Abstract
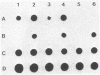
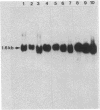
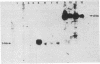
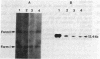
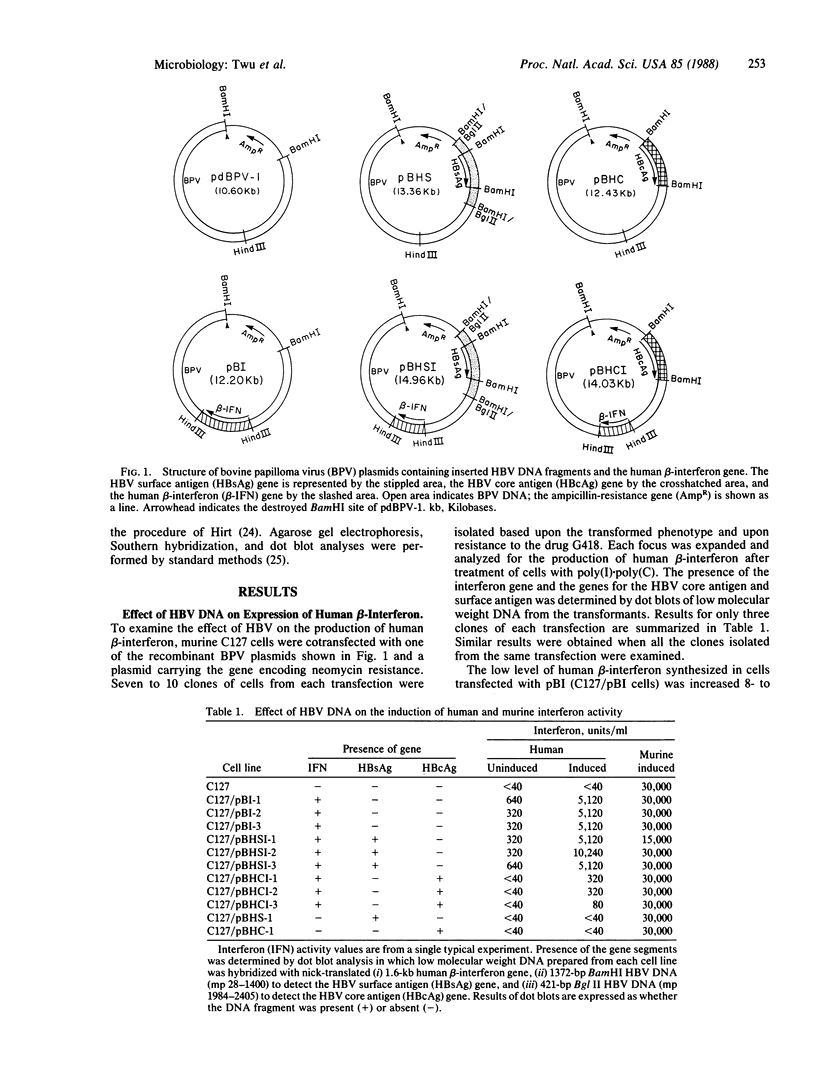
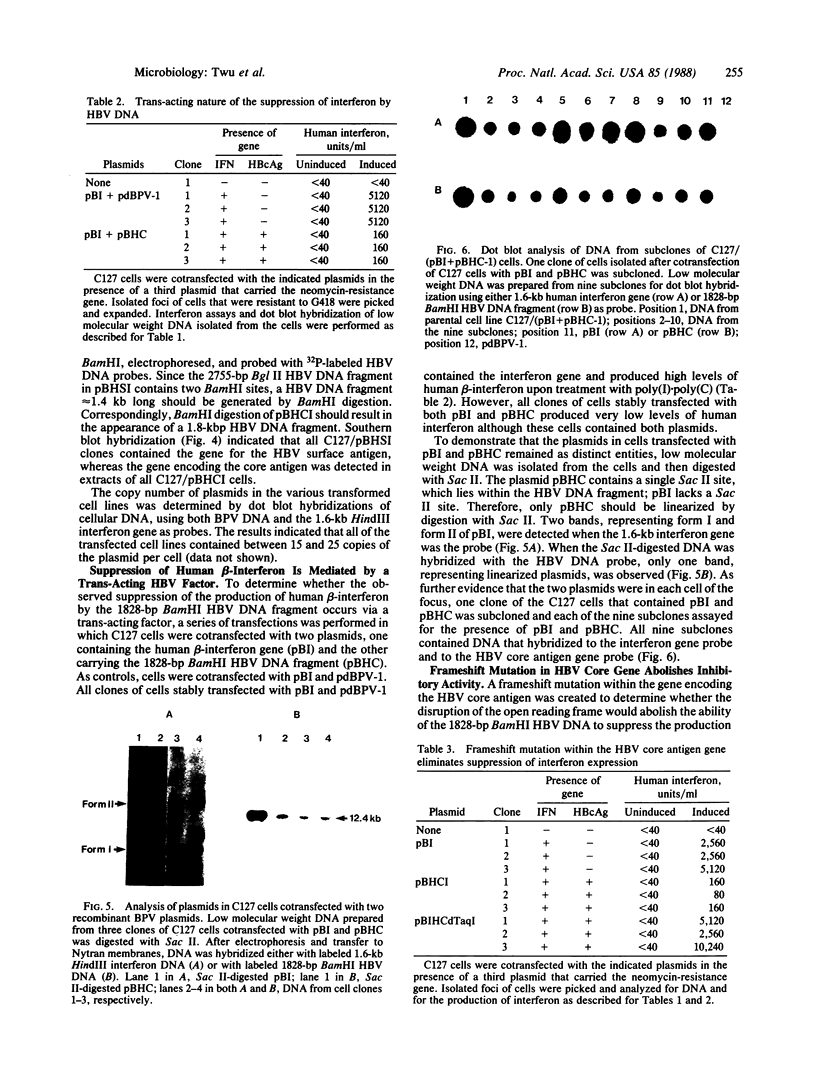
To determine whether hepatitis B virus (HBV) regulates the expression of the human beta-interferon gene, a series of recombinant bovine papilloma virus plasmids containing the human interferon gene and various fragments of the HBV genome were constructed and used to transform C127 cells, a murine fibroblast line. Analysis of the DNA from transformed C127 cells indicated that the interferon gene was intact and that the plasmids replicated as stable multicopy elements. The 1828-base-pair BamHI HBV DNA fragment containing the core antigen gene, but not the 2755-base-pair Bgl II HBV DNA fragment encoding both the surface antigen and the X antigen, suppressed the production of human beta-interferon. No effect by any of the recombinant plasmids on the synthesis of murine interferon was detected. The suppression of human beta-interferon by HBV occurs via a trans-acting factor. A frameshift mutation within the HBV core gene alleviates the inhibitory activity; thus we infer that the core protein is this factor or is crucially associated with this activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S. S., Blumberg B. S., Gerstley B. J., London W. T., Millman I., Sutnick A. I., Loeb L. A. Lymphocyte transformation and hepatitis. I. Impairment of thymidine incorporation and DNA polymerase activity. Proc Soc Exp Biol Med. 1971 Sep;137(4):1498–1502. doi: 10.3181/00379727-137-35818. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Cummings I. W., Browne J. K., Salser W. A., Tyler G. V., Snyder R. L., Smolec J. M., Summers J. Isolation, characterization, and comparison of recombinant DNAs derived from genomes of human hepatitis B virus and woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1842–1846. doi: 10.1073/pnas.77.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley F. J., Fox R. A., Sherlock S. Cellular immunity and hepatitis-associated, Australia antigen liver disease. Lancet. 1972 Apr 1;1(7753):723–726. doi: 10.1016/s0140-6736(72)90234-6. [DOI] [PubMed] [Google Scholar]
