Abstract
Neurons and glia are highly susceptible to reactive oxygen species that play a key role in various neurodegenerative diseases. Menadione, a synthetic derivative of vitamin K, induces reactive oxygen generation. Quercetin one of the most ubiquitous bioflavonoids in food of plant origin, has strong antioxidant activities on different cell types, however recent studies demonstrated that it has also prooxidant and cytotoxic potentials. We examined the action of pre- and co-treatment of quercetin on menadione induced glial toxicity. The primary mixed glial cells obtained from 1 to 3 day old rat brain were pretreated with 10, 25, 100 or 250 μM quercetin for 1 h, washed out and 10, 25, 50, 75 or 100 μM menadione was added for 6 h. The other group of cells was treated with respective doses of quercetin combined simultaneously with the same doses of menadione for 6 h. The cells were washed and incubated for additional 24 h for recovery period and the viability was measured by using MTT assay. Menadione was dose-dependently toxic to glia cells and pretreatment with respective quercetin doses for 1 h could not eliminate this toxicity. Although 10 and 25 μM quercetin combined with 10 and 25 μM menadione could not change, 100 and 250 μM quercetin together with 10 or 25 μM menadione for 6 h increased further the menadione induced toxicity. We conclude that when combined with menadione, quercetin at high doses could be toxic to primary rat glia cells in culture.
Keywords: Glia, Quercetin, Menadione, MTT, In vitro
Introduction
The brain is particularly vulnerable to oxidative damage as a result of its high oxygen consumption rate. It utilizes 20% of the total oxygen consumed though it comprises only 2% of the body weight (Gitika et al. 2006). The membrane lipids in the brain are rich in polyunsaturated fatty acid side chains which are especially sensitive to free radical attacks (Cafe et al. 1995; Leuther et al. 2001). Oxidative stress leads to enhance production of reactive oxygen species (ROS), which can modify DNA, proteins, lipids and carbohydrates in cells resulting in various neurological disorders such as trauma, ischemia, Alzheimer’s and Parkinson’s diseases (Floyd 1999). Therefore, a large number of studies focus on oxidative damage as a common mechanism of neurotoxicity.
Acute cardiac and renal toxicity of menadione, a synthetic derivative of vitamin K, in rat at 25, 50, 100 and 150 mg/kg were reported with a dose–response relationship (Chiou et al. 1997) however, 1 mM menadione killed all cultured rat cortical astrocytes in 6 h (Abe and Saito 1996) and as low as 10 μM menadione decreased viability of bovine aortic endothelial cells in 8 h (Takahashi et al. 2009). Menadione is a strong oxidizing agent that can generate a great quantity of ROS when it enters cells (Lamson and Plaza 2003). The cytotoxic effect of menadione is thought to be mediated through its one or two electron reduction to semiquinone or hydroquinone radicals, which subsequently enter redox cycle with molecular oxygen to produce ROS and oxidative stress (Ngo et al. 1999). Because of these reasons, menadione has been widely used as an oxidant to study oxidative stress in mammalian systems (Lee et al. 2001).
Quercetin belongs to a group of plant pigments called flavonoids and is a convenient compound of major human dietary constituents of vegetables, tea, fruit and wine (Lamson and Brignall 2000). Quercetin is generally considered to have strong antioxidant potency and provides protection against oxidative injury in cultured cells (Boots et al. 2007). The antioxidant properties of quercetin might be via an ability to chelate transition metal ions, such as iron and copper, catalyze electron transport and scavenge ROS (Choi et al. 2003). Pretreatment of quercetin protects against hydrogen peroxide (Saito et al. 2004), glucose oxidase (Lee et al. 2003) and menadione (Aherne and O’Brien 2000; Park et al. 2003) induced toxicity in different cell types. It was reported that both pre- and co-treatment of quercetin with different exitotoxins showed a neuroprotective activity on mouse primary cortical culture (Ha et al. 2003). Although the multiple activities of quercetin were believed to be due to its strong antioxidant properties (Lee et al. 2001), recent studies indicated that it has prooxidant and cytotoxic effects on different cell types (Matsuo et al. 2005). The dose levels of quercetin in long term animal studies suggest the addition of 200–500 mg/day quercetin to diet without any toxic effect (Harwood et al. 2007) however, the concentration at which cultured cell growth was inhibited by IC50 ranged from 7 nM (Lamson and Brignall 2000) to 303 μM (Matsuo et al. 2005). In light of these findings, we postulated to examine first time whether pre-treatment and co-treatment of quercetin can prevent menadione induced toxicity in rat primary glial cells in vitro.
