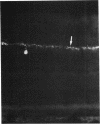
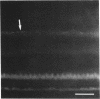
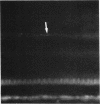
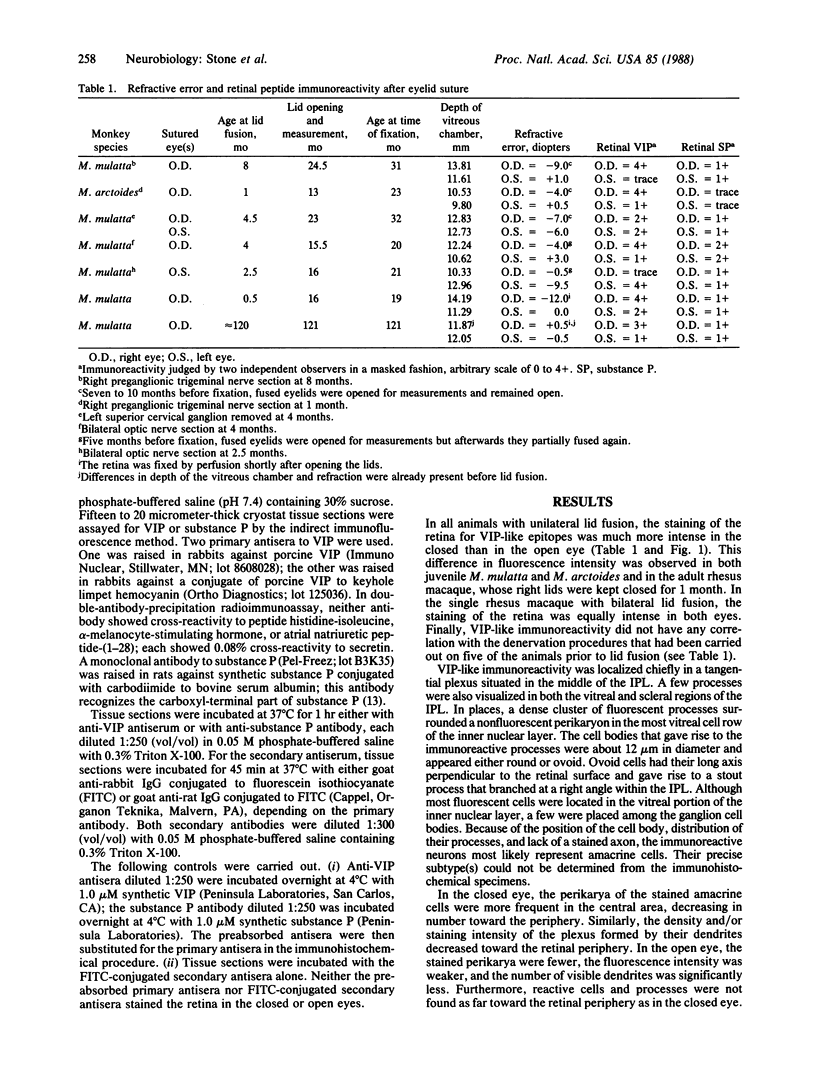
Abstract
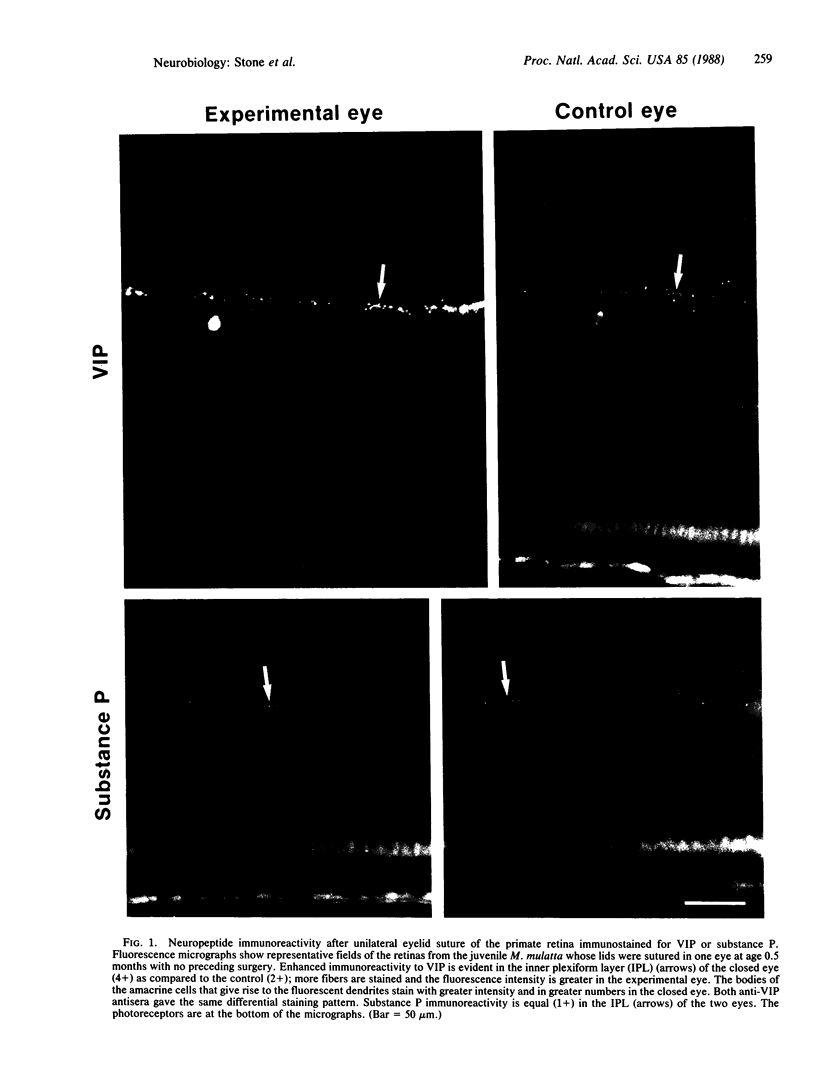
Lids were fused in six neonatal and one adult macaque monkey (Macaca mulatta and Macaca arctoides) and were kept fused for 1 to 18.5 months. The juvenile macaques, but not the adult one, developed myopia due to excessive elongation of the eye. In all animals, the immunohistochemical reactivity of the retina for vasoactive intestinal polypeptide (VIP) was much higher in the closed than in the open eyes. The neuropeptide was localized to the perikaryon and dendrites of amacrine cells. No difference was observed in substance P immunoreactivity between open and closed eyes, suggesting that the observed effect is selective. The change in VIP immunoreactivity could be the result of an increase in peptide synthesis, a decrease in peptide release, or a combination of the two. These results indicate that VIP may play a part in the regulation of postnatal ocular growth.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuello A. C., Galfre G., Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S. H., Kennedy M. B. Immunoreactivity for a calmodulin-dependent protein kinase is selectively increased in macaque striate cortex after monocular deprivation. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1536–1540. doi: 10.1073/pnas.83.5.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt C. S., Stone R. D., Fromer C., Billson F. A. Monocular axial myopia associated with neonatal eyelid closure in human infants. Am J Ophthalmol. 1981 Feb;91(2):197–200. doi: 10.1016/0002-9394(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Johnson C. A., Post R. B., Chalupa L. M., Lee T. J. Monocular deprivation in humans: a study of identical twins. Invest Ophthalmol Vis Sci. 1982 Jul;23(1):135–138. [PubMed] [Google Scholar]
- LeVay S., Wiesel T. N., Hubel D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980 May 1;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Nathan J., Kiely P. M., Crewther S. G., Crewther D. P. Disease-associated visual image degradation and spherical refractive errors in children. Am J Optom Physiol Opt. 1985 Oct;62(10):680–688. doi: 10.1097/00006324-198510000-00003. [DOI] [PubMed] [Google Scholar]
