Abstract
Steroid hormones act in the central and peripheral nervous systems to regulate a variety of functions, including development, cell proliferation, cognition and behavior. Many of these effects of steroid hormones are mediated by their respective receptors, which are members of the nuclear receptor superfamily of transcriptional activators. A variety of cell culture studies reveal that nuclear receptor coactivators are recruited to the steroid receptor complex and are critical in modulating steroid-dependent transcription. Thus, in addition to the availability of the hormone and its receptor, the expression of nuclear receptor coactivators is essential for modulating steroid receptor mediated transcription. This review will discuss the significance of nuclear receptor coactivators in modulating steroid-dependent gene expression in the central and peripheral nervous systems and the regulation of behavior.
Keywords: steroid receptor coactivator-1, SRC-1, steroid hormones, estrogen receptor, progestin receptor, androgen receptor, brain development, sex behavior, spinal cord, peripheral nervous system, Schwann cells, astrocytes
Introduction
Steroid hormones act throughout the body, including in the central and peripheral nervous systems, to profoundly influence physiology and behavior. These hormones elicit changes in brain and other target tissues by binding to their respective receptors, which are members of the nuclear receptor superfamily (Mangelsdorf et al, 1995). Receptors for estrogens (ER) and progestins (PR) can regulate gene transcription via a classic, genomic mechanism. Nuclear receptor coregulators, consisting of coactivators and corepressors, are critical in modulating the transcriptional activity of these steroid receptors, as well as other nuclear receptors (O'Malley, 2006; Rosenfeld et al, 2006). While ER and PR can function in brain independent of ligand and at the membrane to rapidly activate cytoplasmic signaling pathways (Olesen et al, 2005; Kelly and Ronnekleiv, 2008; Mani, 2008; Micevych and Mermelstein, 2008; Vasudevan and Pfaff, 2008; Tetel and Lange, 2009), these receptors can also modulate behavior and physiology by acting through classic, genomic mechanisms. This review will highlight some of the recent findings on the role of nuclear receptor coactivators in genomic mechanisms of steroid action in the mammalian central and peripheral nervous systems and in behavior. For other reviews on coactivators, and detailed discussions of the function of coactivators in bird brain, please see the reviews by Duncan et al. (2009) and Charlier (2009) in this issue.
Steroid receptor structure and genomic mechanisms of action
Steroid receptors, including ER, PR and AR, have a modular domain structure consisting of an amino-terminal region (N-domain), a central DNA binding domain (DBD) and a carboxy-terminal ligand binding domain (LBD) (Mangelsdorf et al., 1995). In general, steroid receptors have two transcriptional activation domains: one in the amino terminal (AF-1) and one in the carboxyl terminal LBD (AF-2) (Tora et al, 1989). Intracellular ER exist in two forms, α and β, which are transcribed from different genes (Jensen et al, 1968; Kuiper et al, 1996). These subtypes differ in their abilities to bind a variety of ligands (Kuiper et al, 1997), distribution in brain (Shughrue et al., 1997; Osterlund et al., 1998; Greco et al., 2001; Mitra et al., 2003), and functions in brain and behavior (Ogawa et al, 1998; Bodo and Rissman, 2006). In rodents and many other species (Schott et al., 1991), PR are expressed in two forms, the full-length PR-B and the N-terminally truncated PR-A, that are transcribed from the same gene. Thus, PR-A and PR-B have identical DBDs and LBDs and differ only in the length of the N-terminus. Under certain cell and promoter contexts, in vitro studies indicate that human PR-B is a stronger transcriptional activator than PR-A (Tung et al, 1993; Vegeto et al., 1993), due to an additional AF domain in the N-terminus of PR-B (Sartorius et al., 1994). PR-A and PR-B appear to have distinct functions in reproductive behavior and physiology (Mulac-Jericevic and Conneely, 2004; Mani et al., 2006).
In the classic genomic mechanism of steroid action (Figure 1), steroid receptors in the absence of hormone are complexed with several chaperone molecules, including heat shock protein (hsp)90 (Pratt et al., 2004). Upon binding hormone, steroid receptors undergo a conformational change that allow receptors to dimerize (DeMarzo et al., 1991). These activated receptor dimers bind directly to specific steroid response elements (SREs) and SRE-like sequences in the promoter regions of target genes (Mangelsdorf et al., 1995). Binding of receptors to DNA increases or decreases gene transcription by altering the rate of recruitment of general transcription factors and influencing the recruitment of RNA polymerase II to the initiation site (Kininis et al 2007). Thus, it is thought that steroids can act in the nervous system via their respective receptors to alter neuronal gene transcription, resulting in profound changes in behavior and physiology (Pfaff, 2005; Blaustein and Mani, 2006).
Figure 1. Classic ligand-dependent genomic mechanism of action of steroid receptors and some of the effects of nuclear receptor coactivators in the central (CNS) and peripheral (PNS) nervous systems.
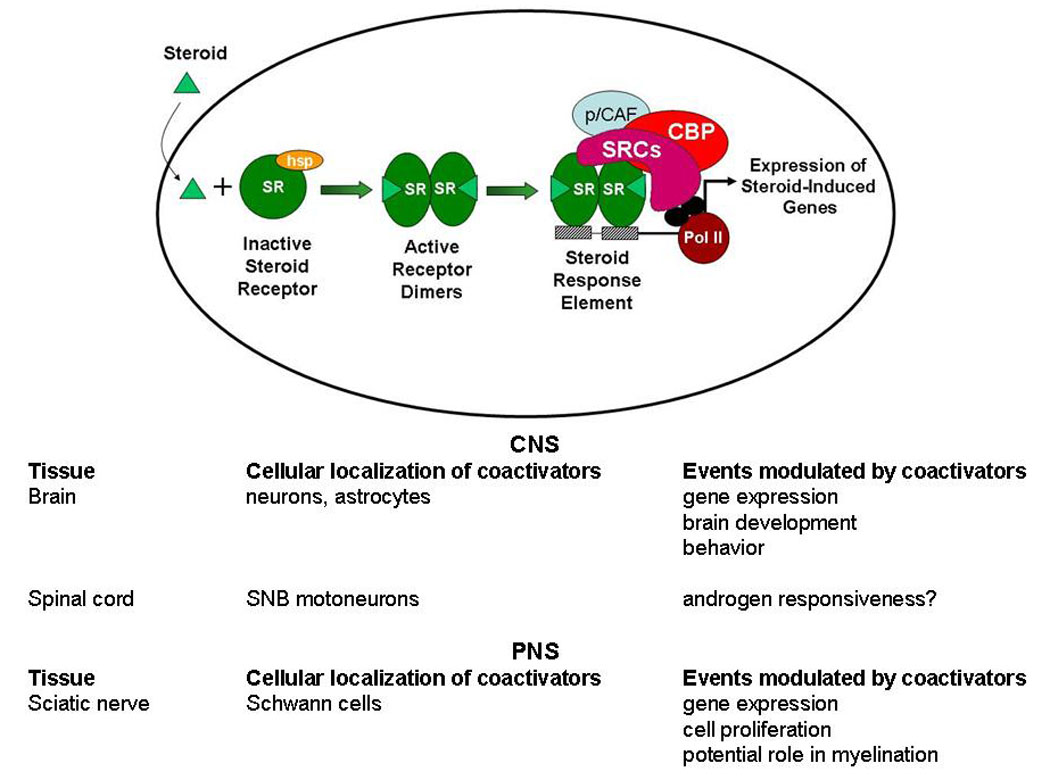
SR, steroid receptor; hsp, heat shock proteins; SRE, steroid response element; SRCs, steroid receptor coactivator family (p160s); CBP, CREB-binding protein; p/CAF, p300/CBP associated factor; Pol II, RNA polymerase II; SNB, spinal nucleus of the bulbocavernosus.