Materials and methods
All the reagents were obtained from Sigma and the glial cells from whole brains of 1–3 day old Spraque-Dawley rats as described previously (Lopez et al. 2007). Briefly, the cells collected from 4 rats were combined and then cultured in a humidified atmosphere of 5% CO2, at 37 °C in 25 cm2 flasks. The culture medium consisted of Dulbecco’s Modified Eagle’s Medium (DMEM), 10% fetal bovine serum (FBS) and 1% penicilin–streptomycine solution. The cells were determined as glia by using glial fibrillary acidic protein antibody by immunohistochemical staining. When confluence was achieved, primary glial cells were dissociated with trypsinization and centrifuged at 1,000 rpm, 4 °C for 5 min. The supernatant was discarded and the samples were counted with a Coulter counter. The glial cells were seeded in 96 well plates (2 × 104 cells/well) for 24 h in 8 wells for the control and 8 wells for each tested drug dose. To minimize the protective effect of FBS on the quercetin action, FBS ratio in culture medium was adjusted for 1% (Rouzaire-Dubois et al. 1993). Menadione (menadione sodium bisulfate) was dissolved in DMEM. Quercetin was dissolved in dimethylsulfoxide (DMSO) and diluted in DMEM to the highest concentration of 0.01% (v/v). All test compounds were prepared immediately prior to use and protected from light.
Experimental groups:
Control group: Culture medium.
Menadione group: Treated with 10, 25, 50, 75 or 100 μM menadione for 6 h.
Quercetin group: Treated with 10, 25, 100 or 250 μM quercetin for 6 h.
Pretreatment group: Pretreated with respective quercetin doses for 1 h and then respective menadione doses were added for 6 h.
Combination group: Respective doses of quercetin and menadione were applied simultaneously for 6 h.
After 6 h cells were washed, fed with fresh medium for 24 h and then viability was measured by colorimetric assay with 3-(4,5 dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT, Mossmann 1983). The optical density read at 550 nm from the drug treated wells was converted to a percentage of living cells against the control using the following formula: Absorbance of treated cells in each well × 100/the mean absorbance of control cells. Data were expressed as the mean percent fraction of control ± standard error of mean (SEM). Statistical significance was ascertained by one way analysis of variance, followed by Tukey’s multiple comparison tests. The results are means of at least three independent assays and a p value less than 0.05 was considered to be significant.
Results
The highest concentration of DMSO or the treatment of primary glial cells with respective concentrations of quercetin alone for 6 h did not change the cell viability rate (data not shown).
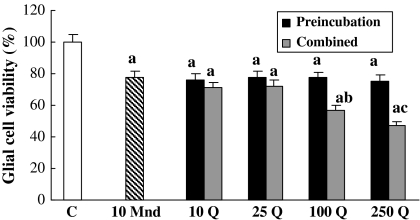
When compared to the control, 10 μM menadione applied alone for 6 h decreased the cell viability by about 22% (Fig. 1). The pretreatment of glia with respective quercetin doses could not eliminate this 10 μM menadione caused toxicity. The combination of 10 μM menadione with either 10 or 25 μM quercetin did also not change significantly the menadione toxicity, however 100 and 250 μM quercetin increased this toxicity further by about 21 and 31% more, respectively.
Fig. 1.
The role of combined and pre-treatment of quercetin concentrations on 10 μM menadione toxicity for 6 h on glial cell survival. C control, Mnd menadione, Q quercetin. a, different from control p < 0.001; b, different from 10 μM menadione p < 0.05; c, different from 10 μM menadione p < 0.01
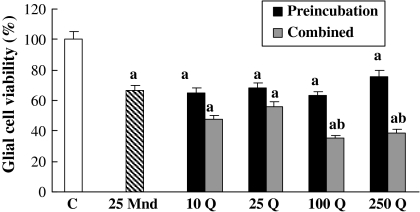
After exposure of glial cells to 25 μM menadione alone for 6 h, cell viability was calculated to be 34% lower than the control (Fig. 2). Pretreatment of the cells with respective quercetin doses could not again eliminate 25 μM menadione caused toxicity. Either 10 or 25 μM quercetin combined simultaneously with 25 μM menadione could not affect, but 100 and 250 μM quercetin decreased further cell viability about 27 and 31% more, respectively.
