Abstract
Inflammation arises in the CNS from a number of neurodegenerative and oncogenic disorders, as well as from ischemic and traumatic brain injuries. These pathologies give rise to increased levels of extracellular adenine nucleotides which, via activation of a variety of cell surface P2 purinergic receptors, influence the inflammatory activities of responding immune cells. One P2 receptor subtype in particular, the P2X7 receptor, potentiates the release of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) from macrophage-like cells. It is also thought to contribute to secondary brain injury by inducing neuronal cell death. Therefore, antagonism of this receptor could have significant therapeutic impact on all disorders, not just CNS, to which excessive inflammatory activities contribute. The use of currently available P2X7 receptor antagonists for the treatment of CNS inflammation has been limited to the generally non-selective antagonists PPADS, oxidized ATP, Brilliant Blue G, suramin, calmidizolium, and KN-62. However, the recent patents and development of novel P2X7 receptor antagonists, as discussed in this review, will provide new tools both for clinical and research purposes. Here we discuss compounds for which patents have been applied since 2006, from the following categories: benzamide inhibitors, bicycloheteroaryl compounds, acylhdranzine antagonists, biaromatic P2X7 antagonists, heterocyclic compounds and amide derivatives, and aromatic amine antagonists.
Keywords: P2X7, antagonist, inflammation, CNS, nucleotides
INTRODUCTION
Acute traumatic and ischemic injury to the brain and spinal cord often result in irrecoverable, widespread neuronal death. Moreover, secondary injury, resulting from inflammation present hours to days after the initial insult, exacerbates the primary injury causing further CNS damage [1]. Thus, therapeutics which interfere with inflammatory processes in the CNS may have significant benefit for minimizing tissue damage and promoting neuronal survival after the primary insult. Secondary injury, consisting of an exaggerated and prolonged inflammatory response, is potentially maintained by continued cell lysis and/or the release of pro-inflammatory cytokines by neighboring glial cells [2]. In cases of CNS trauma, ischemia, neurodegenerative disease or malignant tumor growth, increased levels of extracellular nucleotides in the microenvironment are well-established [3–5]. These nucleotides and their metabolites, the endogenous ligands for P2 nucleotide receptors (traditionally known as purinergic receptors), may be responsible for contributing to secondary neuronal injury by inducing apoptosis and prolonging inflammation. Many of these activities of adenine nucleotides are mediated through P2X receptors, and in particular, through the activation of the P2X7 receptor subtype [3, 6]. In Table (1) we list a number of inflammatory disorders with which P2X receptors are associated.
Table 1.
P2X Related Diseases
| Disease | P2X Receptor Involvement | Citation |
|---|---|---|
| Alzheimer's Disease | P2X7 receptors up regulated around β-amyloid plaques P2X7 receptors stimulate excess superoxide production |
[68, 121– 123] |
| Asthma | Found loss of function P2X7 genotype related to virus-induced loss of asthma control |
[124] |
| Amyotrophic Lateral Sclerosis |
P2X7 increases in microglia in end stage SOD1 animals Blocking P2X4 extends the life span of SOD1 mice High P2X4 levels in degenerating motor neurons in spinal cord ventral horns |
[68, 125– 127] |
| Chaga's Disease | P2X7 receptor associated cell permeabilization | [128] |
| Chlamydia | Role for P2X7 in disease resistance | [129, 130] |
| Chronic Heart Failure | Upregulation of P2X6 | [131] |
| Diabetes | Increased P2X7 receptor-induced pore formation and apoptosis in retinal microvasculature |
[132] |
| Epilepsy | P2X2,4 decreased in hippocampus of seizure prone gerbils Evidence of microglial activation after status epilepticus |
[133, 134] |
| Erectile Dysfunction | P2X1 knockout mice show a loss of sympathetic co-transmission in the vas deferens |
[135, 136] |
| Inflammatory Bowl Disease |
Upregulation of P2Y6 in T lymphocytes and increase in P2X7 induced cytokine expression |
[137–139] |
| Interstitial Cystitis | Upregulation of P2X2, 3 in urotheilal cells | [140, 141] |
| Ischemia | P2X7 antagonists reduce infarct size | [142] |
| Major Depressive Disorder | Single-nucleotide polymorphism in P2X7 significantly associated | [143] |
| Migraine | P2X receptors involved in vasodilation phase | [144] |
| Multiple Sclerosis | P2X5, 6 absent in white and grey matter in the frontal cortex of MS tissue P2X7 deficient mice are more susceptible than wild type |
[61, 125, 145–147] |
| Neuroblastoma | P2X7 mediates proliferation | [148] |
| Neuropathic Pain | P2X7 receptor antagonism reduces pain P2X4 receptor activation increases microglial activation and pain levels P2X4 receptor antagonists reduce pain P2X4 deficient mice lack mechanical hyperalgesia |
[81, 82, 149–151] |
| Parkinson’s Disease | Disrupted cells stimulate P2X7 dependent cell death leading to pathogenesis of the disease Augmented P2X1, 3, 4, 6 proteins in lesions |
[128, 152– 154] |
| Polycystic Kidney Disease | Purinergic signaling increases cyst expansion | [137] |
| Rheumatoid Arthritis | P2X7 activation increases leukocyte function and cartilage damage Use of a P2X7 antagonist reduces inflammation and associated pain |
[155, 156] |
| Tuberculosis | P2X7 loss of function polymorphism increase susceptibility for TB reactivation | [157] |
The type and severity of CNS insult dictates the levels of extracellular nucleotides present in brain tissue. Because P2 receptors have different affinities for different nucleotides, not all receptors will be activated in all conditions; however, ATP is an agonist for most P2 receptors. ATP is released in the brain by several normal physiologic mechanisms [7, 8 ], including co-release with other neurotransmitters from neurons [9–13] and release from nearby cells through membrane channels or gap junctions [11, 15–17]. In this regard, ATP is necessary for propagation of calcium waves between astrocytes [14–19]. Importantly, intracellular ATP concentrations average 3–5mM in most CNS cells [20], and its release into the extracellular space from lysed and damaged cells both within and surrounding the lesion or insult can lead to abnormally high nucleotide levels in the extracellular microenvironment. For example, during hypoxia or ischemia, extracellular ATP levels have been measured in the micromolar range by tissue microdialysis [7, 8], although levels in discrete areas are presumably higher still. And in tumors, extracellular ATP levels are chronically high primarily due to their release from regions of cellular necrosis within the tumor itself as well as from dead and injured cells as the tumor grows into healthy tissue. In addition, the levels and activities o f ectonucleotidases (the extracellular enzymes that degrade nucleotides) are also strongly regulated by pathological processes; their expression is greatly suppressed in gliomas [21, 22] and in infarcted tissue following embolic ischemia [23]. ATP can also be released from platelets [24] and erythrocytes [25, 26] that infiltrate the lesioned tissue, increasing the ways by which nucleotide levels, and their durations of action, can be influenced in the CNS. Although adenine nucleotides perform a number of important regulatory roles in the maintenance of CNS homeostasis and health, their prolonged increase can also contribute to tissue damage and pathology.
ATP is postulated to serve as a “danger signal” to alert and recruit immune cells to the site of tissue damage [27, 28] following injury. However, this protective measure often goes awry, as chronic extracellular nucleotide elevation activates the P2X7 receptor which is thought to contribute to secondary neuronal damage. Of all known P2 receptors, the P2X7 receptor requires the highest nucleotide levels for activation [29], and because of its unique functional properties (described below), it has become a prime therapeutic target for decreasing secondary brain injury. We discuss below its characteristics, and activities which make it a good therapeutic target for minimizing secondary brain damage. In addition, we provide a description of novel P2X7 antagonists that have been recently developed/patented.
