Abstract
Concentrations of extracellular glycine in the central nervous system are regulated by Na+/Cl−-dependent glycine transporters, GLYT1 and GLYT2. N-Arachidonylglycine (NAGly) is an endogenous inhibitor of GLYT2 with little or no effect on GLYT1 and is analgesic in rat models of neuropathic and inflammatory pain. Understanding the molecular basis of NAGly interactions with GLYT2 may allow for the development of novel therapeutics. In this study, chimeric transporters were used to determine the structural basis for differences in NAGly sensitivity between GLYT1 and GLYT2 and also the actions of a series of related N-arachidonyl amino acids. Extracellular loops 2 and 4 of GLYT2 are important in the selective inhibition of GLYT2 by NAGly and by the related compounds N-arachidonyl-γ-aminobutyric acid and N-arachidonyl-d-alanine, whereas only the extracellular loop 4 of GLYT2 is required for N-arachidonyl-l-alanine inhibition of transport. These observations suggest that the structure of the head group of these compounds is important in determining how they interact with extracellular loops 2 and 4 of GLYT2. Site-directed mutagenesis of GLYT2 EL4 residues was used to identify the key residues Arg531, Lys532, and Ile545 that contribute to the differences in NAGly sensitivity.
Introduction
Glycine is an inhibitory neurotransmitter in the spinal cord and brain stem (1) and plays important roles in motor reflex circuits and in regulation of pain sensory neurons (2). Regulation of glycine concentrations by glycine transporters plays a key role in maintaining the dynamic signaling process between neurons (3–5), and recent studies suggest that pharmacological manipulation of glycine transporters may provide a means for treating neurological disorders such as neuropathic pain (6). Furthermore, manipulation of endogenous processes that influence glycine transporter function may also provide leads in the development of novel therapeutic approaches in the treatment of pain.
N-Arachidonylglycine (NAGly)4 is an endogenous derivative of arachidonic acid that is produced in highest concentrations in the spinal cord (7). Intrathecal injection of NAGly provides analgesia in rat models of neuropathic pain (8) and also inflammatory pain (9). We have previously characterized the actions of NAGly on the glycine transporter GLYT2 and found that it inhibits GLYT2 with an IC50 of 3 μm, with little or no activity at GLYT1 or the GABA transporter GAT1 at concentrations up to 100 μm (10). Therefore, NAGly may be considered an endogenous inhibitor of GLYT2 that has the capacity to modulate nociception. It is of interest to understand the molecular basis of NAGly/GLYT2 interactions to further develop the analgesic potential of this class of compounds.
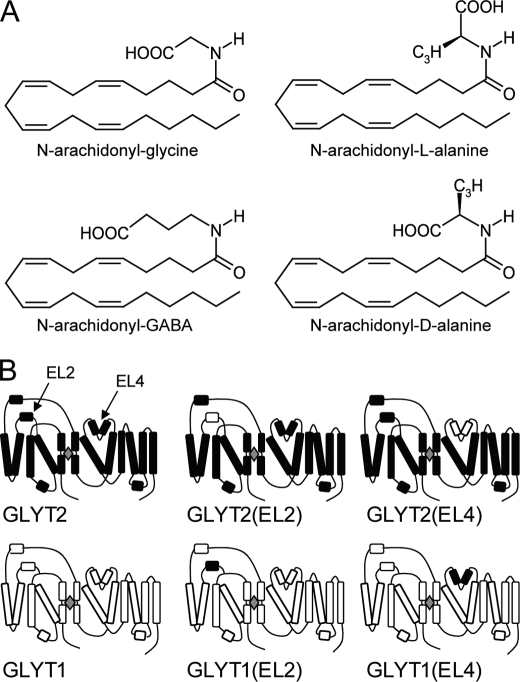
Glycine transporters are members of the neurotransmitter Na+ symporter family of transporters that includes transporters for GABA, dopamine, serotonin, and norepinephrine (11). The crystal structure of a bacterial homolog of this family, LeuTAa, has recently been determined in the presence and absence of a noncompetitive blocker, clomipramine (12, 13). There are two lines of evidence that suggest that extracellular loops 2 and 4 may play important roles in formation of noncompetitive inhibitor binding sites. First, one of the crucial changes in the structure of LeuTAa when bound to clomipramine is that extracellular loop 4 (EL4) is displaced relative to the structure in the absence of the inhibitor (12, 13). Second, it has previously been demonstrated that Zn2+ ions noncompetitively inhibit glycine transport by GLYT1, and histidine residues located on extracellular loops 2 and 4 have been shown to mediate these effects (14). This suggests that restrictions or alterations in the movement of these extracellular loops may provide a mechanism for inhibiting glycine transport. GLYT2 contains a significantly larger extracellular loop 2 (EL2) than GLYT1 (supplemental Fig. 1), and we hypothesized that this loop may contain structural features that mediate the actions of NAGly. In this study, we demonstrate that EL2 and EL4 of GLYT2 are required for NAGly inhibition. The related compound N-arachidonyl-l-alanine (NALAla) also inhibits GLYT2, but although EL4 of GLYT2 is required for this activity, EL2 is not. We also investigated the specificity of interactions by utilizing the additional related compounds N-arachidonyl-γ-aminobutyric acid (NAGABA) and N-archidonyl-d-alanine (NADAla). Furthermore, we have shown that the GLYT2 EL4 residues Arg531, Lys532, and Ile545 influence NAGly and NALAla sensitivity. The structures of the N-arachidonyl-amino acids (NAAAs) used in this study are depicted (see Fig. 1A).