Fig. 2.
The effects of pre-treatment and co-treatment of quercetin concentrations on 25 μM menadione toxicity for 6 h on glial cell survival. a, different from control, p < 0.001; b, different from 25 μM menadione p < 0.001
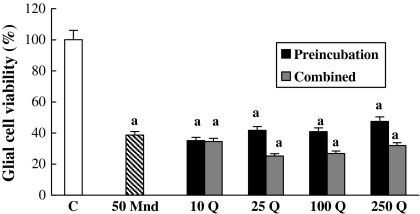
As shown in Fig. 3, alone 50 μM menadione reduced the cell viability by about 61%. Although the combination of respective quercetin doses with 50 μM menadione decreased the glia viability, data were not statistically significant. Pretreatment of respective quercetin doses with 50 μM menadione caused some unsignificant changes in the cell viability.
Fig. 3.
The action of combined and pre-treatment of quercetin concentrations on 50 μM menadione toxicity for 6 h on glial cell survival. a, different from control, p < 0.001
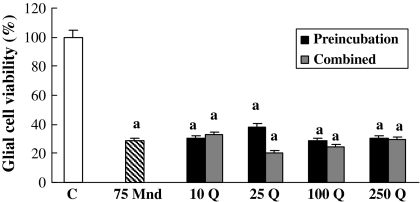
Since 75 and 100 μM menadione doses induced almost the same toxic ratio, 100 μM menadione induced toxicity is not shown. Since 75 μM menadione killed almost 71% of the cells within 6 h, we believe that there was not left enough number of cells to be influenced by either combination or pretreatment with respective doses of quercetin (Fig. 4).
Fig. 4.
The pre-treatment and co-treatment effects of quercetin concentrations on 75 μM menadione toxicity for 6 h on glial cell survival. a, different from control, p < 0.001
Discussion
The present study was designed to determine effects of pretreatment and combined treatment of quercetin on menadione induced toxicity on primary rat glial cells. We preferred menadione because of the major mediator of oxidative stress and usually chosen as a suitable source of ROS for the study of their biological role (Lee et al. 2001).
We found that quercetin alone at respective doses was not able to alter the number of living glial cells in 6 h. Jagadeeswaran et al. (2000) demonstrated that 10–40 μg/ml quercetin did not affect cell viability, morphological changes and lactate dehydrogenase activity of African green monkey kidney cells in 48 h. Likewise, 200 μM quercetin did not change cell viability in 24 h for both human colonic adenocarcinoma cells (Caco-2) and human hepatocellular carcinoma cells HepG2 (Aherne and O’Brien 1999). In another study, concentrations of quercetin less than 100 μM were not toxic, but higher than 100 μM was highly cytotoxic by accelerating the generation of H2O2 and superoxide in human lymphocytes in 30 min (Yen et al. 2003). It was also reported that 100 μM or more quercetin reduced the cell viability of rat aortic smooth muscle cells (Shih et al. 2004) and mouse neuroblastoma × glioma hybrid cells in a concentration dependent manner in 48 h (Rouzaire-Dubois et al. 1993). Depending on dose and free radical source, quercetin induced hydrogen peroxide and super oxide anion in human lung embryonic fibroblast and human umbilical vein endothelial cultured cells in 24 h (Matsuo et al. 2005). Thus, quercetin can exert different effects (anti-/prooxidant) depending on concentration, exposure time, cell type and oxidative balance.