THE P2 RECEPTOR FAMILY & P2X7-DEPENDENT CYTOLYSIS
The P2 nucleotide receptors are plasma membrane proteins that are subdivided into two classes, P2Y and P2X, based primarily on agonist specificity and predicted transmembrane topologies. P2Y receptors (currently 8 members: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14), are hetero-trimeric G-protein coupled, seven transmembrane spanning proteins for which both ATP and UTP are ligands. P2X receptors, (7 members known: P2X1–P2X7), are trimeric, ATP-gated cation channels [30]. The P2X7 receptor is exceptional in that its activation requires concentrations of nucleotides in the millimolar range as opposed to the micromolar levels sufficient to activate other P2 receptor family members [29]. Moreover, it was long thought that the P2X7 receptor was the only P2X receptor that did not heterotrimerize with other P2X subunits [29]. However, recently, it was shown that P2X7 receptor subunits can functionally interact with P2X4 subunits in macrophages [31]. The heteromeric P2X4/P2X7 receptor appears to have pharmacologic properties of both homomeric receptor subtypes [31]. For example, channel conductance through the heteromeric receptor is sensitive to both ivermectin, a positive P2X4 modulator, and a P2X4 antagonist TNP-ATP. Conversely, the P2X7 antagonist BBG also blocks the heteromeric receptor, suggesting that P2X7 antagonists may be able to interfere with both homo- and heteromeric P2X7 receptor function. This increases the therapeutic utility of P2X7 receptor antagonists since P2X4 receptors have also been implicated in neuropathic pain [32]. However, in both microglia and macrophages, very recently, it was shown that the preferential assembly of P2X4 and P2X7 receptors is homomeric, and that the predominant interactions between P2X4 and P2X7 receptor subunits occur between trimeric receptor complexes, not within individual complexes [33, 34], so the pharmacologic profile of these complexes is not yet clear. Additional studies are needed.
Another distinguishing characteristic of this receptor is its ability to mediate reversible permeablization of the plasma membrane [35], provided ATP stimulation is of short duration. P2X7 activation evokes currents carried primarily by Ca2+, K+, and Na+ cations [36–45]. Prolonged activation of P2X7 receptors causes the formation of a large, irreversible, non-selective pore [46, 47] capable of allowing molecules less than 1 kDa in size to enter the cell. Dyes such as ethidium bromide, propidium iodide, and quinolinium fluorescent molecules such as YO-PRO-1 [35, 48, 49] are commonly used to study the P2X7 associated pore, which is now thought to result from an interaction between the P2X7 receptor and pannexin [50, 51], a recently discovered hemi-channel protein that can function in an unpaired conformation. The important role of pannexins in transmitting signals for apoptosis and necrosis in ischemia has been recently reviewed [52].
Prolonged P2X7 receptor activation causes necrotic lysis, membrane permeablization and subsequent loss of cellular homeostasis, similar to that of bacterial toxins or soluble immune factors such as complement or perforin. However, P2X7 receptors can also induce a more organized form of cellular apoptosis including characteristic membrane blebbing, cell shrinkage, nuclear condensation and DNA fragmentation [53]. Whether a cell undergoes necrosis or apoptosis in response to P2X7 activation likely depends on the cell type and the duration and dose of ATP to which it is exposed. The P2X7 receptor is therefore thought to be central to the development of secondary brain and spinal cord injury when levels of extracellular ATP are chronically elevated.
P2X7 INFLAMMATORY RESPONSE
P2X7 nucleotide receptors are widely distributed. They are found on hematopoietic cells [54], taste bud cells [55], immune cells such as macrophages and microglia [5, 56, 57], as well as in certain populations of neurons, such as spinal cord motor neurons [58–60]. Within the brain there is also some evidence for P2X7 receptor expression in both oligodendrocytes [61] and astrocytes [62], although aspects of this remain controversial [63]. Several studies indicate a role for this receptor in regulating immune cell inflammatory responses in the CNS [44, 64–66]. For example, P2X7 receptors are up-regulated on microglia at the site of ischemic damage after middle cerebral artery occlusion [67]. They are also up-regulated in microglia and astrocytes around β-amyloid plaques in Alzheimer disease [68], and in reactive astrocytes from brain autopsy sections of multiple sclerosis lesions [69]. Moreover, P2X7 receptors are also involved in superoxide generation [68], and the processing and release of mature cytokines including interleukin (IL)-1α, IL-1β and IL-18 [5, 38, 69, 70] from macrophages and microglia. The processing and release of IL-1β is the best studied of these P2X7 receptor cytokine effects, which is now known to involve the rapid activation of caspase-1 [71, 72], in both an inflammasome-dependent and inflammasome-independent manner, depending upon the cell type [73, 74]. Inhibiting P2X7 receptor activities in hippocampal slice cultures strongly decreases IL-1β levels following LPS treatment [75], and their antagonism in vivo also decreases LPS-induced neuron damage [76]. In a study that used cortical trauma instead of LPS to induce inflammation in vivo, a P2X7 receptor antagonist was also beneficial [77]. These studies and others, support the utility of P2X7 receptor antagonists in treating brain inflammation.
It should be mentioned here that a role for P2X7 receptors in promoting neuropathic pain has also been suggested. Because P2X7 receptor activation promotes mature IL-1β release, and IL-1β is implicated in promoting hyperalgesia [78–80], compounds that antagonize these receptors may also be beneficial for treating neuropathic pain [81, 82]. Indeed P2X7 receptor deletion [83] and pharmacologic antagonists have recently been shown to decrease pain in different rodent models [82, 84–87].
LABORATORY AND MEDICINAL APPLICATIONS
At present, available pharmacological ligands for the P2X7 receptor suffer from poor receptor specificity [35]. For example, BzATP, the most potent known agonist for P2X7 receptors [88], more strongly activates P2X1, P2X4 and P2Y11 receptor subtypes than P2X7 [29, 58, 89–91]; this lack of pharmacologic specificity, also true of P2X7 receptor antagonists, complicates the therapeutic use of these ligands. Therefore, the aim of new drug discovery in this regard, is to develop P2X7 antagonists with greater functional selectivity, higher affinities and lower IC50 values, to identify more potent antagonists with fewer side effects. It is necessary to note here however, that it is probable that P2X7 antagonists will have differential effects depending on the cell type targeted and the disease state for which it they are used.