- Enoch T., Zinn K., Maniatis T. Activation of the human beta-interferon gene requires an interferon-inducible factor. Mol Cell Biol. 1986 Mar;6(3):801–810. doi: 10.1128/mcb.6.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Hill D. A., Walsh J. H., Purcell R. H. Failure to demonstrate circulating interferon during incubation period and acute stage of transfusion-associated hepatitis. Proc Soc Exp Biol Med. 1971 Mar;136(3):853–856. doi: 10.3181/00379727-136-35379. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kinoshita R., Miura K., Suzuki S., Uchino H. Lack of correlation between interferon production of mononuclear cells and virus replication in chronic hepatitis B virus infection. J Clin Microbiol. 1979 Dec;10(6):923–925. doi: 10.1128/jcm.10.6.923-925.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDENMANN J., BURKE D. C., ISAACS A. Studies on the production, mode of action and properties of interferon. Br J Exp Pathol. 1957 Oct;38(5):551–562. [PMC free article] [PubMed] [Google Scholar]
- Maupas P., Melnick J. L. Hepatitis B infection and primary liver cancer. Prog Med Virol. 1981;27:1–5. [PubMed] [Google Scholar]
- Ohori H., Shimizu N., Yamada E., Onodera S., Ishida N. Immunological and morphological properties of HBeAg subtypes (HBeAg/1 and HBeAg/2) in hepatitis B virus core particles. J Gen Virol. 1984 Feb;65(Pt 2):405–414. doi: 10.1099/0022-1317-65-2-405. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Pillot J. HBc and HBe antigenicity and DNA-binding activity of major core protein P22 in hepatitis B virus core particles isolated from the cytoplasm of human liver cells. J Virol. 1985 Feb;53(2):543–551. doi: 10.1128/jvi.53.2.543-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitrine A., Chousterman S., Chousterman M., Naveau S., Thang M. N., Chaput J. C. Lack of in vivo activation of the interferon system in HBsAg-positive chronic active hepatitis. Hepatology. 1985 Mar-Apr;5(2):171–174. doi: 10.1002/hep.1840050202. [DOI] [PubMed] [Google Scholar]