Pretreatment of the cells with respective quercetin doses for 1 h had no influence on menadione caused toxicity on our rat primary glial cells. The treatment time was modified from the study of Gitika et al. (2006). Our study was first in terms of testing the effect of quercetin on menadione toxicity in primary mixed glial cells. Pretreatment of mouse thymocytes in cultures with 50 μM quercetin for 2 h inhibited glucose oxidase mediated apoptosis (Lee et al. 2003). Saito et al. (2004) showed that 1 h pretreatment with 1 μM quercetin prevented 20 μM H2O2 induced chromosomal damage in human B lymphoblastoid cells (WIL2-NS) in 30 min. Moreover, pretreated with 50 μg/mL quercetin for 30 min and followed by treatment with 20 μM menadione for 12 h, quercetin had significantly protective capacity in H9c2 cells against oxidative stress induced by menadione in vitro (Park et al. 2003). In another study, preincubation of 50 μM quercetin for 24 h protected against 10 μM menadione-induced DNA single strand breaks by acting both as metal chelator and radical scavenger in Caco-2 (Aherne and O’Brien 2000). In contrast, Bestwick and Milne (2001) reported that preincubation with quercetin concentrations of 10 μM or more for 45 min protects against 20 μM menadione induced DNA single strand breaks, conversely exacerbate membrane damage in HL-60 cells. It seems that the effect of quercetin pretreatment may depend on the concentration of quercetin, exposure time, cell type and/or culture conditions.
Another result of our study is that simultaneous combination of menadione with higher doses of quercetin further increased menadione toxicity. We did not encounter with any published data about combined treatment of quercetin with menadione on cultured glial cells. Ha et al. (2003) reported that both pre- and co-treatment with 1–10 μM concentrations of quercetin did not have a neuroprotective effect; however concentrations of quercetin of 30–100 μM protected against neurotoxicity induced by different excitotoxins in mouse primary cortical cultures for 20 and 24 h. We have found in another study that 100 μM H2O2 alone decreased the glial cell number by 75% in culture however, exposed for 3 h simultaneously with the same amount of H2O2, both 75 and 100 μM quercetin eliminated this toxic effect by 15% (unpublished data). Vafeiadou et al. (2008) demonstrated that quercetin is rapidly conjugated to glutathione (GSH) within glial cells. Depletion of GSH concentration and glutathione reductase activity via quercetin implies the weakness of antioxidant defense system and causes oxidative damage. A dysfunction of glutathione metabolites has been found to be also important in most neurodegenerative diseases (Choi et al. 2003). In addition, menadione also decreases the GSH level significantly in human hepatocytes and fibroblasts (Morrison et al. 1985) and, 5 hepatoma cell lines and 4 different carcinoma cells (Wu et al. 1993). Furthermore, some investigators reported that intracellular metabolic degradation of quercetin forms different reactive and cytotoxic metabolites via enzymatic or non-enzymatic pathways in various cell types (Awad et al. 2001, 2002; Boots et al. 2007). It is possible that produced in the presence of menadione, these metabolites may play an important role on quercetin toxicity in the cells.
In conclusion, the present study indicate that while pretreatment with quercetin for 1 h had no effect against menadione toxicity, combined treatment of menadione with the higher doses of quercetin increased menadione toxicity further on primary rat glial cells in vitro.
Acknowledgments
This study was supported by a grant (number: 200719023) from Eskisehir Osmangazi University Scientific Research Projects Committee. These results were presented in Meeting ‘Free Radicals and Nutrition: basic mechanisms and clinical applications’ (Berlin, 05-09.07.2008) and abstract was printed in Free Radical Research vol: 42, Supplement 1, July 2008, S82.