FIGURE 1.
Structures of compounds and schematic diagrams of transporters. A, chemical structures of the N-arachidonyl amino acids investigated in this study. B, a schematic diagram of the topology of the wild type and chimeric transporters GLYT2, GLYT2(EL2), GLYT2(EL4), GLYT1, GLYT1(EL2), and GLYT1(EL4). Black indicates the regions of the transporter from GLYT2, and white indicates the regions from GLYT1.
EXPERIMENTAL PROCEDURES
Chemicals
NAGly, NALAla, and NAGABA were obtained from Cayman Chemical Company. Synthesis of the d-alanine analogue was performed using a procedure adapted from Bezuglov et al. (15). This involved initial formation of the mixed anhydride derived from arachidonic acid and isobutyl chloroformate in acetonitrile at 0 °C followed by reaction with the d-alanine methyl ester hydrochloride. Saponification of the ester provided the desired compound in modest yield as confirmed by 1H NMR spectroscopy (see supplemental data for a more detailed description). All NAAAs were stored at −20 °C in ethanol, and stocks were diluted on the day of use and kept on ice. Restriction enzymes were purchased from Promega Industries Ltd. QIAprep Miniprep and QIAquick Spin kits were obtained from Qiagen. All other chemicals were obtained from Sigma unless stated otherwise.
Construction of GLYT Chimeras
GLYT1 and GLYT2 transporters were used to construct chimeric transporters in which EL2 and/or EL4 were switched. A variation of the fusion PCR technique, described by Shevchuk et al. (16), was used. Supplemental Table 1 shows the DNA sequences of the oligonucleotide primers used to construct the chimeras. Briefly, PCR amplification of fragments of GLYT1 and GLYT2 were performed using tailed oligonucleotides to introduce sequences homologous to GLYT2 or GLYT1, respectively, at the desired chimeric junction sites. Individual fragments were “fused” together in a separate reaction, with the overlapping homologous regions of each fragment serving as priming sites for elongation by DNA polymerase (see supplemental Fig. 2). The resulting chimeras were then amplified with oligonucleotides containing restriction enzyme recognition sites to facilitate subcloning into pOTV (oocyte transcription vector) (17). The sequences of the junction sites were confirmed by DNA sequencing. Fig. 1B is a schematic diagram of the predicted topology of each of the chimeras. Chimeras are named according to the parent transporter with the region switched in brackets. For example, GLYT2(EL4) refers to a chimera that is derived from GLYT2 but contains EL4 from GLYT1, and GLYT1(EL2) refers to a chimera that is derived from GLYT1, with EL2 from GLYT2.
Generation of GLYT2 Point Mutations
All residues in EL4 of GLYT2 that differed from GLYT1 were mutated to the corresponding residue in GLYT1. Eleven mutations in GLYT2 were constructed using the QuikChange mutagenesis kit (Stratagene) including E530H, R531L, K532G, N534D, I535V, E536S, N537R, Q541H, I545L, V549A, and R556L.
Oocyte Preparation and Electrophysiology
The wild type, chimera, and point mutation cDNAs were linearized with SpeI and cRNA transcribed with T7 RNA polymerase and capped with 5′-7-methyl guanosine using the mMessage mMachine kit (Ambion Inc.). Oocytes were harvested from Xenopus laevis, as previously described (18) with all procedures in accordance with the Australian National Health and Medical Research Council guidelines for the prevention of cruelty to animals. 50 nl of cRNA was injected into defoliculated, stage V oocytes and incubated at 16 °C in standard frog ringers solution (ND96; 96 mm NaCl, 2 mm KCl, 1 mm MgCl2, 1.8 mm CaCl2, 5 mm HEPES, pH 7.55) supplemented with 2.5 mm sodium pyruvate, 0.5 mm theophylline, 50 μg/ml gentamicin. 2–5 days later, glycine and sarcosine transport current recordings were made using the two-electrode voltage clamp technique with a GeneClamp 500 amplifier (Axon Instruments, Foster City, CA) interfaced with a MacLab 2e chart recorder (ADI Instruments, Sydney, Australia) using Chart software (ADI Instruments).
Concentration-dependent glycine and sarcosine transport current measurements were fitted to an equation derived from the Michaelis-Menten equation,
where I is the current, Imax is the current produced by a maximal concentration of substrate, and EC50 is the concentration of substrate required to produce half-maximal response.
NAGly, NALAla and NAGABA inhibit GLYT2 with little or no activity on GLYT1 (10). The actions of these compounds and also of NADAla on the chimeric transporters were assessed by applying a fixed concentration of glycine at approximately the EC50 for the particular transporter together with the NAAA at concentrations up to 30 μm. It was not possible to use higher concentrations of the NAAAs because of the capacity of these compounds to form micelles at higher concentrations and to cause irreversible changes in the resting current of the oocyte. The concentration response data were fitted to Equation 2,
where IC50 is the concentration at which half maximal inhibition by the NAAA tested is produced, and C is the fraction of current remaining in the presence of a maximal concentration of inhibitor. IC30 values are presented because complete inhibition was not achieved for a number of chimeras at concentrations up to 30 μm. If the estimated IC30 value is greater than 30 μm, then it is presented as >30 μm.