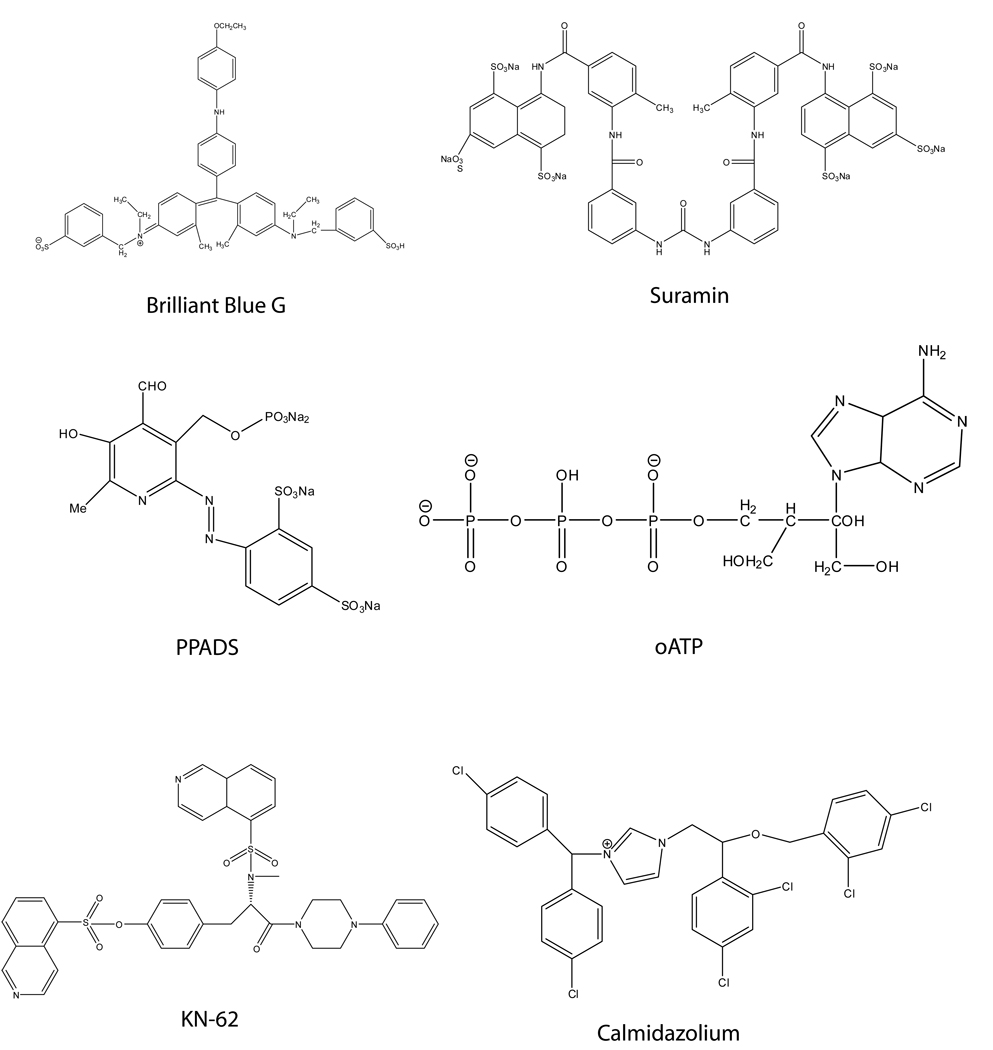
Currently available P2X7 receptor antagonists can be classified into four main groups [29]. The first group contains ions, such as calcium, copper, magnesium, zinc, and protons, all of which inhibit ATP-evoked currents through the P2X7 receptor channel. The second group (Fig. (1)) includes generic or non-selective P2X receptor antagonists such as suramin, pyridoxal-phosphate-6-azophenyl-2',4'-disulfonate (PPADS), and Brilliant Blue G (BBG). Third, are the compounds that contain two large organic cations, i.e., the isoquinoline KN-62 (1-(N,O-bis[5-isoquinolinesulfonyl]-N-methyl-L-tyrosyl)-4-phenylpiperazine) and the imidazole calmidazolium (Fig. (1)) which function to block currents through P2X7 receptors and thereby prevent their activity. Finally, monocolonal antibodies are used to block P2X7 receptor function in vitro.
Figure 1.
General P2X7 Antagonists
Most compounds known to inhibit at least some P2X7-mediated CNS inflammatory responses fall within the second and third classification groups: the non-selective P2 receptor antagonists and the calmidizolium/imidazole classes Fig. (1). All of these compounds, except oATP, are competitive antagonists, but their clinical use is complicated by a lack of information about their pharmacokinetic and pharmacodynamic properties since they were designed as scientific tools for the study of P2X receptors, often in vitro, and not as therapeutics. Therefore, when used in vivo, they are subject to degradation by ectonucleotidases and other enzymes that metabolize them and change or reduce their functionality, situations with which these agents were not originally designed to contend. Additionally, the current, commercially available antagonists display a great deal of species variability. For example, KN-62, first developed as a specific cell-permeable inhibitor of the autophosphorylation of Ca2+/calmodulin-dependent protein kinase II (CAMK II) [92], inhibits dye uptake and calcium mobilization after activation of human P2X7 receptors, but it shows no inhibition at rat P2X7 receptors [93]. In contrast, BBG has been shown to be significantly more effective at inhibiting rat P2X7 receptors than human [94]. Prior to the development of the compounds by Abbott Laboratories including A-740003, A-438079 and most recently, A-839977 (Fig. (2) [81]), BBG was the most specific P2X7 antagonist available; it has nanomolar affinity at rat P2X7 receptors, but it also inhibits P2X2 and some P2Y receptors in the low micromolar range [48, 95, 96].
Figure 2.
Specific P2X7 Antagonist A-839977
Nonetheless, treatment of secondary neuronal injury using the less selective P2 receptor antagonists PPADS and oATP have shown promise. When administered one hour after injury, both compounds significantly reduced motor neuron cell death and improved recovery of motor function in rats subjected to spinal cord injury using a weight drop impact model [3]. Indeed, the idea that administering a P2X receptor antagonist as a means of preventing secondary injury after spinal cord injury or ischemia resulting from stroke was patented in 2005 (US20050164975A1). In this patent, the authors showed that P2X7 antagonism, in a microenvironment containing high concentrations of extracellular nucleotides (i.e. after a weight drop trauma), is beneficial in preventing further CNS damage [97].
Although there are no clinical trials currently underway to treat inflammation in CNS related disorders, there are ongoing trials using P2X7 receptor antagonists to reduce inflammation and pain in peripheral pathologies. In this regard, Evotec recently announced its launch of a new research program aimed at developing novel P2X7 receptor antagonists for the treatment of inflammation in diseases such as rheumatoid arthritis, irritable bowel syndrome, chronic obstructive pulmonary disease, as well as pain; their compounds have progressed to late stage pre-clinical development so far. (Please see Table (1) for a list of central and peripheral disorders in which P2X receptors are implicated). For the treatment of rheumatoid arthritis, Pfizer has an ongoing clinical trial with a P2X7 receptor antagonist to prevent IL-1β and IL-18 release. Another clinical trial using a P2X7 receptor antagonist is being conducted by GlaxoSmithKline to assess the benefit of treating inflammatory pain with a P2X7 antagonist [98]. Lastly, the AstraZeneca compound AZD9056 is currently in phase 2 clinical trials for the treatment of rheumatoid arthritis [98], suggesting that this drug may have significant therapeutic benefit for peripheral inflammatory disorders. Targeting P2X7 receptors for the treatment of inflammation in the CNS or periphery is still in its infancy, so this remains a relatively unexplored and pharmacologically lucrative area.
In this regard, 220 patent applications have been filed with the United States Patent and Trademark Office since 2001 related to P2X7 receptor modulation of the inflammatory response. Among these, 150 have been filed within the last three years, indicating the rapidity with which this area of focus is expanding [99]. Among these 150 patents, at least 16 directly address the regulation of CNS inflammation by P2X7 receptor antagonists (Table (2)). This does not include the many other patents examining regulation of inflammation via P2X7 receptors in other, peripheral organ systems. In an effort to focus the following discussion, we will summarize the patent applications since 2006 which provide a means to attenuate the CNS inflammatory response by antagonizing P2X7 receptors and their subsequent release of pro-inflammatory cytokines. Very nice comprehensive discussions on the medicinal chemistry aspects of current P2X7 receptor antagonists and their therapeutic uses have been recently presented [84, 100, 101]. The novel P2X7 antagonists to be discussed here fall into 6 major classes based on chemical structure. Each will be discussed below in succession: 1) benzamide inhibitors, 2) bicycloheteroaryl compounds, 3) acylhdranzine antagonists, 4) biaromatic antagonists, 5) heterocyclic compounds and amide derivatives and lastly, 6) aromatic amines.