- Nilsson S. F., Bill A. Vasoactive intestinal polypeptide (VIP): effects in the eye and on regional blood flows. Acta Physiol Scand. 1984 Aug;121(4):385–392. doi: 10.1111/j.1748-1716.1984.tb07470.x. [DOI] [PubMed] [Google Scholar]
- O'Leary D. J., Millodot M. Eyelid closure causes myopia in humans. Experientia. 1979 Nov 15;35(11):1478–1479. doi: 10.1007/BF01962795. [DOI] [PubMed] [Google Scholar]
- Rabin J., Van Sluyters R. C., Malach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci. 1981 Apr;20(4):561–564. [PubMed] [Google Scholar]
- Raviola E., Wiesel T. N. An animal model of myopia. N Engl J Med. 1985 Jun 20;312(25):1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- Raviola E., Wiesel T. N. Effect of dark-rearing on experimental myopia in monkeys. Invest Ophthalmol Vis Sci. 1978 Jun;17(6):485–488. [PubMed] [Google Scholar]
- Robb R. M. Refractive errors associated with hemangiomas of the eyelids and orbit in infancy. Am J Ophthalmol. 1977 Jan;83(1):52–58. doi: 10.1016/0002-9394(77)90191-x. [DOI] [PubMed] [Google Scholar]
- Stefanini M., De Martino C., Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967 Oct 14;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Suzuki R., Kobayashi S. Different effects of substance P and vasoactive intestinal peptide on the motor function of bovine intraocular muscles. Invest Ophthalmol Vis Sci. 1983 Dec;24(12):1566–1571. [PubMed] [Google Scholar]
- Tornqvist K., Uddman R., Sundler F., Ehinger B. Somatostatin and VIP neurons in the retina of different species. Histochemistry. 1982;76(2):137–152. doi: 10.1007/BF00501917. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Copenhagen D. R. Control of retinal sensitivity. 3. Lateral interactions at the inner plexiform layer. J Gen Physiol. 1974 Jan;63(1):88–110. doi: 10.1085/jgp.63.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science. 1972 Mar 3;175(4025):1008–1010. doi: 10.1126/science.175.4025.1008. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Raviola E. Increase in axial length of the macaque monkey eye after corneal opacification. Invest Ophthalmol Vis Sci. 1979 Dec;18(12):1232–1236. [PubMed] [Google Scholar]
- Wiesel T. N., Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977 Mar 3;266(5597):66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]