RESULTS
NAGly Is a Noncompetitive Inhibitor of GLYT2
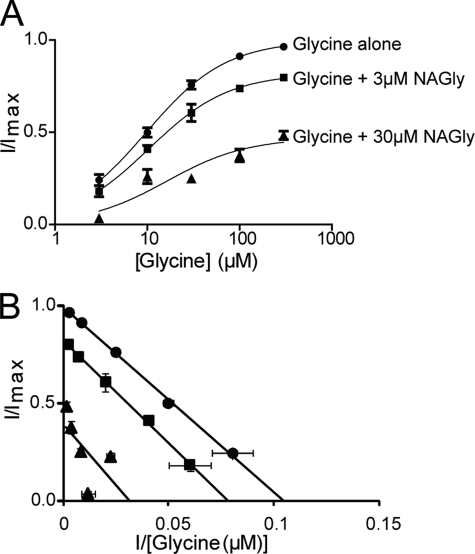
The aim of this study was to investigate the molecular basis for the differences in NAAA inhibition between the human glycine transporters GLYT1 and GLYT2. We have previously established that NAGly inhibits GLYT2 and suggested that it is a noncompetitive inhibitor of transport (10). We have characterized the noncompetitive nature of the inhibition more fully by measuring the glycine concentration dependent transport currents mediated by GLYT2 in the presence of varying NAGly concentrations. Although the Imax for glycine transport is reduced by NAGly in a concentration-dependent manner, the EC50 for glycine transport currents is not changed for the different NAGly concentrations (Fig. 2). Thus, NAGly is a noncompetitive inhibitor of GLYT2.
FIGURE 2.
NAGly is a non-competitive inhibitor of GLYT2. A, glycine concentration-dependent transport currents. The EC50 for glycine (●) is 10.2 ± 0.8 μm (n = 7) and in the presence of 3 μm (■) and 30 μm (▴) NAGly the EC50 of glycine is 11 ± 1 μm (n = 3) and 18 ± 9 μm (n = 4), respectively. The Imax of glycine alone is reduced to 82% (n = 3) and 48% (n = 4) when in the presence of 3 μm and 30 μm NAGly, respectively. Errors are S.E. of the mean. B, Eadie-Hofstee plot including glycine alone (●) and in the presence of 3 μm (■) and 30 μm (▴) NAGly (n ≥3).
Substrate Transport by Wild Type and Chimeric Transporters
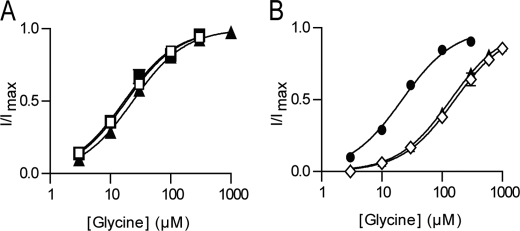
We have focused on the role of EL2 and EL4 in determining differences in NAAA sensitivity by creating chimeric transporters in which EL2, EL4, or both loops are switched between GLYT1 and GLYT2 (Fig. 1B). Application of glycine to oocytes expressing GLYT2, GLYT2(EL2), and GLYT2(EL4) generates glycine concentration dependent transport currents (Fig. 3A). EC50 values for glycine transport currents mediated by GLYT2(EL2) and GLYT2(EL4) are similar to GLYT2 (Table 1). Application of up to 3 mm glycine to oocytes expressing the double chimera GLYT2(EL2,EL4) do not generate measurable currents, and this chimera was not further characterized. Sarcosine (N-methyl-glycine), a GLYT1 selective substrate (19–21), is not transported by either of the GLYT2-based chimeras. Therefore, EL2 and EL4 of GLYT1 do not appear to play a role in substrate selectivity.
FIGURE 3.
Glycine concentration-dependent transport currents for wild type and chimeric transporters. A, GLYT2 (■), GLYT2(EL2) (□), and GLYT2(EL4) (▴) (n = 5/curve). B, GLYT1 (●), GLYT1(EL4) (▴), and GLYT1(EL2,EL4) (◇). All currents were measured at −60 mV, n = 5, with error bars representing S.E. For most curves, the S.E. errors were smaller than the symbols. See Table 1 for EC50 values for each transporter.
TABLE 1.