Molecular mechanisms of nuclear receptor coactivators
Nuclear receptor coregulators, consisting of coactivators and corepressors, are critical for the appropriate modulation of nuclear receptor-dependent transcription (O′Malley, 2006; Rosenfeld et al., 2006). In addition, these coregulators have been implicated in a variety of human diseases, including cancer and neurological disorders (Lonard et al., 2007). Nuclear receptor coactivators dramatically increase the transcriptional activity of nuclear receptors through a variety of mechanisms (Figure 1), including acetylation, methylation, phosphorylation and chromatin remodeling (O’Malley, 2006; Rosenfeld et al., 2006). Under most conditions, steroid receptors associate with coactivators when bound to an agonist, but not when bound to an antagonist or in the absence of ligand (Oñate et al., 1995; O′Malley, 2006; Rosenfeld et al., 2006) but compare with the findings of other studies using antagonists (Webb et al., 1998; Dutertre and Smith, 2003). Corepressors interact with nuclear receptors when bound to antagonists or unliganded and decrease nuclear receptor transcription (Rosenfeld et al., 2006). Given that over 300 coregulators have been identified to date (Lanz et al., 2008), this review will focus on only a few of the nuclear receptor coactivators that have been more widely studied in the nervous system and behavior. For an in-depth discussion of corepressors in hormone action in brain, please see the review by Anthony Auger in this issue.
Nuclear receptor coactivators of steroid receptors
The p160 family
Steroid receptor coactivator-1 (SRC-1, also known as NcoA-1/RIP160) was one of the first coactivators found to interact and function with hormone-bound steroid receptors (Oñate et al., 1995). SRC-1 is a member of a larger family of p160 coactivators that includes SRC-2 (GRIP1/TIF2/NCoA-2) (Voegel et al., 1996) and SRC-3 (AIB1/TRAM-1/p/CIP/ACTR/RAC3) (Anzick et al., 1997). The SRC family of coactivators physically interacts with steroid receptors, including ER and PR (Oñate et al., 1995; O′Malley, 2006). In cell culture, hormone-induced transactivation of PR is reduced by coexpression of ERα, presumably due to squelching or sequestering of shared coactivators (Oñate et al., 1995). This squelching can be reversed by over-expression of SRC-1, suggesting that coactivators are a limiting factor required for full transcriptional activation of receptors. In further support, over-expression of SRC-1 relieves thyroid hormone receptor induced inhibition of ERα-mediated transcription in a neuroendocrine model (Vasudevan et al., 2001).
While much is known about the molecular mechanisms of nuclear receptor coactivators from a variety of in vitro studies, recent work in knock-out mice has revealed much about the role of coactivators in hormone action in vivo. SRC-1 null mice, while fertile, have decreased responsiveness in progesterone target tissues (Xu et al., 1998), delayed development of cerebellar Purkinje cells (Nishihara et al., 2003) and partial resistance to thyroid hormone (Weiss et al., 1999). However, it should be noted that SRC-2 is up-regulated in many steroid sensitive tissues, including brain, suggesting that increased expression of this coactivator compensates for the loss of SRC-1 (Xu et al., 1998). This compensation by one member of the p160 family for the loss of another appears to be unique to the SRC-1 mutant. SRC-1 is also critical in maintaining energy balance by regulating both energy intake and expenditure (Wang et al., 2006). It is interesting to note that SRC-1 exists in at least two isoforms, SRC-1a and the truncated SRC-1e (Kalkhoven et al., 1998), which appear to have different functions (Kalkhoven et al., 1998; van der Laan et al., 2008).
SRC-2 also enhances transcriptional activity of a variety of nuclear receptors, including ER, PR and AR (Voegel et al., 1996). Studies with SRC-2 null mice indicate that this coactivator is important in fertility and ductal branching in mammary gland (Gehin et al, 2002; Mukherjee et al., 2007). Microarray analysis of uteri from SRC-2 null mice reveal that SRC-2 is involved in the ability of progesterone to repress specific genes involved in a variety of functions, including cell cycle and immunity (Jeong et al, 2007). Recently, SRC-2 has been found to be essential in the regulation of glucose production via its function as a coactivator of the orphan nuclear receptor RORα (Chopra et al., 2008). While SRC-1/SRC-2 double null mutants die at birth, analysis of single mutants suggests that SRC-2 is more critical for prenatal growth (Mark et al., 2004).
SRC-3/AIB1, which is amplified in human breast tumors, coactivates ER, PR and other nuclear receptors (Anzick et al., 1997). Female SRC-3 null mice, while fertile, have delayed puberty, longer estrous cycles, ovulate fewer eggs and have impaired mammary gland development (Xu et al, 2000; Han et al, 2006). Using chromatin immunoprecipitation assays, GnRH stimulated more efficient recruitment of SRC-3 by PR, on the PRE of a luciferase reporter gene of the gonadotropin α subunit gene promoter, than progesterone (An et al, 2006). These findings suggest that phosphorylation of PR and its interaction with SRC-3 and binding to DNA may play an important role in the possible ligand-independent activation of PR by GnRHs.
Other coactivators of steroid receptors
CREB binding protein (CBP) is a transcriptional activator of cAMP response element-binding protein (CREB) and a coactivator of nuclear receptors, including ER and PR (Smith et al., 1996). Interestingly, mutation of the CBP gene and the subsequent decrease in cAMP-dependent transcription causes Rubinstein-Taybi syndrome, which results in severe mental retardation and a variety of physiological deformities in humans (Petrij et al, 1995). In mice, mutations of CBP result in impaired memory and similar physical deformities (Oike et al, 1999). A variety of in vitro studies indicate that SRC-1 and CBP act synergistically to enhance ER and PR transcriptional function and activity (Smith et al, 1996; Tetel et al, 1999; Liu et al., 2001). CBP and p300/CBP associated factor (p/CAF), as well as SRC-1, possess histone acetyltransferase activity and aid in chromatin remodeling (Kamei et al, 1996; Spencer et al, 1997).
Steroid receptor RNA activator (SRA) is an interesting coactivator in that it functions as an RNA transcript to enhance transcriptional activity of steroid receptors (Lanz et al., 1999; Cavarretta et al., 2002). While liganded ER reduced PR transcriptional activation, addition of SRA reversed this squelching effect of ER (Lanz et al., 1999). SRA mRNA expressed at high levels in liver, skeletal muscle and heart, and at lower levels in brain and placenta (Lanz et al., 1999). Over-expression of SRA in transgenic mice reveals a role for SRA in estrogen-induced expression of PR in mammary gland (Lanz et al., 2003).
Given that there are many other coactivators that are known to interact with steroid receptors (e.g. ERAP 140, TRAP220, E6-AP and PGC-1), coactivator function in hormone action is becoming increasingly complex (O'Malley, 2006; Rosenfeld et al., 2006). Adding another layer to this complexity, coactivator function can be profoundly modulated through a variety of post-translational modifications, including phosphorylation, acetylation, methylation and sumoylation (Han et al, 2009).