Table 2.
Major Patents/Applications Since 2006 Targeting P2X7 Receptors
| Patent Number | Compound Class | Authors | Corporate Affiliation |
|---|---|---|---|
| US20070142329A1 [102] | Benzamide Inhibitor | Dombroski, M.A.; Duplantier, A.J.; Subramanyam, C. | Pfizer Inc. |
| US20070281939A1 [103] | Benzamide Inhibitor | Dombroski, M.A.; Duplantier, A.J. | Pfizer Inc. |
| US20060217430A1 [104] | Benzamide Inhibitor | Dombroski, M.A.; Duplantier, A.J.; Subramanyam, C. | Pfizer Inc. |
| US20080039478A1 [105] | Bicycloheteroaryl Compounds | Kelly, M.G.; Kincaid, J. | Evotec |
| US20070225324A1 [106] | Bicycloheteroaryl Compounds | Kelly, M.G.; Kincaid, J.; Fang, Y.; He, J.; Cao, Y.; Kaub, C.; Gowlugari, S.; Wang, Z. |
Evotec |
| US20070197565A1 [107] | Bicycloheteroaryl Compounds | Kelly, M.G.; Kincaid, J. | Evotec |
| US20060217448A1 [108] | Bicycloheteroaryl Compounds | Kelly, M.G.; Kincaid, J. | Evotec |
| US20060276505A1 [109] | Acylhydrazine Antagonist | Nelson, D.W.; Jarvis, M.F.; Carroll, W.A. | Abbott Laboratories |
| US20080146612A1 [111] | Biaromatic P2X7 Antagonist | Thompson, T.; Willis, P. | Astrazeneca AB |
| US20080132550A1 [112] | Heterocyclic Ion Channel Blocker | Shum, P.; Gross, A.; Ma, L.; McGarry, D.G.; Merriman, G.H.; Rampe, D.; Ringheim, G.; Sabol, J.S.; Volz, F.A. |
Aventis Pharmaceuticals Inc. |
| US20080009541A1 [113] | Amide Derivative | Chambers, L.J.; Gleave, R.; Senger, S.; Walter, D.S. | GlaxoSmithKline |
| US20070259920A1 [116] | Aromatic Amine Antagonists | Carroll, W.A.; Perez-Medrano, A.; Li, T. | Abbott Laboratories |
| US2007015842A1 [117] | Aromatic Amine Antagonists | Carroll, W.A.; Florjancic, A.S.; Perez-Medrano, A.; Peddi, S. | Abbott Laboratories |
| US20080076924A1 [118] | Aromatic Amine Antagonists | Betschmann, P.; Carroll, W.A.; Ericsson, A.M.; Fix-Stenzel, S.R.; Friedman, M.; Hirst, G., C.; Josephsohn, N.S.; Li, B.; Perez-Medrano, A.; Morytko, M.J.; Rafferty, P.; Chen, H. |
Abbott Laboratories |
1) Benzamide Inhibitors
Pfizer filed three separate patents US20070142329A1, US20070281939A1, and US20060217430A1 in 2006 and 2007, related to the use of three similar compounds. Interestingly, the benzamide structure is similar to that of the potent P2X7 receptor agonist BzATP, consistent with their suggested targeting of P2X7 receptors [102–104]. These drugs are structurally related, but differ primarily in modifications/substitutions to their structures in the R1, R2 and R3 groups as indicated in the summary of invention claims. In cultured human monocytes, these compounds were found to be efficacious for inhibiting P2X7-dependent pore activity (as assessed by YO-PRO and ethidium bromide uptake) and blockade of IL-1β release.
2) Bicycloheteroaryl Compounds
This class of compounds contains a lipophilic group attached to an amide, linked to an aromatic heterocycle bearing another, often polar or H-bonding group. The increase in lipophilicity of these compounds was often due to the addition of an adamantine group, which also serves to increase drug absorption into the blood stream. In four patents US20080039478A1, US20070225324A1, US20070197565A1 and US20060217448A1, all of which are derivatives of the bicycloheteroaryl compounds, the inventors show the ability of these compounds to inhibit IL-1β̣ release in the human monocytic cell line THP-1 and in peripheral blood mononuclear cells (PBMCs); the EC50 values varied depending on the compound examined [105–108]. Each patent provides a table classifying the percent inhibition of IL-1β release relative to untreated cells. Compounds demonstrating inhibition of IL-1β release by 75% or greater were considered to have significant therapeutic potential; multiple drugs with similar structures displayed different capacities to inhibit IL-1β̣ release. Each patent also examined other readouts of P2X7 receptor inhibition such as the ability to reduce neuropathic pain, delay progression of experimental autoimmune encephalomyelitis (EAE), and block P2X7-dependent pore formation and related ion channel currents. These readouts provide additional means by which to assess the breadth of P2X7 receptor functions modulated by these antagonists. Because the lipophilicity of these drugs is increased, they possess outstanding promise for delivery to the CNS, where the ability to cross the blood barrier is often a challenge.
3) Acylhdranzine antagonists
Similar to those discussed above, patent US20060276505A1, filed by Abbott Laboratories, also bases the utility of selected acyl hydrazide and N’-quinoline acyl hydrazide compounds to decrease inflammation in animal models, on their ability to inhibit IL-1β release. These studies were performed both in the THP-1 monocytic cell line and in vivo [109]. The greatest inhibition of IL-1β release was demonstrated by the 3-chloro-1-adamantyl variation of the quinoline derived acyl hydrazide (summarized by Nelson et al. [110]). Additional acyl compounds were tested using a murine model in which IL-1β levels were decreased and latency of paw withdrawal was increased, indicating effective reductions in tactile allodynia, and hyperalgesia when tested using the Ching and CFA models. Antagonist activity at both the human and rat P2X7 receptor was shown to be similar for one quinoline derived acyl hydrazide compound derivative (1-(4 methoxyphenyl)cyclohexyl).
4) Biaromatic P2X7 Antagonists
Another variation of P2X7 receptor antagonists is presented within patent US20080146612A1, where inventors use a substituted biaromatic group [111]. The addition of the cyclohexylmethyl or cyclohephylmethal groups to the biaromatic-amide derivatives allows for high P2X7 receptor antagonist activity as assessed by their ability to inhibit BzATP-induced P2X7-dependent pore activity as assessed by ethidium bromide uptake. Only compounds able to significantly inhibit dye uptake were included in the patent, however, other measurements of P2X7 receptor function were not presented.
5) Heterocyclic Compounds & Amide Derivatives
The heterocyclic compounds in patents US20080132550A1 and US20080009541A1 both target P2X7 ion channel function. The compound in US20080132550A1 was evaluated for its ability to antagonize the P2X7 receptor using pore formation and Ca2+ influx in HEK293 cells expressing recombinant human P2X7 receptors [112]. Of note, this patent was the only one to examine the ability to prevent ischemic brain damage following a 2 hour ischemic episode and 24 hour recovery period. In addition to direct examination of the ischemic brains, functional tests including elicited forelimb placing, postural reflex and shoulder push resistance were performed.