The EC50 of substrate and IC30 values (±S.E.) for NAAA inhibition of glycine transport by wild type and chimeric transporters
| Transporter | EC50 |
IC30 |
||||
|---|---|---|---|---|---|---|
| Glycine | Sarcosine | NAGly | NALAla | NADAla | NAGABA | |
| μm | μm | |||||
| GLYT2 | 18 ± 2 | 3.4 ± 0.6 | 5.9 ± 0.7 | 17 ± 2 | 22 ± 4 | |
| GLYT2(EL2) | 19 ± 2 | 28 ± 4 | 6 ± 1 | >30 | >30 | |
| GLYT2(EL4) | 24 ± 2 | >30 | >30 | >30 | >30 | |
| GLYT1 | 22 ± 2 | 13 ± 1 | >30 | >30 | >30 | >30 |
| GLYT1(EL4) | 139 ± 2 | 228 ± 11 | >30 | 21 ± 2 | >30 | 35 ± 9 |
| GLYT1(EL2,EL4) | 176 ± 8 | 209 ± 8 | >30 | 22 ± 5 | ||
Application of glycine to oocytes expressing either GLYT1(EL4) or GLYT1(EL2,EL4) generates concentration dependent transport currents that are similar to wild type GLYT1 (Fig. 3B). Application of up to 3 mm glycine to GLYT1(EL2) generates very small currents that are too unreliable to accurately characterize. The EC50 values for glycine transport currents mediated by GLYT1(EL4) and GLYT1(EL2,EL4) are increased by ∼7-fold compared with both GLYT1 and GLYT2 (Fig. 3B and Table 1). The GLYT1-selective substrate, sarcosine, generates transport currents for GLYT1, GLYT1(EL4), and GLYT1(EL2,EL4) but not for GLYT2 and the GLYT2-based chimeras (Table 1). These results indicate that switching the EL2 and EL4 between GLYT1 and GLYT2 does not influence relative substrate selectivity but does moderately reduce the affinity of substrates in the GLYT1-based chimeras.
The Effects of the NAAAs on GLYT2 and the GLYT2-based Chimeric Transporters
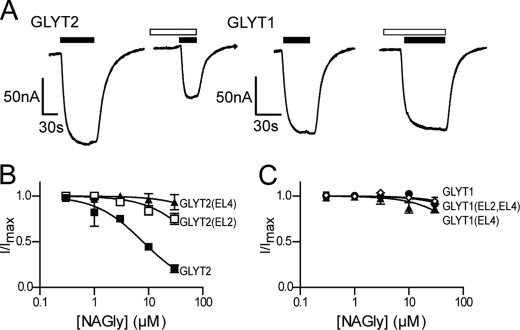
NAGly, NALAla, and NAGABA inhibit GLYT2 but have little or no activity at GLYT1 at concentrations up to 100 μm (Table 1) (10). Glycine was applied to GLYT2- and the GLYT2-based chimeras at approximately the EC50 concentration (30 μm) for each transporter followed by co-application of glycine with the NAAAs at concentrations up to 30 μm. We did not test concentrations above 30 μm, as the holding current at −60 mV does not remain stable, which may be due to the formation of micelles by the NAAAs. Upon washout of NAAAs for 2 min, reapplication of glycine generated transport currents that were similar to control transport currents measured prior to NAAA application (data not shown).
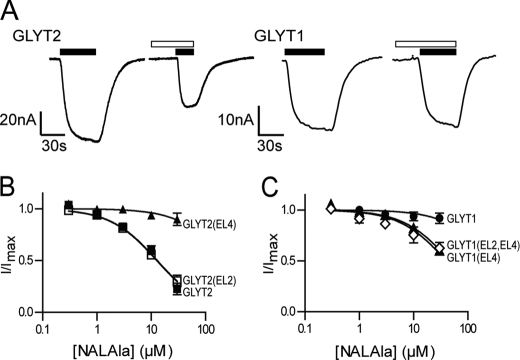
Glycine transport by GLYT2 is inhibited by NAGly (Fig. 4, A and B), NALAla (Fig. 5A and B), and NAGABA (Table 1) in a concentration-dependent manner with IC30 values of 3.4 ± 0.6 μm; 5.9 ± 0.7 μm; and 22 ± 4 μm, respectively. The four NAAAs do not affect glycine transport by GLYT1 with IC30 values >30 μm. We tested the activity of the NAAAs on the chimeric transporters to identify domains responsible for conferring this differential sensitivity. GLYT2(EL2) showed reduced sensitivity to NAGly and NAGABA, with IC30 values of 28 ± 4 μm (Fig. 4B) and >30 μm (Table 1) respectively, compared with wild type GLYT2. In contrast, NALAla inhibits glycine transport by GLYT2(EL2) with similar potency to that for GLYT2 (Fig. 5B). These results suggest that EL2 of GLYT2 is important for NAGly and NAGABA selectivity but not for NALAla.
FIGURE 4.
The effect of NAGly on both wild type and chimeric transporters. A, representative current traces from oocytes clamped at −60 mV expressing either GLYT2 or GLYT1 when 30 μm glycine (closed bars) is applied alone and in the presence of 10 μm NAGly (open bars). B, NAGly concentration-dependent modulation of 30 μm glycine transport currents for GLYT2 (■), GLYT2(EL2) (□), and GLYT2(EL4) (▴) (n ≥5/curve). C, NAGly concentration-dependent modulation of glycine transport currents for GLYT1 (●), GLYT1(EL4) (▴), and GLYT1(EL2,EL4) (◇), respectively (n ≥5/curve). In the cases of GLYT1(EL4) and GLYT1(EL2,EL4), the glycine concentration used was 200 μm and 300 μm, respectively. For most concentration response curves, the S.E. errors were smaller than the symbols.
FIGURE 5.