References
- Abe K, Saito H (1996) Menadione toxicity in cultured rat cortical astrocytes. Jpn J Pharmacol 72:299–306. doi:10.1254/jjp.72.299 [DOI] [PubMed]
- Aherne SA, O’Brien NM (1999) Protection by the flavonoids myricetin, quercetin, and rutin against hydrogen peroxide-induced DNA damage in Caco-2 and Hep G2 cells. Nutr Cancer 34:160–166. doi:10.1207/S15327914NC3402_6 [DOI] [PubMed]
- Aherne SA, O’Brien NM (2000) Mechanism of protection by the flavonoids, quercetin and rutin, against tert-butylhydroperoxide- and menadione-induced DNA single strand breaks in Caco-2 cells. Free Radic Biol Med 29:507–514. doi:10.1016/S0891-5849(00)00360-9 [DOI] [PubMed]
- Awad HM, Boersma MG, Boeren S, Van Bladeren PJ, Vervoort J, Rietjens IMCM (2001) Structure—activity study on the quinone/quinone methide chemistry of flavonoids. Chem Res Toxicol 14:398–408. doi:10.1021/tx000216e [DOI] [PubMed]
- Awad HM, Boersma MG, Boeren S, Van Der Woude H, Van Zanden J, Van Bladeren PJ, Vervoort J, Rietjens IMCM (2002) Identification of o-quinone/quinone methide metabolites of quercetin in a cellular in vitro system. FEBS Lett 520:30–34. doi:10.1016/S0014-5793(02)02754-0 [DOI] [PubMed]
- Bestwick CS, Milne L (2001) Quercetin modifies reactive oxygen levels but exerts only partial protection against oxidative stress within HL-60 cells. Biochim Biophys Acta 1528:49–59. doi:10.1016/S0304-4165(01)00167-2 [DOI] [PubMed]
- Boots AW, Li H, Schins RPF, Duffin R, Heemskerk JWM, Bast A, Haenen GRMM (2007) The quercetin paradox. Toxicol Appl Pharmacol 222:89–96. doi:10.1016/j.taap.2007.04.004 [DOI] [PubMed]
- Cafe C, Torri C, Bertorelli L, Tartara F, Tancioni F, Gaetani P, Baena RRY, Marzatio F (1995) Oxidative events in neuronal and glial cell-enriched fractions of rat cerebral cortex. Free Radic Biol Med 19:853–857. doi:10.1016/0891-5849(95)00086-D [DOI] [PubMed]
- Chiou TJ, Zhang J, Ferrans VJ, Tzeng WF (1997) Cardiac and renal toxicity of menadione in rat. Toxicology 124:193–202. doi:10.1016/S0300-483X(97)00162-5 [DOI] [PubMed]
- Choi EJ, Chee KH, Lee BH (2003) Anti-and prooxidant effects of chronic quercetin administration in rats. Eur J Pharmacol 482:281–285. doi:10.1016/j.ejphar.2003.09.067 [DOI] [PubMed]
- Floyd RA (1999) Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med 222:236–245 [DOI] [PubMed]
- Gitika B, Sai Ram M, Sharma K, Ilavazhagan G, Banerjee PK (2006) Quercetin protects C6 glial cells from oxidative stress induced by tertiary-butylhydroperoxide. Free Radic Biol Med 40:95–102. doi:10.1080/10715760500335447 [DOI] [PubMed]
- Ha HJ, Kwon YS, Park SM, Shin T, Park JH, Kim HC, Kwon MS, Wie MB (2003) Quercetin attenuates oxygen-glucose deprivation- and excitotoxin-induced neurotoxicity in primary cortical cell cultures. Biol Pharm Bull 26:544–546. doi:10.1248/bpb.26.544 [DOI] [PubMed]
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC (2007) A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol 45:2179–2205. doi:10.1016/j.fct.2007.05.015 [DOI] [PubMed]
- Jagadeeswaran R, Thirunavukkarasu C, Gunasekaran P, Ramamurty N, Sakthisekaran D (2000) In vitro studies on the selective cytotoxic effect of crocetin and quercetin. Fitoterapia 71:395–399. doi:10.1016/S0367-326X(00)00138-6 [DOI] [PubMed]
- Lamson DW, Brignall MS (2000) Antioxidants and cancer part 3: quercetin. Altern Med Rev 5:196–208 [PubMed]
- Lamson DW, Plaza SM (2003) The anticancer effects of vitamin K. Altern Med Rev 8:303–318 [PubMed]