- Sackstein R., Colten H. R. Molecular regulation of MHC class III (C4 and factor B) gene expression in mouse peritoneal macrophages. J Immunol. 1984 Sep;133(3):1618–1626. [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A., Jameel S., Mapoles J. Transcriptional control elements of hepatitis B surface antigen gene. Proc Natl Acad Sci U S A. 1986 Feb;83(3):566–570. doi: 10.1073/pnas.83.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Lin L. S., Wiranowska-Stewart M., Cantell K. Elimination of size and charge heterogeneities of human leukocyte interferons by chemical cleavage. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4200–4204. doi: 10.1073/pnas.74.10.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Ikram H., Much M. I., Harley E. J., Hollinger B. Prevalence of hepatitis B virus infection and hepatocellular carcinoma in Chinese-Americans. J Infect Dis. 1978 Jun;137(6):822–829. doi: 10.1093/infdis/137.6.822. [DOI] [PubMed] [Google Scholar]
- Taylor P. E., Zuckerman A. J. Non-production of interfering substances by serum from patients with infectious hepatitis. J Med Microbiol. 1968 Nov;1(2):217–219. doi: 10.1099/00222615-1-2-217. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Charnay P., Vyas G. N. Biology of hepatitis B virus. Science. 1981 Jul 24;213(4506):406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tolentino P., Dianzani F., Zucca M., Giacchino R. Decreased interferon response by lymphocytes from children with chronic hepatitis. J Infect Dis. 1975 Oct;132(4):459–461. doi: 10.1093/infdis/132.4.459. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F., Schenker S., Combes B. Absence of circulating interferon in patients with infectious and serum hepatitis. Proc Soc Exp Biol Med. 1968 May;128(1):251–253. doi: 10.3181/00379727-128-32989. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Winter G. C., McCarthy C. F., Read A. E., Yoffey J. M. Development of macrophages in phytohaemagglutinin cultures of blood from patients with idiopathic steatorrhoea and with cirrhosis. Br J Exp Pathol. 1967 Feb;48(1):66–80. [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Zinn K., Mellon P., Ptashne M., Maniatis T. Regulated expression of an extrachromosomal human beta-interferon gene in mouse cells. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4897–4901. doi: 10.1073/pnas.79.16.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]