The effect of NALAla on both wild type and chimeric transporters. A, representative current traces from oocytes clamped at −60 mV expressing either GLYT2 or GLYT1 when 30 μm glycine (closed bars) is applied alone and in the presence of 10 μm NALAla (open bars). B, NALAla concentration-dependent modulation of 30 μm glycine transport currents for GLYT2 (■), GLYT2(EL2) (□), and GLYT2(EL4) (▴) (n ≥5/curve). C, NALAla concentration-dependent modulation of glycine transport currents for GLYT1 (●), GLYT1(EL4) (▴), and GLYT1(EL2,EL4) (◇), respectively (n ≥5/curve). In the cases of GLYT1(EL4) and GLYT1(EL2,EL4), the glycine concentration used was 200 μm and 300 μm, respectively. For most concentration response curves, the S.E. errors were smaller than the symbols.
The differential sensitivity of the GLYT2(EL2) chimera to NAGly, NALAla, and NAGABA suggests that the conformation and size of the head group of these compounds may play an important role in their ability to bind to GLYT2. The carboxyl groups of NAGly and NAGABA may have the capacity to adopt a larger range of conformations than the carboxyl group of NALAla due to its chiral center, which may impose constraints on the orientation of the head group. To investigate the role of the head group in determining the specificity of the interactions with the wild type and chimeric transporters, we synthesized NADAla and tested its activity. NADAla inhibits GLYT2 with an IC30 of 17 ± 2 μm and does not inhibit glycine transport by GLYT1 (Table 1). We also tested this compound on the GLYT1 and GLYT2 chimeric transporters and found that although NADAla has a reduced affinity for the transporters, it has a similar profile to NAGly; it did not inhibit glycine transport by GLYT2(EL2) or GLYT2(EL4) (Table 1). These results are in contrast to those obtained with NALAla, which only appears to interact with EL4 of GLYT2 and indicates that the position of the head group is important for interactions between the NAAAs and EL2.
The Effect of the NAAAs on the GLYT1-based Chimeric Transporters
Glycine was applied to GLYT1, GLYT1(EL4), and GLYT1(EL2,EL4) at approximately the EC50 for each transporter (30 μm, 200 μm, and 300 μm, respectively) followed by co-application of glycine with the NAAAs at concentrations up to 30 μm. None of the four NAAAs tested affect glycine transport by wild type GLYT1 (Table 1). We then investigated whether the insertion of EL2 and/or EL4 from GLYT2 into GLYT1 could introduce NAAA sensitivity. The GLYT1(EL4) chimera maintains similar sensitivity to NAGly and NADAla as GLYT1 (no activity), but is inhibited by NALAla and NAGABA, albeit with reduced potency to that of GLYT2 (Fig. 5C and Table 1). We did not test the effects of the NAAAs on the GLYT1(EL2) chimera because we could not measure reliable glycine transport currents. However, the GLYT1(EL2,EL4) double chimera behaved in a similar manner to GLYT1(EL4); it is insensitive to NAGly (Fig. 4C) but is inhibited by NALAla (Fig. 5C). NADAla and NAGABA were not tested on GLYT1(EL2,EL4) because their potency is lower than NAGly and NALAla, and any changes in the small glycine currents by GLYT1(EL2,EL4) would be too small to measure accurately. The results from the GLYT2 and GLYT1 chimera studies demonstrate that EL4 of GLYT2 is important for NAGly, NALAla, NAGABA, and NADAla selectivity, whereas EL2 of GLYT2 is required for NAGly, NAGABA, and NADAla selectivity but not NALAla.
Point Mutations in GLYT2
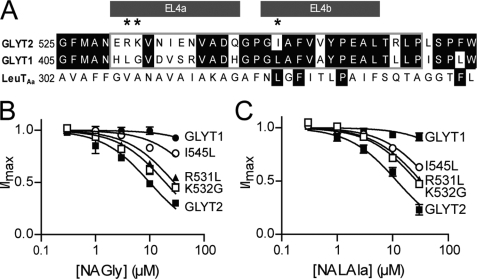
We have focused our mutation studies on the EL4 domain of GLYT2 for a number of reasons. First, NAGly, NALAla, NADAla, and NAGABA all interact with EL4 of GLYT2, whereas not all of the NAAAs interact with EL2. Second, EL4 has also been implicated in the mechanism of inhibition of transport by other transporters including the bacterial homolog, LeuTAa (12, 22, 23) and appears to play an important role in NAAA GLYT2 selectivity. Third, the GLYTs share 60% amino acid sequence identity in EL4 with the most diverse region being EL4a compared with EL4b (Fig. 6A). In contrast, EL2 is very divergent between GLYT1 and GLYT2 and is considerably larger in GLYT2 than GLYT1 (see supplemental Fig. 1). To identify the residues in EL4 that play a role in determining the differential sensitivity of GLYT2 compared with GLYT1, 11 mutations in GLYT2 were constructed: E530H, R531L, K532G, N534D, I535V, E536S, N537R, Q541H, I545L, V549A, and R556L. All residues in EL4 of GLYT2 that differed from GLYT1 were mutated to the corresponding residue in GLYT1 (Fig. 6A). The 11 GLYT2 EL4 point mutations transported glycine in a concentration-dependent manner similar to GLYT2 (Table 2) and did not transport 1 mm sarcosine. Therefore, these mutations do not appear to affect substrate transport affinity or selectivity.