Function of nuclear receptor coactivators in the central nervous system and behavior
Coactivator expression in brain and spinal cord
While much is known about the molecular mechanisms of nuclear receptor coactivators from a variety of cell culture studies as discussed above, we are beginning to understand their role in hormone action in the nervous system. SRC-1 mRNA and protein are expressed at high levels in the cortex, hypothalamus, hippocampus and cerebellum of mice, rats and guinea pigs (Misiti et al., 1998; Auger et al, 2000; Martinez de Arrieta et al., 2000; Meijer et al., 2000; Ogawa et al., 2001; Molenda et al., 2002; Nishihara et al, 2003; Setiawan et al, 2004). In addition, the SRC-1 isoform, SRC-1a, is found in high levels in the hypothalamus, whereas SRC- 1e levels are higher in the nucleus accumbens, thalamus, and amygdala (Meijer et al., 2000). SRC-2 is also expressed at high levels in the hypothalamus and hippocampus, while SRC-3 is expressed predominantly in the hippocampus (Apostolakis et al, 2002; Nishihara et al., 2003; McGinnis et al., 2007). In addition to the p160s, other nuclear receptor coactivators, including CBP, TRAP220 and ERAP140, are expressed at high levels in steroid-sensitive brain regions (Stromberg et al., 1999; Auger et al, 2002b; Galeeva et al, 2002; Molenda et al, 2002; Shao et al., 2002)
In order for coactivators to function with steroid receptors, they must be expressed in the same cells. Estradiol (E)-priming dramatically increases the expression of PR in a variety of brain regions, including the medial preoptic area (MPOA), the ventromedial nucleus of the hypothalamus (VMN), the arcuate nucleus (ARC) and the midbrain central gray (MCG) (MacLusky and McEwen, 1978; Blaustein and Turcotte, 1989; Warembourg et al, 1989; Lauber et al., 1991; Scott et al., 2002; Brinton et al., 2008). Indeed, we found that SRC-1 is expressed in the majority of E-induced PR cells in reproductively-relevant brain regions, including the VMN, MPOA and ARC (Tetel et al., 2007). In addition, the majority of E-induced PR cells in these same brain regions coexpress CBP. Given that virtually all estradiol-induced PR cells in the hypothalamus contain ERα (Blaustein and Turcotte, 1989; Warembourg et al., 1989), these findings suggest that these specialized cells represent functional sites of interaction between ovarian steroid receptors and coactivators (SRC-1 and CBP) in brain (Tetel et al., 2007).
Expression of coactivators has also been studied in the rodent spinal cord. The spinal nucleus of the bulbocavernosus (SNB) is a sexually dimorphic cluster of motoneurons, with males having more motoneurons than females (Johansen et al., 2004; Sengelaub and Forger, 2008). In male rats, the SNB motoneurons innervate the bulbocavernosus and levator ani muscles that attach to the penis. The SNB, which is important in male sex behavior, is androgen-responsive and undergoes androgen-dependent changes across development. SRC-1, SRC-2, CBP and p300 are highly expressed in androgen-responsive SNB motoneurons (Matsumoto, 2002; Monks et al, 2003; O'Bryant and Jordan, 2004; Ranson et al, 2005). Interestingly, the number and area of SNB motoneurons were not different in SRC-1 null mice compared with wild-type littermates, suggesting that SRC-1 is not essential for the development and maintenance of this sexually dimorphic neuromuscular system (Monks et al., 2003). It may be that SRC-1 does not function in AR mediated transcription in the SNB or that the loss of SRC-1 was compensated by another coactivator such as SRC-2, which is up-regulated in many tissues in SRC-1 null mice (Xu et al., 1998). In normal aging, there is a decrease in the number and size of SNB motoneurons, as well as a decrease in male copulatory behavior (Johansen et al, 2004; Sengelaub and Forger, 2008). A decrease in SRC-1 and CBP expression was detected in the SNB of aging rats, which may contribute to some of the age-related decreases in the SNB and in male copulatory behavior (Matsumoto, 2002; Ranson et al., 2003).
Regulation of coactivator expression in the CNS
The presence or absence of a coactivator(s) within an individual cell is likely to be critical for responsiveness of a particular receptor system. Therefore, studying the regulation of coactivator expression is essential to understanding hormone action in the nervous system. A number of studies indicate that hormones can regulate coactivator expression. SRC-1 is expressed in a sexually dimorphic manner in the pituitary gland, with males having higher mRNA (Misiti et al, 1998) and protein (Bousios et al, 2001) levels than females. Ovariectomy decreases SRC-1 expression in the VMH, while estradiol reverses this effect (Mitev et al, 2003). In the hypothalamus of cycling female rats, SRC-1 levels were lowest during diestrus, and highest at proestrus and estrus (Camacho-Arroyo et al., 2005). Interestingly, the endocrine disruptor 4-methylbenzylidene camphor (4-MBC), which has estrogenic activity and also interferes with the thyroid axis, increases SRC-1 mRNA in the VMH and MPOA of female rats (Maerkel et al, 2007). This effect of 4-MBC could further accentuate its estrogenic effect and alter other nuclear receptor signaling pathways. In males, testosterone treatment does not influence SRC-1 expression in the rat hypothalamus (McGinnis et al., 2007) or the hamster MPOA, BNST, ARC and amygdala (Tetel et al., 2004). In contrast, testosterone decreases SRC-2 expression in hypothalamus of male rats (McGinnis et al., 2007). Finally, thyroid hormone reduces SRC-1 expression in neonatal mouse cerebellum (Ramos and Weiss, 2006) and rat cortex and dentate gyrus (Iannacone et al, 2002).
In addition to gonadal steroids, it appears that glucocorticoids and stress regulate SRC-1 expression. Treatment of male rats with the synthetic glucocorticoid, dexamethasone, reduces SRC-1 mRNA in brain, but has no effects on SRC-2, SRC-3, CBP or p/CAF levels (Kurihara et al., 2002). Chronic exposure of adrenalectomized male rats to high levels of corticosterone decreases SRC-1e mRNA in the anterior pituitary, but does not alter SRC-1 mRNA levels in the hippocampus (Meijer et al., 2005). In rats, acute restraint stress decreases SRC-1 expression in the male and female hypothalamus and male frontal cortex, and increases SRC-1 levels in the male pituitary and the female hippocampus (Bousios et al, 2001). Taken together, these studies suggest that coactivators are important in modulating the glucocorticoid-mediated stress response in a brain region- and sex-specific manner.
Daylength has profound effects on neuroendocrine function, including the regulation of reproduction (Bittman et al., 1990). In male Siberian hamsters, we found that short days reduced SRC-1 expression in the posteromedial BNST and posterodorsal medial amygdala (Tetel et al., 2004). In addition, SRC-1 levels in the hippocampus, hindbrain and optic lobes fluctuate through the day in Japanese quail (Charlier et al., 2006); and see the review by Charlier in this issue). Given that both Siberian hamsters and Japanese quail have seasonal cycles, this photoperiodic regulation of SRC-1 may contribute to androgen regulation of seasonal reproduction.
Finally, studies suggest that degradation of coactivators is an important aspect of their regulation. Treatment with a 26S proteasome inhibitor increases SRC-1 expression in the hypothalamus, POA and hippocampus (Villamar-Cruz et al., 2006). In addition, this same treatment up-regulated PR and ERβ in the same brain regions, while ERα was increased only in the POA. These findings suggest that degradation of important components of steroid action, the receptors and coactivators, are regulated in a brain region-specific manner.