Particularly interesting is patent US20080009541A1, which was designed to specifically block the binding of ATP to the ligand binding domain of the P2X7 receptor [113]. This heterocylic amide derivative is unlike any of the other P2X7 receptor antagonists discussed up to this point, which do not target specific domains or motifs in the P2X7 receptor protein. The targeting ability of the compound patented was tested both in vivo and in vitro. The first model employed the use of human recombinant P2X7 receptor-expressing HEK cells. They tested the ability of the bi-aromatic compound to modulate intracellular calcium levels after BzATP treatment. In a second model, they tested its ability to reduce acute inflammatory and neuropathic pain in rats. The ability to directly antagonize the binding of ATP to P2X7 receptors provides for a range of applications. In addition to their therapeutic potential, these drugs will likely also have use in the laboratory due to the limited availability of drugs that show specificity towards the P2X7 receptor [114]. Of the P2X7 targeting drugs currently available, all exert effects on other P2X receptors, if not some P2Y receptors as well. For example, oATP, suramin, BBG and PPADS inhibit most other P2X and P2Y receptors. In addition, many of these non-selective antagonists also block certain ectonucleotidases, a number of intracellular enzymes, and a variety of other ligand-gated ion channels [29, 115]. The recently developed heterocyclic amine, A-839977 (Fig. (2)), by Abbott Laboratories appears to be the most specific drug available to date, but further study is needed to confirm this. The availability of these heterocyclic compounds in the future is sure to be an excellent tool for therapeutic treatment of ischemic injury as well as basic P2X7 receptor research.
6) Aromatic Amine Antagonists
Lastly, Abbott Laboratories has three patents filed for aromatic amines that inhibit P2X7 receptors US20070259920A1, US20070105842A1 and US20080076924A1. These three patents describe drugs to be used for the treatment of a variety of CNS-related inflammatory diseases [116–118]. Compared to the other compounds mentioned above, these drugs are structurally distinct because their basic structures contain aromatic amine functional groups. Again, as with many of the other P2X7 antagonists discussed above, these drugs were shown to inhibit IL-1β release in vitro and exert anti-nociceptive effects in vivo as a measure of their antagonist activities at P2X7 receptors.
CURRENT & FUTURE DEVELOPMENTS
Evidence of a role for P2X7 receptors in neuroinflammation and neurodegeneration is becoming increasingly clear, both in vivo and in vitro [54]. Antagonists of these receptors may therefore be effective for the treatment of inflammation associated with progressive, neurodegenerative conditions; a recent review by Prof. Burnstock provides an in-depth summary of many currently available P2 receptor agonists and antagonists, and their potential uses in the treatment of CNS disorders [119]. In light of these new tools, it is important to keep in mind that expected antagonist effects in vivo may be different from those in in vitro pre-clinical studies due to the variable extracellular environments encountered in different disease states. For example, P2X7 inhibition studies by suramin, KN-62, oATP, PPADS, and BBG have IC50 values that vary by 10- to 20- fold based on the agonist used, and the extracellular conditions to which they are exposed [48, 96, 120]. Thus, when comparing the efficacy of different P2X7 receptor antagonists, one must take care not to directly compare absolute IC50 values from study to study, as many factors may influence the reported values. Another caveat in the search for effective P2X7 antagonists is the consideration of target specificity. The ability to target a specific receptor without cross-reactivity will allow for focused treatment with minimal side effects. Lastly, many of the studies presented in the above patent applications dealt primarily with the ability of these novel antagonists to decrease cytokine release from human monocytes following LPS stimulation. Although LPS is an important tool for manipulating CNS inflammation in animal models, P2X7 antagonism in a more physiological setting of neurodegenerative or ischemic inflammation may not be identical. However, the patents discussed here do represent a promising start and a significant advancement in the discovery of tools useful for both research and clinical inquiries into the role of P2X7 receptors in CNS inflammation. These antagonists will also assuredly have beneficial effects on the many other disease states influenced by aberrant P2X7 receptor activation as well.
Acknowledgements
We would like to thank Dr. Khurshid Zaman and the staff at Recent Patents on CNS Drug Discovery for their assistance in locating applicable patents. This work was supported by NIH grant R01 NS049033 (JJW), T32HL007654 (SAF) and R25 GM083252 (MAC).
Footnotes
Conflict of Interest
The authors do not have any conflicts of interest to declare.
REFERENCES
- 1.Golding EM, Robertson CS, Bryan RM., Jr The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin Exp Hypertens. 1999;21(4):299–332. doi: 10.3109/10641969909068668. [DOI] [PubMed] [Google Scholar]
- 2.Kerschensteiner MME, Hohlfeld R. Neuroimmune crosstalk in CNS diseases. Neuroscience. 2009;158(3):1122–1132. doi: 10.1016/j.neuroscience.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Arcuino G, Takano T, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 4.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS ONE. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Feuvre R, Brough D, Rothwell N. Extracellular ATP and P2X7 receptors in neurodegeneration. Eur J Pharmacol. 2002;447(2–3):261–269. doi: 10.1016/s0014-2999(02)01848-4. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari D, Chiozzi P, Falzoni S, et al. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology. 1997;36(9):1295–1301. doi: 10.1016/s0028-3908(97)00137-8. [DOI] [PubMed] [Google Scholar]
- 7.Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265(3):577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 8.Lutz PL, Kabler S. Release of adenosine and ATP in the brain of the freshwater turtle (Trachemys scripta) during long-term anoxia. Brain Res. 1997;769(2):281–286. doi: 10.1016/s0006-8993(97)00719-1. [DOI] [PubMed] [Google Scholar]
- 9.Dunant Y, Israel M. Neurotransmitter release at rapid synapses. Biochimie. 2000;82(4):289–302. doi: 10.1016/s0300-9084(00)00194-2. [DOI] [PubMed] [Google Scholar]
- 10.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26(8–9):959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 11.Matsuka Y, Neubert JK, Maidment NT, Spigelman I. Concurrent release of ATP and substance P within guinea pig trigeminal ganglia in vivo. Brain Res. 2001;915(2):248–255. doi: 10.1016/s0006-8993(01)02888-8. [DOI] [PubMed] [Google Scholar]
- 12.Kasai Y, Ohta T, Nakazato Y, Ito S. Release of dopamine and ATP from PC12 cells treated with dexamethasone, reserpine and bafilomycin A1. J Vet Med Sci. 2001;63(4):367–372. doi: 10.1292/jvms.63.367. [DOI] [PubMed] [Google Scholar]
- 13.Poelchen W, Sieler D, Wirkner K, Illes P. Co-transmitter function of ATP in central catecholaminergic neurons of the rat. Neuroscience. 2001;102(3):593–602. doi: 10.1016/s0306-4522(00)00529-7. [DOI] [PubMed] [Google Scholar]
- 14.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20(8):2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21(7):2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiga H, Tojima T, Ito E. Ca2+ signaling regulated by an ATP-dependent autocrine mechanism in astrocytes. Neuroreport. 2001;12(12):2619–2622. doi: 10.1097/00001756-200108280-00007. [DOI] [PubMed] [Google Scholar]
