Abstract
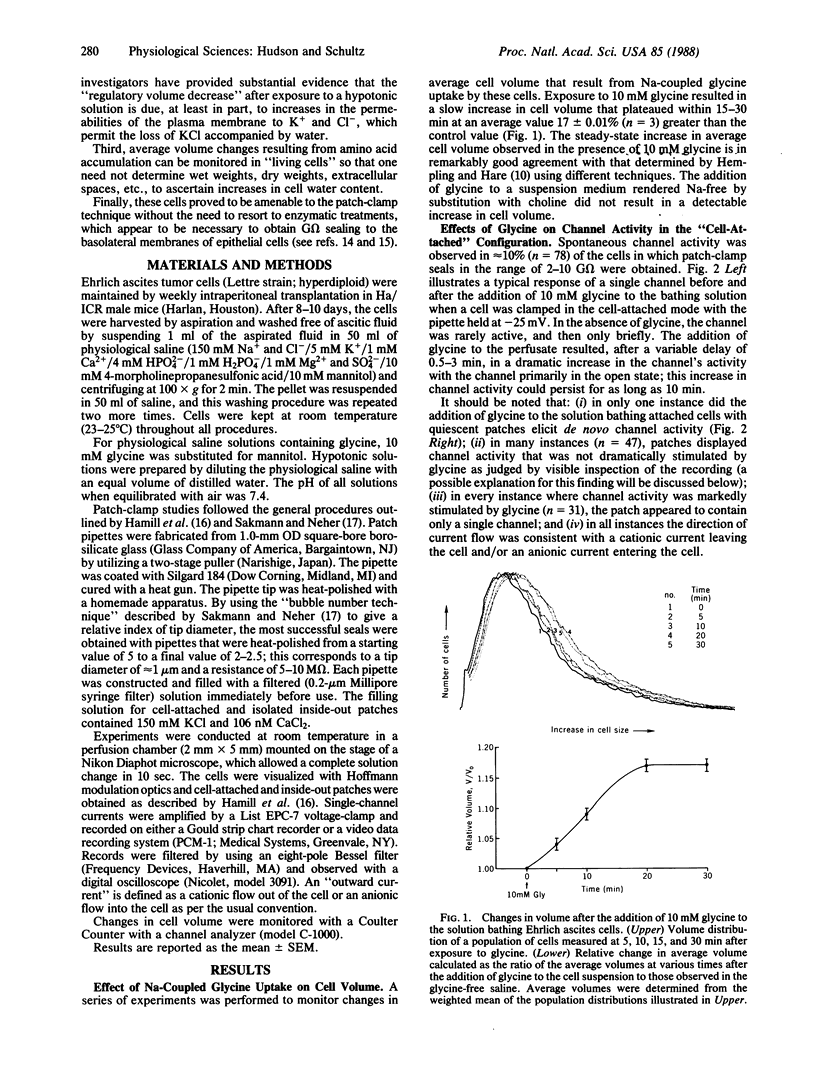
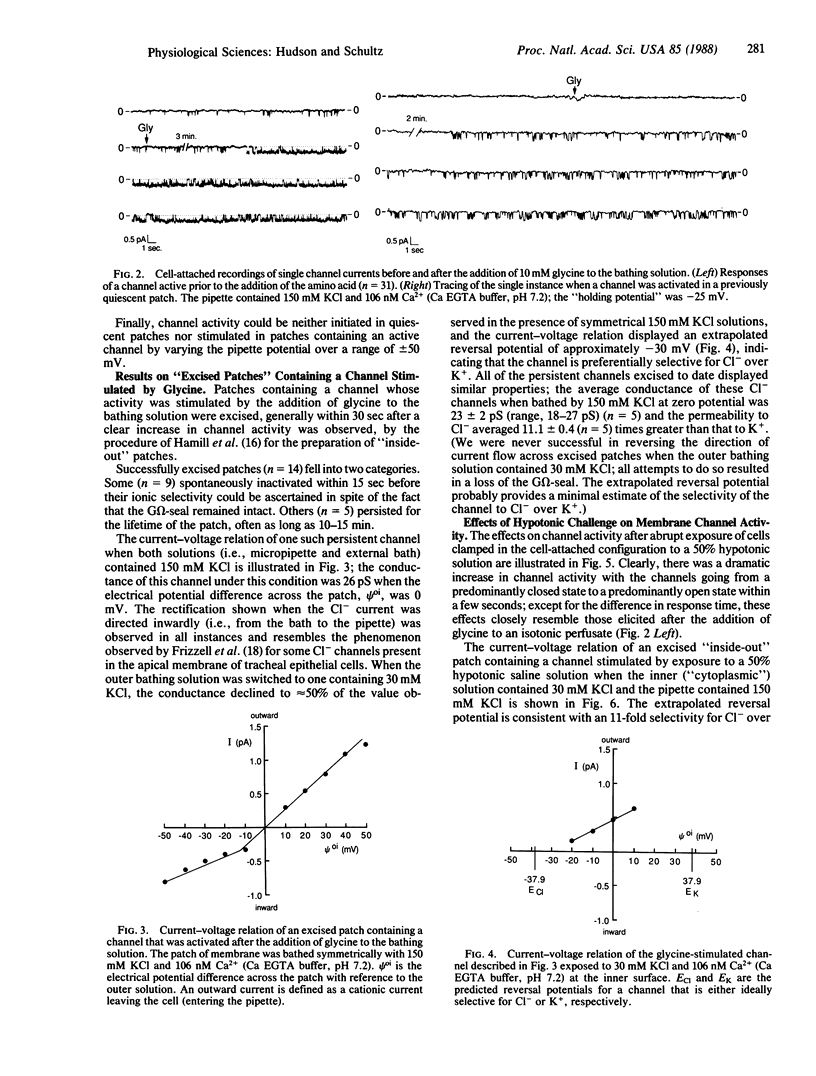
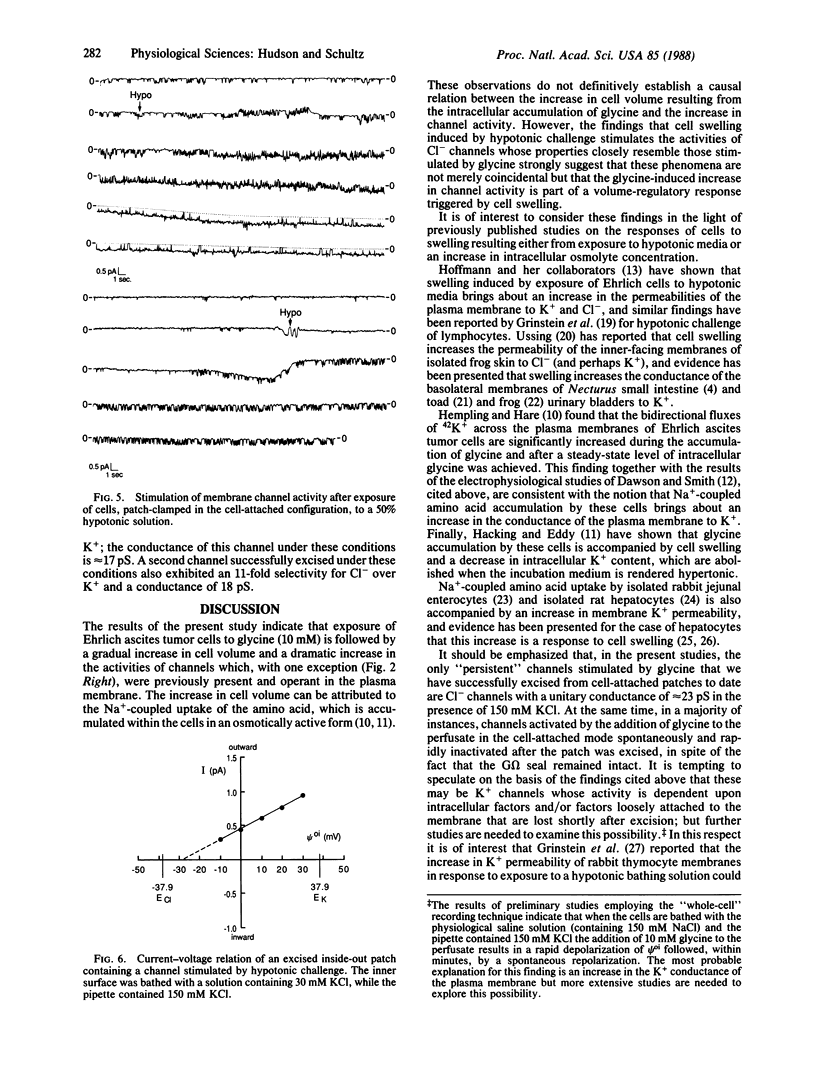
The addition of 10 mM glycine to a physiological saline bathing Ehrlich ascites tumor cells is followed by a slow increase in cell volume that plateaus between 15 and 30 min at a level approximately equal to 17% greater than the control volume; this increase is not observed when glycine is added to cells suspended in a Na+-free saline. The results of studies using the patch-clamp technique in the cell-attached mode indicate that, 0.5-3 min after the addition of glycine to the bathing solution, there is a marked increase in the activity of single channels, which is almost all instances were previously present and operant in the plasma membrane. Successfully excised patches of membrane that contained a channel stimulated by glycine fell into two categories. Some became inactive within 15 sec in spite of the fact that the G omega seal remained intact. Others persisted for the lifetime of the seal. All of the persistent channels had an 11-fold selectivity for Cl- over K+ and a conductance of 23 pS when bathed by symmetrical 150 mM KCl solutions. Although the ionic specificities of the other channels have not been identified, there is reason to suspect that they might be K+ channels whose activities are dependent on factors lost when the patch is excised. Swelling induced by exposing these cells to a 50% hypotonic perfusate stimulated the activities of Cl- channels whose properties closely resemble those stimulated by the addition of glycine to the perfusate, strongly suggesting that the glycine-induced stimulation of Cl- channel activity is part of a volume-regulatory response to cell swelling. If the increase in channel activity induced by the addition of glycine to the perfusate is indeed a response to cell swelling, then this perfusate is indeed a response to cell swelling, then this volume-regulatory response must be extremely sensitive inasmuch as it appears to be "triggered" by an average increase in cell volume that does not exceed 5%.