Coactivators in brain development
A classic example of hormone-dependent sexual differentiation of the brain is the development of the rat sexually dimorphic nucleus (SDN) of the POA, which is 3–4 times larger in males than females (Gorski et al., 1980). In collaboration with Tony Auger and Peg McCarthy, we investigated the role of SRC-1 in hormone-dependent sexual differentiation of the SDN (Auger et al., 2000). On postnatal days (PN) 0–2, the hypothalami of female rat pups were bilaterally infused with antisense oligonucleotides (ODNs) to SRC-1 mRNA or scrambled control ODNs. On PN1, female pups were treated with the aromatizable androgen, testosterone propionate, to increase SDN volume. At PN13, androgenized females treated earlier with antisense to SRC-1 had reduced SDN volumes compared to animals treated with control ODNs. The testosterone surge in male rats just after birth, which can be aromatized to E, suppresses the development of female sexual behavior in adulthood (Whalen and Edwards, 1967; Sodersten, 1978). In addition, this testosterone surge is critical for the development of masculine sexual behavior in the adult rat and is mediated by AR (Whalen and Edwards, 1967). To test if SRC-1 was critical in development of sexual behavior, androgenized female and male rats were treated with SRC-1 antisense or control ODNs on PN0–2 (Auger et al., 2000). Males were castrated in adulthood and following testosterone treatment, were tested for male and female sex behavior. Males and androgenized females treated with SRC-1 antisense displayed higher levels of female sexual behavior than did rats treated with control ODNs. Interestingly, male sexual behavior in these animals did not differ. Thus, these findings suggest that reduction of SRC-1 in brain decreases ER activity, and thus alters brain development and inhibits the defeminizing actions of estrogen during development (Auger et al., 2000).
On the day of birth, CBP is expressed in a dimorphic manner in the mPOA and VMN, suggesting that gonadal steroids alter levels of CBP during development (Auger et al., 2002a). In this same study, testosterone-treated females that received CBP antisense in the hypothalamus on PN0–2 displayed higher levels of lordosis than androgenized females treated with control ODNs. However, CBP antisense treatment did not affect development of male sexual behavior in these androgenized females. Taken together with the previous study, it appears that both SRC-1 and CBP are necessary for the defeminizing actions of ER, but not the masculinizing actions of AR, during early development.
Hormone-dependent gene expression in brain
A classic example of hormone-dependent gene expression is the E-induction of PR in a variety of estrogen-responsive tissues, including brain (MacLusky and McEwen, 1978; Blaustein and Turcotte, 1989; Warembourg et al., 1989; Lauber et al., 1991; Scott et al., 2002; Brinton et al., 2008). Estradiol-induction of PR gene expression in the VMH is important for hormone-dependent female sexual behavior (Pleim et al., 1989). Therefore, we tested the hypothesis that SRC-1 and CBP are critical in modulating ER-mediated transactivation of the PR gene in the VMN. Infusions of antisense ODNs to SRC-1 and CBP mRNA into one side of the VMN of adult female rats reduced the expression of ER-mediated activation of PR gene expression compared to the contralateral control ODN-treated VMN (Molenda et al., 2002). These findings extend previous in vitro studies indicating that SRC-1 and CBP function together to modulate ER activity (Smith et al., 1996). Another study in brain supports our findings of SRC-1 function in ER-mediated induction of PR in the VMN and extend them to include a role of SRC-2, but not SRC-3 (Apostolakis et al., 2002). For a discussion of coactivators in hormone-dependent gene expression in bird brain, see the reviews by Duncan et al. and Charlier in this issue.
In a variety of neuroendocrine cell culture models, the p160 coactivators have been shown to function in GR action. The three p160s are expressed in primary cultures of rat astrocytes (Grenier et al., 2006). Interestingly, expression of SRC-1 and SRC-2 was mainly nuclear, while SRC-3 was expressed predominantly in the lumen of the Golgi apparatus (Grenier et al., 2006). Over-expression and siRNA knockdown experiments using these astrocytes revealed that GR recruited SRC-1e, SRC-2 and to a lesser extent SRC-3, to a minimal glucocorticoid-sensitive reporter gene (Grenier et al., 2006). In further support of this SRC-1 isoform specific effect on GR transcriptional activity, SRC-1a, but not SRC-1e, enhanced hormone-dependent GR-mediated repression of the corticotropin-releasing hormone gene in cell culture (van der Laan et al., 2008). In the astrocyte model, CBP enhanced GR transcriptional activity, while p300 suppressed it (Fonte et al., 2007). Taken together, these findings reveal the complexity with which the p160s can act, in concert with secondary coactivators such as CBP and p300, in a cell type-specific manner to modulate GR responsiveness. To add to this complexity, these results in astrocytes of the CNS differ from those in Schwann cells of the PNS, which are discussed below.
In summary, there is mounting evidence, in vivo and in cell culture, that nuclear receptor coactivators are essential for full steroid receptor transcriptional activity in the central nervous system. These findings indicate that coactivators act in a brain region- and cell type-specific manner to modulate hormone responsiveness in the CNS.
Coactivators and hormone-dependent behaviors
Given that nuclear receptor coactivators are critical for hormone-dependent gene expression in brain, we tested the hypothesis that coactivators act in brain to modulate the expression of hormone-dependent behaviors (Molenda et al., 2002). Female rats treated with antisense to both SRC-1 and CBP mRNA into the VMN displayed reduced levels of hormone-dependent female sexual receptivity compared to scrambled treated controls (Molenda et al, 2002). Another study supported these findings with SRC-1 and extended them to include a role for SRC-2, but not SRC-3, in hormone-dependent lordosis (Apostolakis et al, 2002).
One limitation of the behavioral experiments discussed above is that they do not isolate the effects of coactivators on specific ER- and PR-dependent aspects of female sexual behavior. Therefore, we asked if coactivators function in the two modes of hormone regulated female reproductive behavior in rats (Molenda-Figueira et al., 2006): estrogen-mediated (elicited by estradiol alone) and progesterone-facilitated (requires estradiol priming followed by progesterone) (Blaustein and Mani, 2006). To test the hypothesis that coactivators function in brain to modulate ER-mediated aspects of female reproductive behavior, animals were injected with estradiol only (Molenda-Figueira et al., 2006). Antisense to SRC-1 and CBP infused into the VMN of animals treated with estradiol alone decreased the frequency and intensity of lordosis, suggesting that these coactivators modulate ER-mediated aspects of female sexual behavior. Proceptive behaviors by the female, which serve to solicit interaction by the male, are PR-dependent and include ear-wiggling and hopping and darting (Hardy and DeBold, 1971; Erskine, 1989). Infusion of antisense to SRC-1 and CBP mRNA into the VMN around the time of progesterone administration reduced the frequency of PR-dependent ear-wiggling and hopping and darting (Molenda-Figueira et al., 2006). Thus, it appears that coactivators function in brain to modulate both PR- and ER-specific aspects of hormone-dependent sexual behaviors in rodents.