- 17.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19(2):520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18(21):8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotrina ML, Lin JH, Alves-Rodrigues A, et al. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95(26):15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitz JG. Regulation of cellular ATP release. Trans Am Clin Climatol Assoc. 2007;118:199–208. [PMC free article] [PubMed] [Google Scholar]
- 21.Wink MR, Lenz G, Braganhol E, et al. Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Lett. 2003;198(2):211–218. doi: 10.1016/s0304-3835(03)00308-2. [DOI] [PubMed] [Google Scholar]
- 22.Morrone FB, Oliveira DL, Gamermann P, et al. In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model. BMC Cancer. 2006;6:226. doi: 10.1186/1471-2407-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igarashi H, Yokofujita J, Murakami K, Okada A, AKuroda M. Microglial ecto-Ca(2+)-ATPase activity in a rat model of focal homologous blood clot embolic cerebral ischemia: an enzyme histochemical study. Brain Res Bull. 2003;60(1–2):93–104. doi: 10.1016/s0361-9230(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 24.Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol. 1999;276(1):267–278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- 25.Edwards J, Sprung R, Sprague R, Spence D. Chemiluminescence detection of ATP release from red blood cells upon passage through microbore tubing. Analyst. 2001;126(8):1257–1260. doi: 10.1039/b100519g. [DOI] [PubMed] [Google Scholar]
- 26.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Med Sci Monit. 2001;7(4):669–674. [PubMed] [Google Scholar]
- 27.Kaufmann A, Musset B, Limberg SH, et al. "Host tissue damage" signal ATP promotes non-directional migration and negatively regulates toll-like receptor signaling in human monocytes. J Biol Chem. 2005;280(37):32459–32467. doi: 10.1074/jbc.M505301200. [DOI] [PubMed] [Google Scholar]
- 28.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 29.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 30.Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78(2):113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- 31.Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4 / P2X7 heteromeric receptors. Mol Pharmacol. 2007;72(6):1402–1405. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- 32.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109(1–2):210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Boumechache M, Masin M, Edwardson JM, Gorecki DC, Murrell-Lagnado R. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem. 2009;284(20):13446–13454. doi: 10.1074/jbc.M901255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicke A. Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun. 2008;377(3):803–808. doi: 10.1016/j.bbrc.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 35.Gunosewoyo H, Coster MJ, Kassiou M. Molecular probes for P2X7 receptor studies. Curr Med Chem. 2007;14(14):1505–1523. doi: 10.2174/092986707780831023. [DOI] [PubMed] [Google Scholar]
- 36.Blanchard DK, Hoffman SL, Djeu JY. Inhibition of extracellular ATP-mediated lysis of human macrophages by calmodulin antagonists. J Cell Biochem. 1995;57(3):452–464. doi: 10.1002/jcb.240570311. [DOI] [PubMed] [Google Scholar]
- 37.Erb L, Lustig KD, Ahmed AH, Gonzalez FA, Weisman GA. Covalent incorporation of 3'-O-(4-benzoyl)benzoyl-ATP into a P2 purinoceptor in transformed mouse fibroblasts. J Biol Chem. 1990;265(13):7424–7431. [PubMed] [Google Scholar]
- 38.Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di Virgilio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996;156(4):1531–1539. [PubMed] [Google Scholar]
- 39.Korngreen A, Ma W, Priel Z, Silberberg SD. Extracellular ATP directly gates a cation-selective channel in rabbit airway ciliated epithelial cells. J Physiol. 1998;508:703–720. doi: 10.1111/j.1469-7793.1998.703bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korngreen A, Priel Z. Purinergic stimulation of rabbit ciliated airway epithelia: control by multiple calcium sources. J Physiol. 1996;497:53–66. doi: 10.1113/jphysiol.1996.sp021749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uzlaner N, Priel Z. Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea. J Physiol. 1999;516:179–190. doi: 10.1111/j.1469-7793.1999.179aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisman GA, De BK, Friedberg I, Pritchard RS, Heppel LA. Cellular responses to external ATP which precede an increase in nucleotide permeability in transformed cells. J Cell Physiol. 1984;119(2):211–219. doi: 10.1002/jcp.1041190211. [DOI] [PubMed] [Google Scholar]
- 43.Wiley JS, Jamieson GP, Mayger W, Cragoe EJ, Jr, Jopson M. Extracellular ATP stimulates an amiloride-sensitive sodium influx in human lymphocytes. Arch Biochem Biophys. 1990;280(2):263–268. doi: 10.1016/0003-9861(90)90328-v. [DOI] [PubMed] [Google Scholar]
- 44.Wiley JS, Chen R, Jamieson GP. The ATP4-receptor-operated channel (P2Z class) of human lymphocytes allows Ba2+ and ethidium+ uptake: inhibition of fluxes by suramin. Arch Biochem Biophys. 1993;305(1):54–60. doi: 10.1006/abbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- 45.Wiley JS, Chen JR, Snook MB, Jamieson GP. The P2Z-purinoceptor of human lymphocytes: actions of nucleotide agonists and irreversible inhibition by oxidized ATP. Br J Pharmacol. 1994;112(3):946–950. doi: 10.1111/j.1476-5381.1994.tb13172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khakh BS, Burnstock G, Kennedy C, et al. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53(1):107–118. [PubMed] [Google Scholar]
- 47.Valera S, Hussy N, Evans RJ, et al. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 48.Michel AD, Chessell IP, Humphrey PP. Ionic effects on human recombinant P2X7 receptor function. Naunyn Schmiedebergs Arch Pharmacol. 1999;359(2):102–109. doi: 10.1007/pl00005328. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg TH, Silverstein SC. Extracellular ATP4- promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987;262(7):3118–3122. [PubMed] [Google Scholar]
- 50.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo J. 2006;25(21):5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282(4):2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 52.Bargiotas P, Monyer H, Schwaninger M. Hemichannels in cerebral ischemia. Curr Mol Med. 2009;9(2):186–194. doi: 10.2174/156652409787581646. [DOI] [PubMed] [Google Scholar]
- 53.Di Virgilio F, Chiozzi P, Falzoni S, et al. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5(3):191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- 54.Romagnoli R, Baraldi PG, Cruz-Lopez O, et al. The P2X7 receptor as a therapeutic target. Expert Opin Ther Targets. 2008;12(5):647–661. doi: 10.1517/14728222.12.5.647. [DOI] [PubMed] [Google Scholar]
- 55.Surprenant A, North RA. Signaling at Purinergic P2X Receptors. Annu Rev Physiol. 2009;71:333–339. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 56.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442(7102):527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 57.Volonte C, Amadio S, Cavaliere F, D'Ambrosi N, Vacca F, Bernardi G. Extracellular ATP and neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2(6):403–412. doi: 10.2174/1568007033482643. [DOI] [PubMed] [Google Scholar]
- 58.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 59.Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24(28):6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson CM, Nedergaard M. Emerging challenges of assigning P2X7 receptor function and immunoreactivity in neurons. Trends Neurosci. 2006;29(5):257–262. doi: 10.1016/j.tins.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Matute C, Torre I, Perez-Cerda F, et al. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci. 2007;27(35):9525–9533. doi: 10.1523/JNEUROSCI.0579-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26(5):1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jabs R, Matthias K, Grote A, Grauer M, Seifert G, Steinhauser C. Lack of P2X receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia. 2007;55(16):1648–1655. doi: 10.1002/glia.20580. [DOI] [PubMed] [Google Scholar]
