6770–6773. doi: 10.1073/pnas.0401604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiner MJ, Hess P, Dechraoui MY, McMahon T, Samons MS, Satake M, Yasumoto T, Ramsdell JS, Doucette GJ. Cytotoxic and cytoskeletal effects of azaspiracid-1 on mammalian cell lines. Toxicon. 2005;45:891–900. doi: 10.1016/j.toxicon.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Twiner MJ, Rehmann N, Hess P, Doucette GJ. Azaspiracid shellfish poisoning: a review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Mar. Drugs. 2008;6:39–72. doi: 10.3390/md20080004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Alfonso A, Vieytes MR, Romaris XM, Arevalo F, Botana AM, Botana LM. In vitro and in vivo evaluation of paralytic shellfish poisoning toxin potency and the influence of the pH of extraction. Anal. Chem. 2008a;80:1770–1776. doi: 10.1021/ac7022266. [DOI] [PubMed] [Google Scholar]
- Vale C, Gomez-Limia B, Nicolaou KC, Frederick MO, Vieytes MR, Botana LM. The c-Jun-N-terminal kinase is involved in the neurotoxic effect of azaspiracid-1. Cell. Physiol. Biochem. 2007a;20:957–966. doi: 10.1159/000110456. [DOI] [PubMed] [Google Scholar]
- Vale C, Nicolaou KC, Frederick MO, Gomez-Limia B, Alfonso A, Vieytes MR, Botana LM. Effects of azaspiracid-1, a potent cytotoxic agent, on primary neuronal cultures. A structure-activity relationship study. J. Med. Chem. 2007b;50:356–363. doi: 10.1021/jm061063g. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J. Neurosci. 2000;20:1912–1921. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Wandscheer C, Nicolaou KC, Frederick MO, Alfonso C, Vieytes MR, Botana LM. Cytotoxic effect of azaspiracid-2 and azaspiracid-2-methyl ester in cultured neurons: involvement of the c-Jun N-terminal kinase. J. Neurosci. Res. 2008b;86:2952–2962. doi: 10.1002/jnr.21731. [DOI] [PubMed] [Google Scholar]
- Varming T, Drejer J, Frandsen A, Schousboe A. Characterization of a chemical anoxia model in cerebellar granule neurons using sodium azide: protection by nifedipine and MK-801. J. Neurosci. Res. 1996;44:40–46. doi: 10.1002/(SICI)1097-4547(19960401)44:1<40::AID-JNR5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Vilarino N, Nicolaou KC, Frederick MO, Vieytes MR, Botana LM. Irreversible cytoskeletal disarrangement is independent of caspase activation during in vitro azaspiracid toxicity in human neuroblastoma cells. Biochem. Pharmacol. 2007;74:327–335. doi: 10.1016/j.bcp.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Volmer DA, Brombacher S, Whitehead B. Studies on azaspiracid biotoxins. I. Ultrafast high-resolution liquid chromatography/mass spectrometry separations using monolithic columns. Rapid Commun. Mass Spectrom. 2002;16:2298–2305. doi: 10.1002/rcm.851. [DOI] [PubMed] [Google Scholar]
- Wei L, Xiao AY, Jin C, Yang A, Lu ZY, Yu SP. Effects of chloride and potassium channel blockers on apoptotic cell shrinkage and apoptosis in cortical neurons. Pflugers Arch. 2004;448:325–334. doi: 10.1007/s00424-004-1277-2. [DOI] [PubMed] [Google Scholar]
- Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog. Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]