FIGURE 6.
The effect of NAGly and NALAla on the GLYT2 EL4 mutants R531L, K532G, and I545L. A, an amino acid sequence alignment of GLYT1, GLYT2, and LeuTAa for EL4. B, NAGly concentration-dependent modulation of 30 μm glycine transport currents for GLYT2 (■), R531L (□), K532G (▴), I545L (○), and GLYT1 (●) (n ≥5/curve). C, NALAla concentration-dependent modulation of 30 μm glycine transport currents for GLYT2 (■), R531L (□), K532G (▴), I545L (○), and GLYT1 (●) (n ≥5/curve). For most concentration response curves, the S.E. errors were smaller than the symbols.
TABLE 2.
The EC50 of glycine and IC30 values (±S.E.) for NAGly and NALAla inhibition of glycine transport by wild type and GLYT2 EL4 mutant transporters
| Transporter | EC50 of glycine | IC30 |
|
|---|---|---|---|
| NAGly | NALAla | ||
| μm | μm | ||
| GLYT2 | 18 ± 2 | 3.4 ± 0.6 | 5.9 ± 0.7 |
| GLYT1 | 22 ± 2 | >30 | >30 |
| E530H | 11.2 ± 0.9 | 2.1 ± 0.7 | |
| R531L | 10 ± 4 | 13 ± 2 | 14 ± 1 |
| K532G | 39 ± 2 | 9 ± 1 | 12 ± 1 |
| N534D | 19 ± 2 | 5 ± 1 | |
| I535V | 13 ± 2 | 2.2 ± 0.4 | |
| E536S | 13 ± 3 | 2.3 ± 0.5 | |
| N537R | 10 ± 1 | 4.9 ± 1.5 | |
| Q541H | 12.7 ± 0.8 | 2.6 ± 0.4 | |
| I545L | 8.4 ± 0.8 | 42 ± 7 | 21 ± 1 |
| V549A | 9 ± 1 | 1.3 ± 0.2 | |
| R556L | 6 ± 1 | 8 ± 1 | |
Glycine was applied to the mutant transporters at approximately the EC50 for each transporter (30 μm) followed by co-application of glycine with NAGly at concentrations up to 30 μm. Glycine transport by eight of the mutants was inhibited by NAGly similar to GLYT2 (Table 2). R531L and K532G had reduced sensitivity to NAGly (IC30 values of 13 ± 2 μm and 9 ± 1 μm, respectively) compared with GLYT2 (IC30; 3.4 ± 0.6 μm), whereas I545L had markedly reduced sensitivity to NAGly with an IC30 value of >30 μm (Fig. 6B). R531L, K532G, and I545L were further investigated by determining their sensitivity to NALAla (Fig. 6C). R531L, K532G, and I545L had reduced sensitivities to NALAla with IC30 values of 14 ± 1 μm, 12 ± 1 μm, and 21 ± 1 μm, respectively, compared with GLYT2 (IC30; 5.9 ± 0.7 μm). Therefore, the GLYT2 residues Arg531, Lys532, and Ile545 are required for NAGly and NALAla inhibition of GLYT2.
DISCUSSION
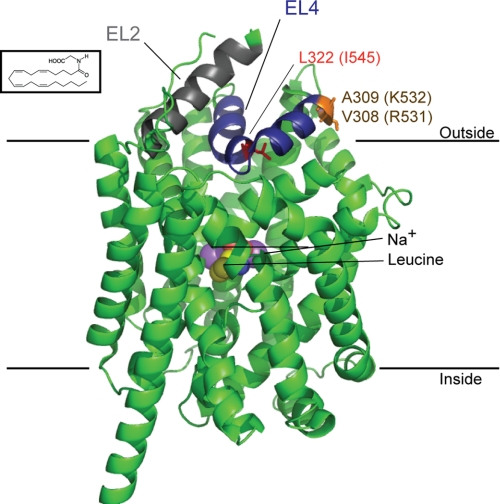
There have been limited studies on how lipids interact with and modulate the functions of neurotransmitter transporters. NAGly, NALAla, and NAGABA have previously been shown to inhibit GLYT2 with a rank order of potency of NAGly ≥ NALAla > NAGABA (10), with little or no activity at GLYT1. In this study, we have utilized chimeric transporters to demonstrate that EL2 and EL4 contain molecular determinants that are responsible for NAAA sensitivity of GLYT2. Furthermore, point mutations in EL4 of GLYT2 demonstrate that the EL4 residues Arg531, Lys532, and Ile545 are required for NAGly inhibition. I545L is a relatively conservative mutation, and yet, it shows the most marked changes in NAGly affinity. In most other transporters in this family, the corresponding residue is a leucine. Interestingly, we have previously shown that NAGly does not inhibit GAT1 (10), and GAT1 also contains a leucine residue at this position. Thus, an isoleucine for leucine difference may play a crucial role in determining how NAGly interacts with the transporter. At this stage, it is difficult to explain why such a conservative mutation causes the most marked changes. Two possibilities are: first, NAGly may bind to Ile545, and the I545L mutation may distort the way that NAGly fits into the binding site; or second, Ile545 is located at the apex of the EL4 loop, and the I545L mutation may alter the conformation of the two arms of EL4 (Fig. 7), which then impacts on the way that this domain interacts with other elements that may be crucial for NAGly binding. The other two residues identified in this study, Arg531 and Lys532, are located at the edge of EL4. It is possible that the carboxyl groups of the NAAAs may interact with these positively charged residues. Further structural studies will be required to fully characterize the specific interaction sites between the NAAAs and GLYT2.