- 64.Ballerini P, Rathbone MP, Di Iorio P, et al. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport. 1996;7(15–17):2533–2537. doi: 10.1097/00001756-199611040-00026. [DOI] [PubMed] [Google Scholar]
- 65.el-Moatassim C, Dornand J, Mani JC. Extracellular ATP and cell signalling. Biochim Biophys Acta. 1992;1134(1):31–45. doi: 10.1016/0167-4889(92)90025-7. [DOI] [PubMed] [Google Scholar]
- 66.Ross PE, Ehring GR, Cahalan MD. Dynamics of ATP-induced calcium signaling in single mouse thymocytes. J Cell Biol. 1997;138(5):987–998. doi: 10.1083/jcb.138.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franke H, Gunther A, Grosche J, et al. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63(7):686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 68.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278(15):13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 69.Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49(2):245–258. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J Immunol. 2000;165(8):4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- 71.Ohtsuki T, Ruetzler CA, Tasaki K, Hallenbeck JM. Interleukin-1 mediates induction of tolerance to global ischemia in gerbil hippocampal CA1 neurons. J Cereb Blood Flow Metab. 1996;16(6):1137–1142. doi: 10.1097/00004647-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176(7):3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 73.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 Receptor Differentially Couples to Distinct Release Pathways for IL-1{beta} in Mouse Macrophage. J Immunol. 2008;180(11):7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 74.Silverman WR, De Rivero Vaccari JP, Locovei S, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernardino L, Balosso S, Ravizza T, et al. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptor-mediated IL-1beta release. J Neurochem. 2008;106(1):271–280. doi: 10.1111/j.1471-4159.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- 76.Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27(18):4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panenka W, Jijon H, Herx LM, et al. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. Journal of Neuroscience. 2001;21(18):7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334(6184):698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 79.Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol. 2000;130(6):1418–1424. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sommer C, Petrausch S, Lindenlaub T, Toyka KV. Neutralizing antibodies to interleukin 1-receptor reduce pain associated behavior in mice with experimental neuropathy. Neurosci Lett. 1999;270(1):25–28. doi: 10.1016/s0304-3940(99)00450-4. [DOI] [PubMed] [Google Scholar]
- 81.Honore P, Donnelly-Roberts D, Namovic M, et al. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1alphabeta knockout mice. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.05.018. epub. [DOI] [PubMed] [Google Scholar]
- 82.Fulgenzi A, Ticozzi P, Gabel CA, et al. Periodate oxidized ATP (oATP) reduces hyperalgesia in mice: involvement of P2X7 receptors and implications for therapy. Int J Immunopathol Pharmacol. 2008;21(1):61–71. doi: 10.1177/039463200802100108. [DOI] [PubMed] [Google Scholar]
- 83.Chessell IP, Hatcher JP, Bountra C, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114(3):386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151(5):571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Honore P, Donnelly-Roberts D, Namovic MT, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319(3):1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- 86.Nelson DW, Sarris K, Kalvin DM, et al. Structure-activity relationship studies on N'-aryl carbohydrazide P2X7 antagonists. J Med Chem. 2008;51(10):3030–3034. doi: 10.1021/jm701516f. [DOI] [PubMed] [Google Scholar]
- 87.McGaraughty S, Chu KL, Namovic MT, et al. P2X7-related modulation of pathological nociception in rats. Neuroscience. 2007;146(4):1817–1828. doi: 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 88.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 89.Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors) Mol Pharmacol. 1995;48(2):178–183. [PubMed] [Google Scholar]
- 90.Bianchi BR, Lynch KJ, Touma E, et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376(1–2):127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 91.Communi D, Robaye B, Boeynaems JM. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999;128(6):1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990;265(8):4315–4320. [PubMed] [Google Scholar]
- 93.Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54(1):22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- 94.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58(1):82–88. [PubMed] [Google Scholar]
- 95.Soltoff SP, McMillian MK, Talamo BR. Coomassie Brilliant Blue G is a more potent antagonist of P2 purinergic responses than Reactive Blue 2 (Cibacron Blue 3GA) in rat parotid acinar cells. Biochem Biophys Res Commun. 1989;165(3):1279–1285. doi: 10.1016/0006-291x(89)92741-1. [DOI] [PubMed] [Google Scholar]
- 96.Chessell IP, Michel AD, Humphrey PP. Effects of antagonists at the human recombinant P2X7 receptor. Br J Pharmacol. 1998;124(6):1314–1320. doi: 10.1038/sj.bjp.0701958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nedergaard M, Goldman SA. 20050164975A1. US. 2005
- 98.United States National Institutes of Health. 2009 July 22; http://clinicaltrials.gov/ct2/home.
- 99.United States Patent and Trademark Office. 2009 June 1; www.uspto.gov.
- 100.Guile SD, Alcaraz L, Birkinshaw TN, et al. Antagonists of the P2X(7) receptor. From lead identification to drug development. J Med Chem. 2009;52(10):3123–3141. doi: 10.1021/jm801528x. [DOI] [PubMed] [Google Scholar]
- 101.Carroll WA, Donnelly-Roberts D, Jarvis MF. Selective P2X(7) receptor antagonists for chronic inflammation and pain. Purinergic Signal. 2009;5(1):63–73. doi: 10.1007/s11302-008-9110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dombroski MA, Duplantier AJ, Subramanyam C. 20070142329A1. US. 2007
- 103.Dombrowski MA, Duplantier AJ. 20070281939A1. US. 2007
- 104.Dombroski MA, Duplantier AJ, Subramanyam C. 20060217430A1. US. 2006
- 105.Kelly MG, Kincaid J. 20080039478A1. US. 2008
- 106.Kelly MG, Kincaid J, Fang Y, et al. 20070225324A1. US. 2007
- 107.Kelly MG, Kincaid J. 20070197565A1. US. 2007
- 108.Kelly MG, Kincaid J. 20060217448A1. US. 2006
- 109.Nelson DW, Jarvis MF, Carroll WA. 20060276505A1. US. 2006
- 110.Nelson DW, Gregg RJ, Kort ME, et al. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem. 2006;49(12):3659–3666. doi: 10.1021/jm051202e. [DOI] [PubMed] [Google Scholar]
- 111.Thompson T, Willis P. 20080146612A1. US. 2008
- 112.Shum P, Gross A, Ma L, et al. 20080132550A1. US. 2008
- 113.Chambers LJ, Gleave R, Senger S, Walter DS. 20080009541A1. US. 2008
- 114.Stokes L, Jiang LH, Alcaraz L, et al. Characterization of a selective and potent antagonist of human P2X(7) receptors, AZ11645373. Br J Pharmacol. 2006;149(7):880–887. doi: 10.1038/sj.bjp.0706933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baraldi PG, Di Virgilio F, Romagnoli R. Agonists and antagonists acting at P2X7 receptor. Curr Top Med Chem. 2004;4(16):1707–1717. doi: 10.2174/1568026043387223. [DOI] [PubMed] [Google Scholar]
- 116.Carroll WA, Perez-Medrano A, Li T. 20070259920A1. US. 2007
- 117.Carroll WA, Florjancic AS, Perez-Medrano A, Peddi S. 20070105842A1. US. 2007
- 118.Betschmann P, Carroll WA, Ericsson AM, et al. 20080076924A1. US. 2008
- 119.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7(7):575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 120.Hibell AD, Thompson KM, Xing M, Humphrey PP, Michel AD. Complexities of measuring antagonist potency at P2X(7) receptor orthologs. J Pharmacol Exp Ther. 2001;296(3):947–957. [PubMed] [Google Scholar]