Interactions between steroid receptor and coactivators from brain
Our lab has begun to take a proteomics-based approach to study the interactions of steroid receptors with coactivators from rat brain. To test the hypotheses that SRC-1 from brain physically associates with PR and ER subtypes in a ligand-dependent manner, we developed pull-down assays with brain tissue from female rats (Molenda-Figueira et al, 2008). SRC-1 from hypothalamic or hippocampal extracts interacted with both GST-tagged PR-A and PR-B when bound to the agonist R5020. In contrast, very little to no SRC-1 from brain associated with PR-A or PR-B in the absence of ligand or in the presence of the selective PR modulator (SPRM), RU486. These findings that interactions between SRC-1 from brain and PR are agonist-dependent support our previous work indicating a role for hypothalamic SRC-1 in PR-dependent female sexual behavior (Molenda-Figueira et al, 2006) and suggest that SRC-1 may contribute to progestin effects in the hippocampus on memory (Sandstrom and Williams, 2001). Interestingly, we found that SRC-1 from hypothalamus or hippocampus interacts more with PR-B, than with PR-A, suggesting a mechanism by which PR-B may be a stronger transcriptional activator than PR-A.
SRC-1 from hypothalamus or hippocampus also interacted with ERα and ERβ when bound to estradiol, which was confirmed by mass spectrometry (Molenda-Figueira et al., 2008). Very little to no association of SRC-1 from brain was detected with ERα or ERβ in the absence of ligand or in the presence of tamoxifen, suggesting this SERM is functioning as an antagonist to prevent receptor-coactivator interactions. These results support our previous findings that SRC-1 action in the hypothalamus is important for maximal ER-mediated transactivation of the PR gene and expression of female sexual behavior (Molenda et al, 2002; Molenda-Figueira et al, 2006). SRC-1 may function with both ER subtypes in the hippocampus to differentially modulate estrogen’s effects on cognition and stress (Fugger et al., 2000; Isgor et al., 2003; Bodo and Rissman, 2006). Interestingly, SRC-1 from the hippocampus interacted equally with ERα and ERβ, while SRC-1 obtained from hypothalamic extracts interacted more with ERα than with ERβ, suggesting that these brain regions have distinct expression patterns of coregulators involved in these important protein-protein interactions. In addition, it is possible that SRC-1 undergoes differential phosphorylation in these two brain regions, leading to distinct patterns of interaction with receptors. Future experiments will need to apply mass spectrometry analysis to determine if different coregulators are present in the receptor-coactivator complex and/or if SRC-1 undergoes differential phosphorylation in a brain region-specific manner.
Our findings of SRC-1 from brain interacting with receptor subtypes differ from some other studies using cell lines alone (Oñate et al., 1998; Cowley and Parker, 1999; Giangrande et al., 2000; Monroe et al., 2003) and emphasize the importance of using biologically-relevant tissue in investigating these receptor-coactivator interactions. It may be that other coregulators and proteins that are present in tissue (e.g. brain) are important for appropriate coactivator interactions with receptor. Understanding how nuclear receptor coactivators function with various steroid receptors, and their subtypes, is critical to understanding how hormones act in different brain regions to profoundly influence physiology and behavior. Ultimately, mass spectrometry analyses of these receptor-coactivator interactions using brain tissue may allow the identification of novel coregulators involved in the steroid receptor complex in brain.
Nuclear receptor coactivator expression and function in the peripheral nervous system
In addition to the CNS, steroids have profound effects in the peripheral nervous system. For example, neuroactive steroids, including progesterone and its derivatives (e.g. dihydroprogesterone, DHP) act in the PNS to elicit changes in Schwann cell proliferation and morphology and functions associated with myelination (Melcangi et al., 2005). Thus, progesterone and other neuroactive steroids have been proposed as therapeutic agents for treatment of disorders involving peripheral neuropathy (Roglio et al., 2008). These neuroactive steroids act in the PNS via classic genomic and non-genomic mechanisms of action (Melcangi et al., 2005). The three p160s and steroid receptor RNA activator (SRA, discussed above) are expressed in rat Schwann cells in culture and in an immortalized cell line of Schwann cells (MSC80 cells) (Cavarretta et al., 2004; Grenier et al., 2004; Melcangi et al., 2005). In MSC80 Schwann cells, the p160s have differential intracellular distribution: SRC-1 displayed hormone-dependent nucleocytoplasmic shuttling, while SRC-2 remained nuclear and SRC-3 was cytoplasmic (Grenier et al., 2006). Interestingly, SRC-1 mRNA levels were increased in MSC80 cells treated with DHP, suggesting that steroids can regulate coactivator expression in the PNS (Cavarretta et al., 2004).
The Melcangi lab has investigated the role of nuclear receptor coactivators in steroid-dependent gene expression in the PNS. In the sciatic nerve of male rats, progesterone and its derivatives increase the expression of the glycoprotein P0 (P0), which is produced exclusively by Schwann cells and is critical in the maintenance of the multilamella structure of PNS myelin (Melcangi et al., 2005). In MCS80 Schwann cells, over-expression of SRC-1 potentiated the DHP-induced increase in P0 expression, while under-expression of SRC-1 eliminated this increase in P0 expression (Cavarretta et al., 2004). These findings suggest that SRC-1 functions in the PNS to regulate progestin-dependent P0 gene expression and maintenance of myelin. The role of SRC-1 and SRA has also been investigated in the proliferation of Schwann cells, which can occur in the absence of steroids in serum-free media. In MCS80 cells, over-expression of SRC-1 attenuated the Schwann cell proliferation, while over-expression of SRA potentiated it (Melcangi et al., 2005). Taken together, these findings indicate that SRC-1 functions in classic genomic mechanism of neuroactive steroid-dependent gene expression in Schwann cells (Cavarretta et al., 2004). Furthermore, it appears that SRC-1 and SRA influence Schwann cell proliferation in a steroid-independent manner in culture (Melcangi et al., 2005).
In the PNS, coactivators modulate glucocorticoid action, which has been reported to have trophic effects on Schwann cells (Grenier et al., 2004). In MCS80 Schwann cells, GR recruits SRC-1a, SRC-1e or SRC-3, but not SRC-2, in the transactivation of a minimal glucocorticoid-sensitive reporter gene containing two GREs. (Grenier et al., 2004; Grenier et al., 2006). However, on a more complex mouse mammary tumor virus promoter, GR recruits SRC-1e and SRC-2, but not SRC-1a or SRC-3 (Grenier et al., 2004). Furthermore, GR recruits only SRC-1e to the promoter of the endogenous target gene, cytosolic aspartate aminotransferase, in MCS80 cells. Interestingly, this GR recruitment of the p160s in Schwann cells differs from that in astrocytes (see above). Finally, while p300 and CBP usually enhance GR transcription, in MCS80 Schwann cells these coregulators either repressed or had no effect, respectively, on GR-mediated transcription (Fonte et al., 2005). Thus, these data indicate that GR recruits the p160s, as well as p300 and CBP, in a highly promoter-specific fashion, as well as in a CNS- and PNS-specific manner.
Conclusions
The mechanisms by which steroids act in a tissue-specific, and cell type-specific, manner is a fundamental issue in neuroendocrinology. Recent investigations indicate that, in addition to the bioavailability of hormone and receptor levels, nuclear receptor coactivators are critical molecules in modulating steroid receptor-mediated transcription. Work in the nervous system and other steroid-sensitive tissues indicates that nuclear receptor coactivators are critical in the fine-tuning of steroid-responsiveness within individual cells (Figure 1). Understanding the recruitment of different coactivator complexes to the promoter, which is likely to be cell- and tissue-specific, will be critical to understanding how hormones function in the nervous system to regulate complex behaviors. In support, recent findings discussed above indicate that coactivators function differently in the central vs. peripheral nervous systems. It is becoming increasingly apparent that the cellular milieu, including the presence or absence of coactivators, is a critical parameter in modulating steroid action in the nervous system and behavior.