FIGURE 7.
Proposed interactions between the NAAAs and GLYT2. The structure of LeuTAa bound by leucine and two sodium ions (purple) with EL2 (gray), EL4 (blue), and the LeuTAa residues corresponding to the GLYT2 EL4 residues Ile545 (red) and Arg531 and Lys532 (orange) are highlighted. A diagram was generated using PyMOL (DeLano Scientific LLC) for Protein Data Bank code 2A65. The structure of NAGly is also presented at the approximate scale relative to the structure of LeuTAa.
The four NAAAs used in this study have the same lipid core structure combined with subtle variations in the head group. The different head groups influence potency of the compounds and also their specificity for the various chimeric transporters. The studies with the GLYT2-based chimeras are the most informative because it is possible to selectively knock out the activity of some of the NAAAs but not all. The inhibitory activity of NAGly, NADAla, and NAGABA on GLYT2(EL2) was greatly reduced compared with wild type GLYT2, which suggests that EL2 contains important binding determinants for these compounds. However, NALAla inhibits GLYT2(EL2) with similar potency to that of GLYT2. In contrast, the inhibitory activity of all four NAAAs is abolished in the GLYT2(EL4) chimera. Thus, EL4 of GLYT2 contains molecular determinants that are required for all four of the NAAAs, whereas EL2 is important for NAGly, NADAla, and NAGABA binding but not NALAla. The differential sensitivity of the GLYT2 chimeras to NALAla and NADAla and the reduced affinity of NADAla are intriguing. The only difference between NALAla and NADAla is the chiral center within the head group, which appears to be crucial in determining interactions with EL2. The chiral center of the two compounds influences the orientation of the carboxyl group relative to the methyl group of the alanine moiety, which may then impact on the way in which the inhibitor fits into the binding site. The different orientation may result in the carboxyl group adopting different positions and thereby interacting with GLYT2 in a different manner. The binding could also be influenced by interactions between the methyl group and hydrophobic residues and or pockets.
EL2 and EL4 have previously been implicated in forming noncompetitive inhibitor binding sites in GLYT1 (14) and also the related bacterial leucine transporter, LeuTAa(12,23), which suggests that the conformation and flexibility of these domains may contribute to a general mechanism for noncompetitive inhibition of this class of transporters. Glycine transporters are predicted to share a similar structure to LeuTAa with the core protein consisting of transmembrane domains 1–10, and are also predicted to share similar mechanisms of transport and inhibition. The crystal structure of LeuTAa in complex with the noncompetitive inhibitor clomipramine reveals possible mechanisms of transport inhibition that may apply to NAGly inhibition of GLYT2 (12). There are at least three conformational states of the transporter: open-to-outside, occluded, and open-to-inside (12). The open-to-outside and open-to-inside states of the transporter are controlled by the extracellular and intracellular gates, respectively. Clomipramine inhibits the transporter by stabilizing the occluded state through interactions with EL4, as well as transmembrane domains 1, 3, 6, and 10. In the clomipramine-bound state of LeuTAa, the tip of EL4 is displaced, and this is thought to contribute to the inhibitory mechanism. The isoleucine residue in EL4 of GLYT2 (Ile545) that is crucial for NAGly inhibition is two residues from the tip of EL4 (Fig. 7). If the formation of an occluded state in GLYT2 is generated by a similar process to LeuTAa, then it is possible that NAAAs interactions with EL4 inhibit glycine transport through a similar mechanism. Tryptophan is a competitive inhibitor of LeuTAa and traps the transporter in an open-to-out conformation (22). Singh et al. (22) determined the crystal structure of LeuTAa in complex with tryptophan and identified four separate tryptophan molecules in the crystal structure. This may represent transient binding sites of the inhibitor, and possibly even the substrate, that form part of the transport pathway from the extracellular space to the primary binding site located in the center of the transporter. Interestingly, one of these tryptophan molecules is bound in a pocket formed by EL2 and EL4. This result emphasizes that EL2 and EL4 are indeed in close proximity in LeuTAa, but also that if similar structures exist in GLYT2, then the NAAAs may sit in a similar cleft in GLYT2. By analogy with the tryptophan model for inhibition of LeuTAa, the NAAAs may inhibit GLYT2 by binding to EL2 and EL4, thereby restricting the movement of the transporter and hence substrate translocation. Furthermore, the role of EL2 and EL4 in forming a possible inhibitor/substrate permeation pathway may explain why the GLYT1(EL4) and GLYT1(EL2,EL4) chimeras have reduced affinity for substrates.