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker-Grunwald T. Potassium permeability and volume control in isolated rat hepatocytes. Biochim Biophys Acta. 1983 Jun 10;731(2):239–242. doi: 10.1016/0005-2736(83)90014-7. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Sepúlveda F. V. Potassium movements associated with amino acid and sugar transport in enterocytes isolated from rabbit jejunum. J Physiol. 1985 Jun;363:271–285. doi: 10.1113/jphysiol.1985.sp015709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N. Exploiting amino acid structure to learn about membrane transport. Adv Enzymol Relat Areas Mol Biol. 1979;49:41–101. doi: 10.1002/9780470122945.ch2. [DOI] [PubMed] [Google Scholar]
- Cooper K. E., Tang J. M., Rae J. L., Eisenberg R. S. A cation channel in frog lens epithelia responsive to pressure and calcium. J Membr Biol. 1986;93(3):259–269. doi: 10.1007/BF01871180. [DOI] [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Cell volume regulation in frog urinary bladder. Fed Proc. 1985 Jun;44(9):2520–2525. [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Interactions of sodium transport, cell volume, and calcium in frog urinary bladder. J Gen Physiol. 1987 May;89(5):687–702. doi: 10.1085/jgp.89.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W. D., Smith T. C. Energetics of Na+-dependent amino acid co-transport in Ehrlich ascites tumor cells. Biochim Biophys Acta. 1987 Feb 12;897(1):5–13. doi: 10.1016/0005-2736(87)90309-9. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Grasset E., Gunter-Smith P., Schultz S. G. Effects of Na-coupled alanine transport on intracellular K activities and the K conductance of the basolateral membranes of Necturus small intestine. J Membr Biol. 1983;71(1-2):89–94. doi: 10.1007/BF01870677. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Sarkadi B., Rothstein A. Induction of 86Rb fluxes by Ca2+ and volume changes in thymocytes and their isolated membranes. J Cell Physiol. 1983 Sep;116(3):352–362. doi: 10.1002/jcp.1041160313. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Dupre A., Rothstein A. Volume regulation by human lymphocytes. Role of calcium. J Gen Physiol. 1982 May;79(5):849–868. doi: 10.1085/jgp.79.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter-Smith P. J., Grasset E., Schultz S. G. Sodium-coupled amino acid and sugar transport by Necturus small intestine. An equivalent electrical circuit analysis of a rheogenic co-transport system. J Membr Biol. 1982;66(1):25–39. doi: 10.1007/BF01868479. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Greger R. Single channel recordings from basolateral and apical membranes of renal proximal tubules. Pflugers Arch. 1984 Aug;401(4):424–426. doi: 10.1007/BF00584348. [DOI] [PubMed] [Google Scholar]
- HEMPLING H. G., HARE D. The effect of glycine transport on potassium fluxes in the Ehrlich mouse ascites tumor cell. J Biol Chem. 1961 Sep;236:2498–2502. [PubMed] [Google Scholar]
- Hacking C., Eddy A. A. The accumulation of amino acids by mouse ascites-tumour cells. Dependence on but lack of equilibrium with the sodium-ion electrochemical gradient. Biochem J. 1981 Feb 15;194(2):415–426. doi: 10.1042/bj1940415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K. Role of separate K+ and Cl- channels and of Na+/Cl- cotransport in volume regulation in Ehrlich cells. Fed Proc. 1985 Jun;44(9):2513–2519. [PubMed] [Google Scholar]
- Kristensen L. O. Energization of alanine transport in isolated rat hepatocytes. Electrogenic Na+-alanine co-transport leading to increased K+ permeability. J Biol Chem. 1980 Jun 10;255(11):5236–5243. [PubMed] [Google Scholar]
- Kristensen L. O., Folke M. Volume-regulatory K+ efflux during concentrative uptake of alanine in isolated rat hepatocytes. Biochem J. 1984 Jul 1;221(1):265–268. doi: 10.1042/bj2210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J. Y., Hudson R. L., Schultz S. G. Current-voltage relations of sodium-coupled sugar transport across the apical membrane of Necturus small intestine. J Membr Biol. 1986;93(3):205–219. doi: 10.1007/BF01871175. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Hudson R. L., Schultz S. G. Cell swelling increases a barium-inhibitable potassium conductance in the basolateral membrane of Necturus small intestine. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3591–3594. doi: 10.1073/pnas.81.11.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K. R., Hudson R. L., Schultz S. G. Effect of hypertonicity on the increase in basolateral conductance of Necturus small intestine in response to Na+-sugar cotransport. Biochim Biophys Acta. 1986 Feb 13;855(1):193–196. doi: 10.1016/0005-2736(86)90205-1. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Butt A. G., Bowler M. J., Leader J. P., Macknight A. D. Effects of anions on cellular volume and transepithelial Na+ transport across toad urinary bladder. J Membr Biol. 1985;83(1-2):119–137. doi: 10.1007/BF01868744. [DOI] [PubMed] [Google Scholar]
- Lohr J. W., Grantham J. J. Isovolumetric regulation of isolated S2 proximal tubules in anisotonic media. J Clin Invest. 1986 Nov;78(5):1165–1172. doi: 10.1172/JCI112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards N. W., Dawson D. C. Single potassium channels blocked by lidocaine and quinidine in isolated turtle colon epithelial cells. Am J Physiol. 1986 Jul;251(1 Pt 1):C85–C89. doi: 10.1152/ajpcell.1986.251.1.C85. [DOI] [PubMed] [Google Scholar]
- Sackin H. Stretch-activated potassium channels in renal proximal tubule. Am J Physiol. 1987 Dec;253(6 Pt 2):F1253–F1262. doi: 10.1152/ajprenal.1987.253.6.F1253. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Fuisz R. E., Curran P. F. Amino acid and sugar transport in rabbit ileum. J Gen Physiol. 1966 May;49(5):849–866. doi: 10.1085/jgp.49.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T., Mills B., Zorgniotti F. Membrane transport in synchronized Ehrlich ascites tumor cells: uptake of amino acids by the A and L system during the cell cycle. J Cell Physiol. 1976 May;88(1):77–87. doi: 10.1002/jcp.1040880110. [DOI] [PubMed] [Google Scholar]
- Ussing H. H. Volume regulation of frog skin epithelium. Acta Physiol Scand. 1982 Mar;114(3):363–369. doi: 10.1111/j.1748-1716.1982.tb06996.x. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]