- 121.Rampe D, Wang L, Ringheim GE. P2X7 receptor modulation of beta-amyloid- and LPS-induced cytokine secretion from human macrophages and microglia. J Neuroimmunol. 2004;147(1–2):56–61. doi: 10.1016/j.jneuroim.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 122.Kim SY, Moon JH, Lee HG, Kim SU, Lee YB. ATP released from beta-amyloid-stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp Mol Med. 2007;39(6):820–827. doi: 10.1038/emm.2007.89. [DOI] [PubMed] [Google Scholar]
- 123.McLarnon JG, Ryu JK, Walker DG, Choi HB. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol. 2006;65(11):1090–1097. doi: 10.1097/01.jnen.0000240470.97295.d3. [DOI] [PubMed] [Google Scholar]
- 124.Denlinger LC, Shi L, Guadarrama A, et al. Attenuated P2X7 pore function as a risk factor for virus-induced loss of asthma control. Am J Respir Crit Care Med. 2009;179(4):265–270. doi: 10.1164/rccm.200802-293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yiangou Y, Facer P, Durrenberger P, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6(12) doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Casanovas A, Hernandez S, Tarabal O, Rossello J, Esquerda JE. Strong P2X4 purinergic receptor-like immunoreactivity is selectively associated with degenerating neurons in transgenic rodent models of amyotrophic lateral sclerosis. J Comp Neurol. 2008;506(1):75–92. doi: 10.1002/cne.21527. [DOI] [PubMed] [Google Scholar]
- 127.Andries M, Van Damme P, Robberecht W, Van Den Bosch L. Ivermectin inhibits AMPA receptor-mediated excitotoxicity in cultured motor neurons and extends the life span of a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;25(1):8–16. doi: 10.1016/j.nbd.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 128.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 129.Coutinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A, Ojcius DM. Modulation of P2Z/P2X(7) receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol. 2001;280(1):81–89. doi: 10.1152/ajpcell.2001.280.1.C81. [DOI] [PubMed] [Google Scholar]
- 130.Coutinho-Silva R, Stahl L, Raymond MN, et al. Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003;19(3):403–412. doi: 10.1016/s1074-7613(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 131.Banfi C, Ferrario S, De Vincenti O, et al. P2 receptors in human heart: upregulation of P2X6 in patients undergoing heart transplantation, interaction with TNFalpha and potential role in myocardial cell death. J Mol Cell Cardiol. 2005;39(6):929–939. doi: 10.1016/j.yjmcc.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 132.Sugiyama T, Kobayashi M, Kawamura H, Li Q, Puro DG. Enhancement of P2X(7)-induced pore formation and apoptosis: an early effect of diabetes on the retinal microvasculature. Invest Ophthalmol Vis Sci. 2004;45(3):1026–1032. doi: 10.1167/iovs.03-1062. [DOI] [PubMed] [Google Scholar]
- 133.Kang TC, An SJ, Park SK, Hwang IK, Won MH. P2X2 and P2X4 receptor expression is regulated by a GABA(A) receptor-mediated mechanism in the gerbil hippocampus. Brain Res Mol Brain Res. 2003;116(1–2):168–175. doi: 10.1016/s0169-328x(03)00260-2. [DOI] [PubMed] [Google Scholar]
- 134.Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci. 2008;28(37):9133–9144. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dunn PM. Fertility: purinergic receptors and the male contraceptive pill. Curr Biol. 2000;10(8):305–307. doi: 10.1016/s0960-9822(00)00436-x. [DOI] [PubMed] [Google Scholar]
- 136.Gur S, Ozturk B. Altered relaxant responses to adenosine and adenosine 5'-triphosphate in the corpus cavernosum from men and rats with diabetes. Pharmacology. 2000;60(2):105–112. doi: 10.1159/000028354. [DOI] [PubMed] [Google Scholar]
- 137.Schwiebert EM, Wallace DP, Braunstein GM, et al. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol. 2002;282(4):763–775. doi: 10.1152/ajprenal.0337.2000. [DOI] [PubMed] [Google Scholar]
- 138.Kottgen M, Loffler T, Jacobi C, et al. P2Y6 receptor mediates colonic NaCl secretion via differential activation of cAMP-mediated transport. J Clin Invest. 2003;111(3):371–379. doi: 10.1172/JCI16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li GH, Lee EM, Blair D, et al. The distribution of P2X receptor clusters on individual neurons in sympathetic ganglia and their redistribution on agonist activation. J Biol Chem. 2000;275(37):29107–29112. doi: 10.1074/jbc.M910277199. [DOI] [PubMed] [Google Scholar]
- 140.Birder LA, Ruan HZ, Chopra B, et al. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287(5):1084–1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- 141.Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int. 2004;93(9):1344–1348. doi: 10.1111/j.1464-410X.2004.04858.x. [DOI] [PubMed] [Google Scholar]
- 142.Lammer A, Gunther A, Beck A, et al. Neuroprotective effects of the P2 receptor antagonist PPADS on focal cerebral ischaemia-induced injury in rats. Eur J Neurosci. 2006;23(10):2824–2828. doi: 10.1111/j.1460-9568.2006.04825.x. [DOI] [PubMed] [Google Scholar]
- 143.Barden N, Harvey M, Gagne B, et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(4):374–382. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- 144.Burnstock G. The role of adenosine triphosphate in migraine. Biomed Pharmacother. 1989;43(10):727–736. doi: 10.1016/0753-3322(89)90161-3. [DOI] [PubMed] [Google Scholar]
- 145.Chen L, Brosnan CF. Exacerbation of experimental autoimmune encephalomyelitis in P2X7R−/− mice: evidence for loss of apoptotic activity in lymphocytes. J Immunol. 2006;176(5):3115–3126. doi: 10.4049/jimmunol.176.5.3115. [DOI] [PubMed] [Google Scholar]
- 146.Guo LH, Schluesener HJ. Lesional accumulation of P2X(4) receptor(+) macrophages in rat CNS during experimental autoimmune encephalomyelitis. Neuroscience. 2005;134(1):199–205. doi: 10.1016/j.neuroscience.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 147.Matute C. P2X7 receptors in oligodendrocytes: a novel target for neuroprotection. Mol Neurobiol. 2008;38(2):123–128. doi: 10.1007/s12035-008-8028-x. [DOI] [PubMed] [Google Scholar]
- 148.Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V. The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res. 2006;66(2):907–914. doi: 10.1158/0008-5472.CAN-05-3185. [DOI] [PubMed] [Google Scholar]
- 149.Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A. 2009;106(19):8032–8037. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nagata K, Imai T, Yamashita T, Tsuda M, Tozaki-Saitoh H, Inoue K. Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain. 2009;5:20. doi: 10.1186/1744-8069-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ulmann L, Hatcher JP, Hughes JP, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28(44):11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jun DJ, Kim J, Jung SY, et al. Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J Biol Chem. 2007;282(52):37350–37358. doi: 10.1074/jbc.M707915200. [DOI] [PubMed] [Google Scholar]
- 153.Sato A, Arimura Y, Manago Y, et al. Parkin potentiates ATP-induced currents due to activation of P2X receptors in PC12 cells. J Cell Physiol. 2006;209(1):172–182. doi: 10.1002/jcp.20719. [DOI] [PubMed] [Google Scholar]
- 154.Amadio S, Montilli C, Picconi B, Calabresi P, Volonte C. Mapping P2X and P2Y receptor proteins in striatum and substantia nigra: An immunohistological study. Purinergic Signal. 2007;3(4):389–398. doi: 10.1007/s11302-007-9069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Labasi JM, Petrushova N, Donovan C, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168(12):6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 156.Broom DC, Matson DJ, Bradshaw E, et al. Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-amino-pyrrolidin-1-yl)methyl]-2-chloro-benzamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharmacol Exp Ther. 2008;327(3):620–633. doi: 10.1124/jpet.108.141853. [DOI] [PubMed] [Google Scholar]
- 157.Nino-Moreno P, Portales-Perez D, Hernandez-Castro B, et al. P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo patients with pulmonary tuberculosis. Clin Exp Immunol. 2007;148(3):469–477. doi: 10.1111/j.1365-2249.2007.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]