In summary, we have used a series of chimeric GLYT transporters to demonstrate that NAGly, NALAla, NADAla and NAGABA interact with EL4 of GLYT2, while only NAGly, NADAla and NAGABA interact with EL2 of GLYT2. The subtly different structural requirements for NALAla inhibition of transport highlights the role of different conformations of the head groups of these lipid modulators in determining how they bind to GLYT2. We also tested a series of EL4 mutants to demonstrate that the GLYT2 residues Ile545, Arg531 and Lys532 are required for NAGly and NALAla inhibition of GLYT2. This study is the first to describe the molecular determinants for lipid modulation of neurotransmitter transporters and further work may develop the therapeutic potential of this class of compounds.
Acknowledgments
We thank Xin Liu, Gracia Quek, Ken Wyse and the University of Sydney Laboratory Animal Services for assistance in maintaining the Xenopus laevis colony.
This work was supported by an Australian National Health and Medical Research Council (NHMRC) Project Grant.

The on-line version of this article (available at http://www.jbc.org) contains supplemental data, Table 1, and Figs. 1 and 2.
- NAGly
- N-arachidonylglycine
- EL
- extracellular loop
- NAGABA
- N-arachidonyl-γ-aminobutyric acid
- NADAla
- N-arachidonyl-d-alanine
- NAAA
- N-arachidonyl-amino acid
- NALAla
- N-arachidonyl-l-alanine.
REFERENCES
- 1.Aprison M. H., Werman R. (1965) Life Sci. 4, 2075–2083 [DOI] [PubMed] [Google Scholar]
- 2.Lynch J. W. (2004) Physiol. Rev. 84, 1051–1095 [DOI] [PubMed] [Google Scholar]
- 3.Gomeza J., Hülsmann S., Ohno K., Eulenburg V., Szöke K., Richter D., Betz H. (2003) Neuron 40, 785–796 [DOI] [PubMed] [Google Scholar]
- 4.Gomeza J., Ohno K., Hülsmann S., Armsen W., Eulenburg V., Richter D. W., Laube B., Betz H. (2003) Neuron 40, 797–806 [DOI] [PubMed] [Google Scholar]
- 5.Betz H., Gomeza J., Armsen W., Scholze P., Eulenburg V. (2006) Biochem. Soc. Trans. 34, 55–58 [DOI] [PubMed] [Google Scholar]
- 6.Morita K., Motoyama N., Kitayama T., Morioka N., Kifune K., Dohi T. (2008) J. Pharmacol. Exp. Ther. 326, 633–645 [DOI] [PubMed] [Google Scholar]
- 7.Huang S. M., Bisogno T., Petros T. J., Chang S. Y., Zavitsanos P. A., Zipkin R. E., Sivakumar R., Coop A., Maeda D. Y., De Petrocellis L., Burstein S., Di Marzo V., Walker J. M. (2001) J. Biol. Chem. 276, 42639–42644 [DOI] [PubMed] [Google Scholar]
- 8.Vuong L. A., Mitchell V. A., Vaughan C. W. (2008) Neuropharmacol. 54, 189–193 [DOI] [PubMed] [Google Scholar]
- 9.Succar R., Mitchell V. A., Vaughan C. W. (2007) Molecular Pain 3, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiles A. L., Pearlman R. J., Rosvall M., Aubrey K. R., Vandenberg R. J. (2006) J. Neurochem. 99, 781–786 [DOI] [PubMed] [Google Scholar]
- 11.Nelson N. (1998) J. Neurochem. 71, 1785–1803 [DOI] [PubMed] [Google Scholar]
- 12.Singh S. K., Yamashita A., Gouaux E. (2007) Nature 448, 952–956 [DOI] [PubMed] [Google Scholar]
- 13.Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. (2005) Nature 437, 215–223 [DOI] [PubMed] [Google Scholar]
- 14.Ju P., Aubrey K. R., Vandenberg R. J. (2004) J. Biol. Chem. 279, 22983–22991 [DOI] [PubMed] [Google Scholar]
- 15.Bezuglov V., Bobrov M., Gretskaya N., Gonchar A., Zinchenko G., Melck D., Bisogno T., Di Marzo V., Kuklev D., Rossi J. C., Vidal J. P., Durand T. (2001) Bioorg. Med. Chem. Lett. 11, 447–449 [DOI] [PubMed] [Google Scholar]
- 16.Shevchuk N. A., Bryksin A. V., Nusinovich Y. A., Cabello F. C., Sutherland M., Ladisch S. (2004) Nucleic Acids Res. 32, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arriza J. L., Fairman W. A., Wadiche J. I., Murdoch G. H., Kavanaugh M. P., Amara S. G. (1994) J. Neurosci. 14, 5559–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenberg R. J., Mitrovic A. D., Johnston G. A. (1998) Br. J. Pharmacol. 123, 1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guastella J., Brecha N., Weigmann C., Lester H. A., Davidson N. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 7189–7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q. R., López-Corcuera B., Mandiyan S., Nelson H., Nelson N. (1993) J. Biol. Chem. 268, 22802–22808 [PubMed] [Google Scholar]
- 21.Vandenberg R. J., Shaddick K., Ju P. (2007) J. Biol. Chem. 282, 14447–14453 [DOI] [PubMed] [Google Scholar]
- 22.Singh S. K., Piscitelli C. L., Yamashita A., Gouaux E. (2008) Science 322, 1655–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z., Zhen J., Karpowich N. K., Goetz R. M., Law C. J., Reith M. E., Wang D. N. (2007) Science 317, 1390–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]