Acknowledgements
I thank Dr. Roberto Melcangi for helpful discussions on sections of this review. Studies contributed by my laboratory were supported by grants from NSF IBN 0080818 and NIH R01 DK61935. .
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An BS, Selva DM, Hammond GL, Rivero-Muller A, Rahman N, Leung PC. Steroid receptor coactivator-3 is required for progesterone receptor trans-activation of target genes in response to gonadotropin-releasing hormone treatment of pituitary cells. J Biol Chem. 2006;281:20817–20824. doi: 10.1074/jbc.M600743200. [DOI] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Ramamurphy M, Zhou D, Onate S, O'Malley B. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Molecular Endocrinology. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 mediates the development of sex specific brain morphology and behavior. Proceedings of the National Academy of Sciences USA. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger AP, Perrot-Sinai TS, Auger CJ, Ekas LA, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002a;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger CJ, Bentley GE, Auger AP, Ramamurthy M, Ball GF. Expression of Camp response element binding protein-binding protein in the song control system and hypothalamus of adult European starlings (Sturnus vulgaris) Journal of Neuroendocrinology. 2002b;14:805–813. doi: 10.1046/j.1365-2826.2002.00842.x. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Hegarty CM, Layden MQ, Jonassen JA. Influences of Photoperiod on Sexual Behaviour, Neuroendocrine Steroid Receptors and Adenohypophysial Hormone Secretion and Gene Expression in Female Golden Hamsters. JMolecularEndocrinol. 1990;5:15–25. doi: 10.1677/jme.0.0050015. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Turcotte JC. Estradiol-induced progestin receptor immunoreactivity is found only in estrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology. 1989;49:454–461. doi: 10.1159/000125152. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Mani SK. Feminine sexual behavior from neuroendocrine and molecular neurobiological perspectives. In: Blaustein JD, editor. Handbook of Neurochemistry and Molecular Neurobiology. New York: Springer; 2006. pp. 95–150. [Google Scholar]
- Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Bousios S, Karandrea D, Kittas C, Kitraki E. Effects of gender and stress on the regulation of steroid receptor coactivator-1 expression in the rat brain and pituitary. The Journal of Steroid Biocheminstry and Molecular Biology. 2001;78:401–407. doi: 10.1016/s0960-0760(01)00123-6. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: beta with ERbrain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Neri-Gomez T, Gonzalez-Arenas A, Guerra-Araiza C. Changes in the content of steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid hormone receptors in the rat brain during the estrous cycle. The Journal of Steroid Biocheminstry and Molecular Biology. 2005;94:267–272. doi: 10.1016/j.jsbmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Cavarretta IT, Martini L, Motta M, Smith CL, Melcangi RC. SRC-1 is involved in the control of the gene expression of myelin protein Po. J Mol Neurosci. 2004;24:217–226. doi: 10.1385/JMN:24:2:217. [DOI] [PubMed] [Google Scholar]
- Cavarretta ITR, Mukopadhyay R, Lonard DM, Cowsert LM, Bennet CF, O'Malley B, Smith CL. Reduction of coactivator expression by antisense oligodeoxynucleotides inhibits ERa transcriptional activity and MCF-7 proliferation. Molecular Endocrinology. 2002;16(2):253–269. doi: 10.1210/mend.16.2.0770. [DOI] [PubMed] [Google Scholar]
- Charlier TD. Importance of steroid receptor coactivators in the modulation of steroid action on brain and behavior. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.05.004. in press. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Plasticity in the expression of the steroid receptor coactivator-1 in the Japanese quail brain: Effect of sex, testosterone, stress and time of the day. Neuroscience. 2006;172:333–343. doi: 10.1016/j.neuroscience.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O'Malley BW. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science. 2008;322:1395–1399. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley SM, Parker MG. A comparison of transcriptional activation by ER alpha and ER beta. J Steroid Biochem Mol Biol. 1999;69:165–175. doi: 10.1016/s0960-0760(99)00055-2. [DOI] [PubMed] [Google Scholar]
- DeMarzo A, Beck CA, Oñate SA, Edwards DP. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proceedings of the National Academy of Sciences USA. 1991;88:72–76. doi: 10.1073/pnas.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KA, Jimenez P, Carruth LL. The selective estrogen alpha coactivator, RPL7, and sexual differentiation of the songbird brain. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.04.023. in press. [DOI] [PubMed] [Google Scholar]
- Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-Binding Protein (CBP) with estrogen receptor- à: Regulation by phosphorylation sites in the A/B region depends on other receptor domains. Molecular Endocrinology. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: A review. HormsBehav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Fonte C, Trousson A, Grenier J, Schumacher M, Massaad C. Opposite effects of CBP and p300 in glucocorticoid signaling in astrocytes. J Steroid Biochem Mol Biol. 2007;104:220–227. doi: 10.1016/j.jsbmb.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Fonte C, Grenier J, Trousson A, Chauchereau A, Lahuna O, Baulieu EE, Schumacher M, Massaad C. Involvement of {beta}-catenin and unusual behavior of CBP and p300 in glucocorticosteroid signaling in Schwann cells. Proc Natl Acad Sci U S A. 2005;102:14260–14265. doi: 10.1073/pnas.0506930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Galeeva A, Treuter E, Tuohimaa P, Pelto-Huikko M. Comparative distribution of the mammalian mediator subunit thyroid hormone receptor-associated protein (TRAP220) mRNA in developing and adult rodent brain. European Journal of Neuroscience. 2002;16:671–683. doi: 10.1046/j.1460-9568.2002.02115.x. [DOI] [PubMed] [Google Scholar]
- Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Molecular and Cellular Biology. 2002;22:5923–5937. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangrande PH, Kimbrel A, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. MolCell Biol. 2000;20:3102–3115. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: Effects of estradiol reatment. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- Grenier J, Trousson A, Chauchereau A, Cartaud J, Schumacher M, Massaad C. Differential recruitment of p160 coactivators by glucocorticoid receptor between Schwann cells and astrocytes. MolEndocrinol. 2006;20:254–267. doi: 10.1210/me.2005-0061. [DOI] [PubMed] [Google Scholar]
- Grenier J, Trousson A, Chauchereau A, Amazit L, Lamirand A, Leclerc P, Guiochon-Mantel A, Schumacher M, Massaad C. Selective recruitment of p160 coactivators on glucocorticoid-regulated promoters in Schwann cells. Mol Endocrinol. 2004;18:2866–2879. doi: 10.1210/me.2004-0241. [DOI] [PubMed] [Google Scholar]
- Han SJ, Lonard DM, O'Malley BW. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol Metab. 2009;20:8–15. doi: 10.1016/j.tem.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Demayo FJ, Xu J, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. MolEndocrinol. 2006;20:45–55. doi: 10.1210/me.2005-0310. [DOI] [PubMed] [Google Scholar]
- Hardy DF, DeBold JF. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. HormsBehav. 1971;2:287–297. [Google Scholar]
