Abstract
Two beta-tubulin mutants of the unicellular green alga Chlamydomonas reinhardtii have been isolated on the basis of altered sensitivity to the growth-inhibitory effect of colchicine. The two mutations of colR4 and colR15 have been found to be tightly linked, mapping to a previously unmarked site in linkage group XII. The drug-resistance phenotypes of both mutations segregated in genetic crosses with the presence of distinct, acidic variant beta-tubulin isoforms found assembled into the microtubules of the flagella. Analysis of the in vitro translation products of total poly(A)+ RNA from the mutants provided evidence that the variant proteins are altered primary beta-tubulin gene products. Compared to wild type, strains carrying the mutations expressed an increased resistance to the inhibitory effects of colchicine in clonal growth, flagellar assembly, and germination of meiotic products, suggesting that the beta-tubulin altered in the mutants participates in multiple microtubule functions.
Full text
PDF


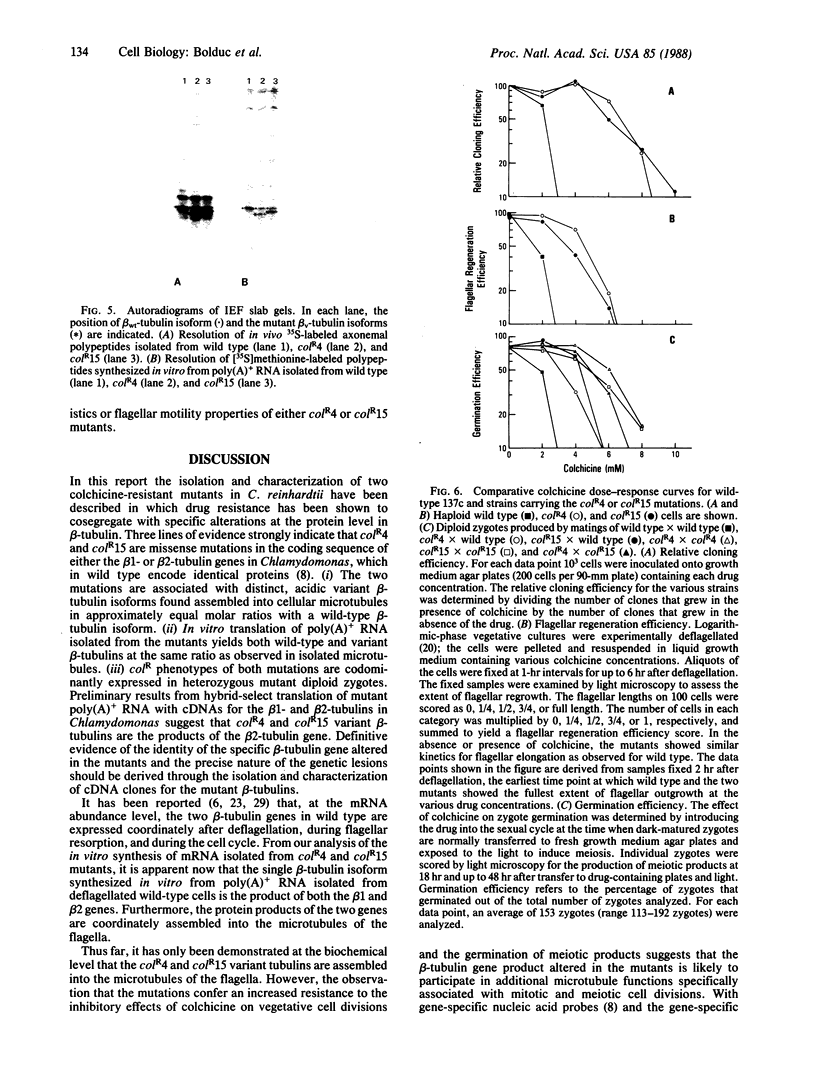

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. M., Huang B., Piperno G., Luck D. J. Central-pair microtubular complex of Chlamydomonas flagella: polypeptide composition as revealed by analysis of mutants. J Cell Biol. 1981 Oct;91(1):69–76. doi: 10.1083/jcb.91.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M., Warr J. R. Colchicine-resistant mutants of Chlamydomonas reinhardi. Exp Cell Res. 1972;71(2):473–475. doi: 10.1016/0014-4827(72)90319-9. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Ares M., Jr, Howell S. H. Cell cycle stage-specific accumulation of mRNAs encoding tubulin and other polypeptides in Chlamydomonas. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5577–5581. doi: 10.1073/pnas.79.18.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke K. J., Young E. E., Buchbinder B. U., Weeks D. P. Coordinate regulation of the four tubulin genes of Chlamydomonas reinhardi. Nucleic Acids Res. 1982 Feb 25;10(4):1295–1310. doi: 10.1093/nar/10.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Sobel M. E., Gottesman M. M. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered beta-tubulin. Cell. 1980 May;20(1):29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Flavin M., Slaughter C. Microtubule assembly and function in Chlamydomonas: inhibition of growth and flagellar regeneration by antitubulins and other drugs and isolation of resistant mutants. J Bacteriol. 1974 Apr;118(1):59–69. doi: 10.1128/jb.118.1.59-69.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Piperno G., Luck D. J. Paralyzed flagella mutants of Chlamydomonas reinhardtii. Defective for axonemal doublet microtubule arms. J Biol Chem. 1979 Apr 25;254(8):3091–3099. [PubMed] [Google Scholar]
- Johnson U. G., Porter K. R. Fine structure of cell division in Chlamydomonas reinhardi. Basal bodies and microtubules. J Cell Biol. 1968 Aug;38(2):403–425. doi: 10.1083/jcb.38.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol. 1983 Jul;97(1):258–263. doi: 10.1083/jcb.97.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE R. P., EBERSOLD W. T. The genetics and cytology of Chlamydomonas. Annu Rev Microbiol. 1960;14:197–216. doi: 10.1146/annurev.mi.14.100160.001213. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. A., Silflow C. D., Wieben E. D., Rosenbaum J. L. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980 Jun;20(2):469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- Luck D., Piperno G., Ramanis Z., Huang B. Flagellar mutants of Chlamydomonas: studies of radial spoke-defective strains by dikaryon and revertant analysis. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3456–3460. doi: 10.1073/pnas.74.8.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morejohn L. C., Bureau T. E., Tocchi L. P., Fosket D. E. Tubulins from different higher plant species are immunologically nonidentical and bind colchicine differentially. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1440–1444. doi: 10.1073/pnas.81.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach M. J., Porro E. B., Yanofsky C. Cloning and characterization of the gene for beta-tubulin from a benomyl-resistant mutant of Neurospora crassa and its use as a dominant selectable marker. Mol Cell Biol. 1986 Jul;6(7):2452–2461. doi: 10.1128/mcb.6.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Luck D. J. Microtubular proteins of Chlamydomonas reinhardtii. An immunochemical study based on the use of an antibody specific for the beta-tubulin subunit. J Biol Chem. 1977 Jan 10;252(1):383–391. [PubMed] [Google Scholar]
- Ringo D. L. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J Cell Biol. 1967 Jun;33(3):543–571. doi: 10.1083/jcb.33.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C. A conditional cell division mutant of Chlamydomonas reinhardii having an increased level of colchicine resistance. Exp Cell Res. 1976 Sep;101(2):251–259. doi: 10.1016/0014-4827(76)90375-x. [DOI] [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M. H., Morris N. R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Chisholm R. L., Conner T. W., Ranum L. P. The two alpha-tubulin genes of Chlamydomonas reinhardi code for slightly different proteins. Mol Cell Biol. 1985 Sep;5(9):2389–2398. doi: 10.1128/mcb.5.9.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow C. D., Lefebvre P. A., McKeithan T. W., Schloss J. A., Keller L. R., Rosenbaum J. L. Expression of flagellar protein genes during flagellar regeneration in Chlamydomonas. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):157–169. doi: 10.1101/sqb.1982.046.01.019. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Rosenbaum J. L. Multiple alpha- and beta-tubulin genes in Chlamydomonas and regulation of tubulin mRNA levels after deflagellation. Cell. 1981 Apr;24(1):81–88. doi: 10.1016/0092-8674(81)90503-1. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Chiang K. S., Kates J. R. Deoxyribonucleic acid replication in meiosis of Chlamydomonas reinhardi. I. Isotopic transfer experiments with a strain producing eight zoospores. J Mol Biol. 1967 Apr 14;25(1):47–66. doi: 10.1016/0022-2836(67)90278-1. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Neff N. F., Botstein D. Isolation and characterization of mutations in the beta-tubulin gene of Saccharomyces cerevisiae. Genetics. 1985 Dec;111(4):715–734. doi: 10.1093/genetics/111.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne P. L. The effects of colchicine on cellular organization in Chlamydomonas. I. Light microscopy and cytochemistry. Am J Bot. 1966 Oct;53(9):908–916. [PubMed] [Google Scholar]
- Warr J. R., Flanagan D., Quinn D. Mutants of Chlamydomonas reinhardii with altered sensitivity to antimicrotubular agents. Exp Cell Res. 1978 Jan;111(1):37–46. doi: 10.1016/0014-4827(78)90234-3. [DOI] [PubMed] [Google Scholar]
- Youngblom J., Schloss J. A., Silflow C. D. The two beta-tubulin genes of Chlamydomonas reinhardtii code for identical proteins. Mol Cell Biol. 1984 Dec;4(12):2686–2696. doi: 10.1128/mcb.4.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]