- Lee JY, Bae ON, Chung SM, Lee MY, Chung JH (2001) Menadione induces endothelial dysfunction mediated by oxidative stress and arylation. Chem-Biol Interact 137:169–183. doi:10.1016/S0009-2797(01)00235-6 [DOI] [PubMed]
- Lee JC, Kim J, Park JK, Chung GH, Jang YS (2003) The antioxidant, rather than prooxidant, activities of quercetin on normal cells: quercetin protects mouse thymocytes from glucose oxidase-mediated apoptosis. Exp Cell Res 291:386–397. doi:10.1016/S0014-4827(03)00410-5 [DOI] [PubMed]
- Leuther S, Eckert A, Müller WE (2001) ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J Neural Transm 108:955–967 [DOI] [PubMed]
- Lopez MVN, Cuadrado MPG, Ruiz-Poveda OMP, Fresno AMVD, Accame MEC (2007) Neuroprotective effect of individual ginsenosides on astrocytes primary culture. Biochim Biophys Acta 1770:1308–1316. doi:10.1016/j.bbagen.2007.06.008 [DOI] [PubMed]
- Matsuo M, Sasaki N, Saga K, Kaneko T (2005) Cytotoxicity of flavonoids toward cultured normal human cells. Biol Pharm Bull 28:253–259. doi:10.1248/bpb.28.253 [DOI] [PubMed]
- Morrison H, Monte DI, Nordenskjöld M, Jernström B (1985) Induction of cell damage by menadione and benzo(a)pyrene-3, 6-quinone in cultures of adult rat hepatocytes and human fibroblasts. Toxicol Lett 28:37–47. doi:10.1016/0378-4274(85)90007-4 [DOI] [PubMed]
- Mossmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Method 65:55–63. doi:10.1016/0022-1759(83)90303-4 [DOI] [PubMed]
- Ngo EO, Sun TP, Chang JY, Wang CC, Chi KW, Cheng AL, Nutter LM (1999) Menadione-induced DNA damage in a human tumor cell line. Biochem Pharma 42:1961–1968 [DOI] [PubMed]
- Park C, So HS, Shin CH, Baek SH, Moon BS, Shin SH, Lee HS, Lee DW, Park RK (2003) Quercetin protects the hydrogen peroxide-induced apoptosis via inhibition of mitochondrial dysfuntion in H9c2 cardiomyoblast cells. Biochem Pharmacol 66:1287–1295. doi:10.1016/S0006-2952(03)00478-7 [DOI] [PubMed]
- Rouzaire-Dubois B, Gérard V, Dubois JM (1993) Involvement of K+ channels in the quercetin-induced inhibition of neuroblastoma cell growth. Pflugers Arch 423:202–205. doi:10.1007/BF00374395 [DOI] [PubMed]
- Saito A, Sugisawa A, Umegaki K, Sunagawa H (2004) Protective effects of quercetin and its metabolites on H2O2-induced chromosomal damage to WIL2-NS cells. Biosci Biotechnol Biochem 68:271–276. doi:10.1271/bbb.68.271 [DOI] [PubMed]
- Shih CM, Lin H, Liang YC, Lee WS, Bi WF, Juan SH (2004) Concentration-dependent differential effects of quercetin on rat aortic smooth muscle cells. Eur J Pharmacol 496:41–48. doi:10.1016/j.ejphar.2004.06.016 [DOI] [PubMed]
- Takahashi K, Shibata T, Oba T, Ishikawa T, Yoshikawa M, Tatsunami R, Takahashi K, Tampo Y (2009) Multidrug-resistance-associated protein plays a protective role in menadione-induced oxidative stress in endothelial cells. Life Sci 84:211–217. doi:10.1016/j.lfs.2008.11.021 [DOI] [PubMed]
- Vafeiadou K, Vauzour D, Rodriguez-Mateos A, Whiteman M, Williams RJ, Spencer JPE (2008) Glial metabolism of quercetin reduces its neurotoxic potential. Arch Biochem Biophys 478:195–200. doi:10.1016/j.abb.2008.07.014 [DOI] [PubMed]
- Wu FYH, Liao WC, Chang HM (1993) Comparison of antitumor activity of vitamins K1, K2 and K3 on human tumor cells by two (MTT and SRB) cell viability assays. Life Sci 52:1797–1804. doi:10.1016/0024-3205(93)90469-J [DOI] [PubMed]
- Yen GC, Duh PD, Tsai HL, Huang SL (2003) Pro-oxidative properties of flavonoids in human lymphocytes. Biosci Biotechnol Biochem 67:1215–1222. doi:10.1271/bbb.67.1215 [DOI] [PubMed]