- Iannacone EA, Yan AW, Gauger KJ, Dowling ALS, Zoeller RT. Thyroid hormone exerts site-specific effects on SRC-1 and NCoR expression selectively in the neonatal rat brain. Molecular and Cellular Endocrinology. 2002;186:49–59. doi: 10.1016/s0303-7207(01)00672-4. [DOI] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in theparaventricular nucleus of hypothalamus regulates the neuroendocrine response to stressand is regulated by corticosterone. Neuroscience. 2003;121:837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- Jensen EV, Suzuki T, Kawasima T, Stumpf WE, Jungblut PW, de Sombre ER. A two-step mechanism for the interaction of estradiol with rat uterus. Proceedings of the National Academy of Sciences USA. 1968;59:632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O'Malley BW, Demayo FJ. The p160 steroid receptor coactivator-2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology. 2007;148:4238–4250. doi: 10.1210/en.2007-0122. [DOI] [PubMed] [Google Scholar]
- Johansen JA, Jordan CL, Breedlove SM. Steroid hormone masculinization of neural structure in rats: a tale of two nuclei. Physiol Behav. 2004;83:271–277. doi: 10.1016/j.physbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Valentine JE, Heery DM, Parker MG. Isoforms of steroid receptor coactivator 1 differ in their ability to potentiate transcription by the oestrogen receptor. EMBO J. 1998;17:232–243. doi: 10.1093/emboj/17.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamicneurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol. 2007;27:5090–5104. doi: 10.1128/MCB.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kurihara I, Shibata H, Suzuki T, Ando T, Kobayashi S, Hayashi M, saito I, Saruta T. Expression and regulation of nuclear receptor coactivators in glucocorticoid action. Mol Cell Endocrinol. 2002;189:181–189. doi: 10.1016/s0303-7207(01)00717-1. [DOI] [PubMed] [Google Scholar]
- Lanz RB, Lonard DM, O'Malley BW. Nuclear receptor coregulators in human diseases. In: Kumar R, O'Malley BW, editors. Nuclear receptor coregulators and human diseases. World Scientific Books; 2008. pp. 1–133. [Google Scholar]
- Lanz RB, Chua SS, Barron N, Soder BM, DeMayo F, O'Malley BW. Steroid receptor RNA activator stimulates proliferation as well as apoptosis in vivo. Molecular and Cellular Biology. 2003;23:7163–7176. doi: 10.1128/MCB.23.20.7163-7176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz RB, McKenna NJ, Oñate SA, Albrecht U, Wong J, Tsai SY, Tsai MJ, O'Malley BW. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97:17–27. doi: 10.1016/s0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Pfaff DW. Sex difference in estradiol regulation of progestin receptor messenger RNA in rat mediobasal hypothalamus as demonstrated by In situ hybridization. Neuroendocrinology. 1991;53:608–613. doi: 10.1159/000125781. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wong J, Tsai SY, Tsai MJ, O'Malley BW. Sequential recruitment of steroid receptor coactivator-1 (SRC-1) and p300 enhances progesterone receptor-dependent initiation and reinitiation of transcription from chromatin. ProcNatlAcadSciUSA. 2001;98:12426–12431. doi: 10.1073/pnas.231474798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O'Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–587. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- Maerkel K, Durrer S, Henseler M, Schlumpf M, Lichtensteiger W. Sexually dimorphic gene regulation in brain as a target for endocrine disrupters: developmental exposure of rats to 4-methylbenzylidene camphor. Toxicol Appl Pharmacol. 2007;218:152–165. doi: 10.1016/j.taap.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S. Progestin receptor subtypes in the brain: The known and the unknown. Endocrinology. 2008;149:2750–2756. doi: 10.1210/en.2008-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Reyna AM, Chen JZ, Mulac-Jericevic B, Conneely OM. Differential response of progesterone receptor isoforms in hormone-dependent and -independent facilitation of female sexual receptivity. Molecular Endocrinology. 2006;20:1322–1332. doi: 10.1210/me.2005-0466. [DOI] [PubMed] [Google Scholar]
- Mark M, Yoshida-Kimoya H, Gehin M, Liao L, Tsai MJ, O'Malley BW, Chambon P, Xu J. Partially redundant functions of SRC-1 and TIF2 in postnatal survival and male reproduction. Proceedings of the National Academy of Sciences USA. 2004;101:4453–4458. doi: 10.1073/pnas.0400234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de Arrieta C, Koibuchi N, Chin WW. Coactivator and corepressor gene expression in rat cerebellum during postnatal development and the effect of altered thyroid status. Endocrinology. 2000;141:1693–1698. doi: 10.1210/endo.141.5.7467. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Age-related changes in nuclear receptor coactivator immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. Brain Res. 2002;943:202–205. doi: 10.1016/s0006-8993(02)02622-7. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figuiera HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiology and Behavior. 2007;92:1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC, Steenbergen PJ, de Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141:2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- Meijer OC, Kalkhoven E, van der LS, Steenbergen PJ, Houtman SH, Dijkmans TF, Pearce D, de Kloet ER. Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology. 2005;146:1438–1448. doi: 10.1210/en.2004-0411. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Cavarretta IT, Ballabio M, Leonelli E, Schenone A, Azcoitia I, Miguel Garcia-Segura L, Magnaghi V. Peripheral nerves: a target for the action of neuroactive steroids. Brain Res Brain Res Rev. 2005;48:328–338. doi: 10.1016/j.brainresrev.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: An emerging mechanism of estrogen action in brain. Molecular Neurobiology. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiti S, Schomburg L, Yen PM, Chin WW. Expression and hormonal regulation of coactivator and corepressor genes. Endocrinology. 1998;139:2493–2500. doi: 10.1210/endo.139.5.5971. [DOI] [PubMed] [Google Scholar]
- Mitev YA, Wolf SS, Almeida OF, Patchev VK. Developmental expression profiles and distinct regional estrogen responsiveness suggest a novel role for the steroid receptor coactivator SRC-l as a discriminative amplifier of estrogen signaling in the rat brain. The FASEB Journal. 2003;17:518–519. doi: 10.1096/fj.02-0513fje. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. Nuclear receptor coactivators function in estrogen receptor- and progestin receptor-dependent aspects of sexual behavior in female rats. Hormones and Behavior. 2006;50:383–392. doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenda-Figueira HA, Murphy SD, Shea KL, Siegal NK, Zhao Y, Chadwick JG, Denner LA, Tetel MJ. Steroid receptor coactivator-1 from brain physically interacts differentially with steroid receptor subtypes. Endocrinology. 2008;149:5272–5279. doi: 10.1210/en.2008-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- Monks DA, Xu J, O'Malley BW, Jordan CL. Steroid receptor coactivator-1 is not required for androgen-mediated sexual differentiation of spinal motoneurons. Neuroendocrinology. 2003;78:45–51. doi: 10.1159/000071705. [DOI] [PubMed] [Google Scholar]
- Monroe DG, Johnsen SA, Subramaniam M, Getz BJ, Khosla S, Riggs BL, Spelsberg TC. Mutual antagonism of estrogen receptors alpha and beta and their preferred interactions with steroid receptor coactivators in human osteoblastic cell lines. JEndocrinol. 2003;176:349–357. doi: 10.1677/joe.0.1760349. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Amato P, Allred DC, DeMayo FJ, Lydon JP. Steroid receptor coactivator 2 is required for female fertility and mammary morphogenesis: insights from the mouse, relevance to the human. Nucl Recept Signal. 2007;5:e011. doi: 10.1621/nrs.05011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- Nishihara E, Yoshida-Kimoya H, Chan C, Liao L, Davis RL, O'Malley BW, Xu J. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. The Journal of Neuroscience. 2003;23:213–222. doi: 10.1523/JNEUROSCI.23-01-00213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 'Bryant EL, Jordan CL. Expression of nuclear receptor coactivators in androgen-responsive and -unresponsive motoneurons. Hormones and Behavior. 2004;47:29–38. doi: 10.1016/j.yhbeh.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 'Malley BW. Molecular biology. Little molecules with big goals. Science. 2006;313:1749–1750. doi: 10.1126/science.1132509. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Nishi M, Kawata M. Localization of nuclear coactivators p300 and steroid receptor coactivator 1 in the rat hippocampus. Brain Research. 2001;890:197–202. doi: 10.1016/s0006-8993(00)03158-9. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. HumMolGenet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146:3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Oñate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai MJ, Edwards DP, O'Malley BW. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. Journal of Biological Chemistry. 1998;273:12101–12108. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van OGJ, Goodman RH, Peters DJ. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–351. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- Pfaff D. Hormone-driven mechanisms in the central nervous system facilitate the analysis of mammalian behaviours. J Endocrinol. 2005;184:447–453. doi: 10.1677/joe.1.05897. [DOI] [PubMed] [Google Scholar]
- Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–1812. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Galigniana MD, Morishima Y, Murphy PJ. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004;40:41–58. doi: 10.1042/bse0400041. [DOI] [PubMed] [Google Scholar]
- Ramos HE, Weiss RE. Regulation of nuclear coactivator and corepressor expression in mouse cerebellum by thyroid hormone. Thyroid. 2006;16:211–216. doi: 10.1089/thy.2006.16.211. [DOI] [PubMed] [Google Scholar]
- Ranson RN, Santer RM, Watson AHD. SRC-1 localisation in lumbosacral spinal cord of male and female Wistar rats. Neuroreport. 2003;14:1821–1824. doi: 10.1097/00001756-200310060-00012. [DOI] [PubMed] [Google Scholar]
- Ranson RN, Santer RM, Watson AH. Biogenic amine and neuropeptide inputs to identified pelvic floor motoneurons that also express SRC-1. Neurosci Lett. 2005;382:248–253. doi: 10.1016/j.neulet.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Roglio I, Giatti S, Pesaresi M, Bianchi R, Cavaletti G, Lauria G, Garcia-Segura LM, Melcangi RC. Neuroactive steroids and peripheral neuropathy. Brain Res Rev. 2008;57:460–469. doi: 10.1016/j.brainresrev.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Molecular Endocrinology. 1994;8:1347–1360. doi: 10.1210/mend.8.10.7854352. [DOI] [PubMed] [Google Scholar]
- Schott DR, Shyamala G, Schneider W, Parry G. Molecular cloning, sequence analyses, and expression of complementary DNA encoding murine progesterone receptor. Biochemistry. 1991;30:7014–7020. doi: 10.1021/bi00242a029. [DOI] [PubMed] [Google Scholar]
- Scott REM, Wu-Peng XS, Pfaff DW. Regulation and expression of progesterone receptor mRNA isoforms A and B in the male and female rat hypothalamus and pituitary following oesterogen treatment. Journal of Neuroendocrinology. 2002;14:175–183. doi: 10.1046/j.0007-1331.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Forger NG. The spinal nucleus of the bulbocavernosus: firsts in androgen-dependent neural sex differences. Horm Behav. 2008;53:596–612. doi: 10.1016/j.yhbeh.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Owen D, McCabe L, Kostaki A, Andrews MH, Matthews SG. Glucocorticoids do not alter developmental expression of hippocampal or pituitary steroid receptor coactivator-1 and −2 in the late gestation fetal guinea pig. Endocrinology. 2004;145:3796–3803. doi: 10.1210/en.2003-1723. [DOI] [PubMed] [Google Scholar]
- Shao W, Halachmi S, Brown M. ERAP140, a conserved tissue-specific nuclear receptor coactivator. Molecular and Cellular Biology. 2002;22:3358–3372. doi: 10.1128/MCB.22.10.3358-3372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Smith CL, Oñate SA, Tsai MJ, O'Malley BW. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proceedings of the National Academy of Sciences USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodersten P. Effects of anti-oestrogen treatment of neonatal male rats on lordosis behaviour and mounting behaviour in the adult. JEndocrinol. 1978;76:241–249. doi: 10.1677/joe.0.0760241. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–197. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Stromberg H, Svensson SP, Hermanson O. Distribution of CREB-binding protein immunoreactivity in the adult rat brain. Brain Res. 1999;818:510–514. doi: 10.1016/s0006-8993(98)01219-0. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Lange CA. Molecular genomics of progestin actions. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2009. pp. 1439–1465. [Google Scholar]
- Tetel MJ, Siegal NK, Murphy SD. Cells in behaviourally relevant brain regions coexpress nuclear receptor coactivators and ovarian steroid receptors. J Neuroendocrinol. 2007;19:262–271. doi: 10.1111/j.1365-2826.2007.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel MJ, Ungar TC, Hassan B, Bittman EL. Photoperiodic regulation of androgen receptor and steroid receptor coactivator-1 in Siberian hamster brain. Molecular Brain Research. 2004;131:79–87. doi: 10.1016/j.molbrainres.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo Molecular. Endocrinology. 1999;13:910–924. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent non-acidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Tung L, Kamel Mohamed M, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Molecular Endocrinology. 1993;7:1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology. 2008;149:725–732. doi: 10.1210/en.2007-1234. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Frontiers in Neuroendocrinology. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Zhu YS, Daniel S, Koibuchi N, Chin WW, Pfaff D. Crosstalk between oestrogen receptors and thyroid hormone receptor isoforms results in differential regulation of the preproenkephalin gene. J Neuroendocrinol. 2001;13:779–790. doi: 10.1046/j.1365-2826.2001.00693.x. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Molecular Endocrinology. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Villamar-Cruz O, Manjarrez-Marmolejo J, Alvarado R, Camacho-Arroyo I. Regulation of the content of progesterone and estrogen receptors, and their cofactors SRC-1 and SMRT by the 26S proteasome in the rat brain during the estrous cycle. Brain ResBull. 2006;69:276–281. doi: 10.1016/j.brainresbull.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJS, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO Journal. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Qi C, Krones A, Woodring P, Zhu X, Reddy JK, Evans RM, Rosenfeld MG, Hunter T. Critical roles of the p160 transcriptional coactivators p/CIP and SRC-1 in energy balance. Cell Metab. 2006;3:111–122. doi: 10.1016/j.cmet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Warembourg M, Jolivet A, Milgrom E. Immunohistochemical evidence of the presence of estrogen and progesterone receptors in the same neurons of the guinea pig hypothalamus and preoptic area. Brain Res. 1989;480:1–15. doi: 10.1016/0006-8993(89)91561-8. [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stallcup MR, Kushner PJ. Estrogen receptor activation function 1 works by binding p160 coactivator proteins Molecular. Endocrinology. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- Weiss RE, Xu J, Ning G, Pohlenz J, O'Malley BW, Refetoff S. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J. 1999;18:1900–1904. doi: 10.1093/emboj/18.7.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen RE, Edwards DA. Hormonal determinants of the development of masculine and feminine behavior in male and female rats. AnatRec. 1967;157:173–180. doi: 10.1002/ar.1091570208. [DOI] [PubMed] [Google Scholar]
- Xu J, Qiu Y, Demayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- Xu J, Liao L, Ning G, Yoshida-Kimoya H, Deng C, O'Malley BW. The steroid receptor coactivator SRC-3 (p/cip/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proceedings of the National Academy of Sciences USA. 2000;97:6379–6384. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]


