Abstract
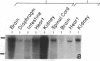
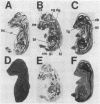
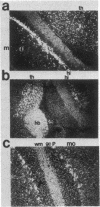
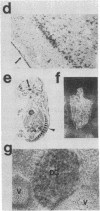
The isolation of multiple Na+,K+-ATPase cDNAs from rat brain has led to the discovery of a family of alpha-isoform genes. Using A1 (alpha), A2 (alpha+), and A3 (alpha III) Na+,K+-ATPase gene probes, we have analyzed the distribution of Na+,K+-ATPase mRNAs in adult and fetal rat tissues by RNA blot and hybridization histochemistry. A1 Na+,K+-ATPase mRNA was found ubiquitously among various tissues, with highest levels in transport epithelial and neural tissues. A2 mRNA was found in adult neural and muscle tissues, and A3 mRNA was found only in neural tissues and fetal heart muscle. Both A1 and A2 mRNAs were less abundant in fetal brain than in adult brain; in contrast, A3 mRNA was abundant at both stages. In situ mapping of brain areas that contain A3 mRNA suggests that this Na+,K+-ATPase isoenzyme is expressed predominantly by neural cells. Analysis of Na+,K+-ATPase proteins generated by cell-free translation of synthetic mRNAs suggests that the A3 protein has properties similar to A2 (alpha+).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. J., Schwartz A., Grupp G., Grupp I., Lee S. W., Wallick E. T., Powell T., Twist V. W., Gathiram P. High-affinity ouabain binding site and low-dose positive inotropic effect in rat myocardium. Nature. 1982 Mar 11;296(5853):167–169. doi: 10.1038/296167a0. [DOI] [PubMed] [Google Scholar]
- Akera T., Ng Y. C., Hadley R., Katano Y., Brody T. M. High affinity and low affinity ouabain binding sites in the rat heart. Eur J Pharmacol. 1986 Dec 16;132(2-3):137–146. doi: 10.1016/0014-2999(86)90598-4. [DOI] [PubMed] [Google Scholar]
- Awgulewitsch A., Utset M. F., Hart C. P., McGinnis W., Ruddle F. H. Spatial restriction in expression of a mouse homoeo box locus within the central nervous system. 1986 Mar 27-Apr 2Nature. 320(6060):328–335. doi: 10.1038/320328a0. [DOI] [PubMed] [Google Scholar]
- Bertoni J. M., Siegel G. J. Development of (Na+-K+)-ATPase in rat cerebrum: correlation with Na+-dependent phosphorylation and K+-paranitrophenylphosphatase. J Neurochem. 1978 Dec;31(6):1501–1511. doi: 10.1111/j.1471-4159.1978.tb06577.x. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Anderson H. C., Palade G. E., Jamieson J. D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986 Aug 15;46(4):623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Charlemagne D., Maixent J. M., Preteseille M., Lelievre L. G. Ouabain binding sites and (Na+,K+)-ATPase activity in rat cardiac hypertrophy. Expression of the neonatal forms. J Biol Chem. 1986 Jan 5;261(1):185–189. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Ernst S. A., Mills J. W. Autoradiographic localization of tritiated ouabain-sensitive sodium pump sites in ion transporting epithelia. J Histochem Cytochem. 1980 Jan;28(1):72–77. doi: 10.1177/28.1.6243324. [DOI] [PubMed] [Google Scholar]
- Firth J. A. Quantitative assessment of Na+,K+-ATPase localization by direct and indirect p-nitrophenyl phosphatase methods. J Histochem Cytochem. 1987 Apr;35(4):507–513. doi: 10.1177/35.4.3029215. [DOI] [PubMed] [Google Scholar]
- Hauger R., Luu H. M., Meyer D. K., Goodwin F. K., Paul S. M. Characterization of "high-affinity" [3H]ouabain binding in the rat central nervous system. J Neurochem. 1985 Jun;44(6):1709–1715. doi: 10.1111/j.1471-4159.1985.tb07158.x. [DOI] [PubMed] [Google Scholar]
- Kashgarian M., Biemesderfer D., Caplan M., Forbush B., 3rd Monoclonal antibody to Na,K-ATPase: immunocytochemical localization along nephron segments. Kidney Int. 1985 Dec;28(6):899–913. doi: 10.1038/ki.1985.216. [DOI] [PubMed] [Google Scholar]
- Kazazoglou T., Renaud J. F., Rossi B., Lazdunski M. Two classes of ouabain receptors in chick ventricular cardiac cells and their relation to (Na+,K+)-ATPase inhibition, intracellular Na+ accumulation, Ca2+ influx, and cardiotonic effect. J Biol Chem. 1983 Oct 25;258(20):12163–12170. [PubMed] [Google Scholar]
- Lytton J. Insulin affects the sodium affinity of the rat adipocyte (Na+,K+)-ATPase. J Biol Chem. 1985 Aug 25;260(18):10075–10080. [PubMed] [Google Scholar]
- Lytton J., Lin J. C., Guidotti G. Identification of two molecular forms of (Na+,K+)-ATPase in rat adipocytes. Relation to insulin stimulation of the enzyme. J Biol Chem. 1985 Jan 25;260(2):1177–1184. [PubMed] [Google Scholar]
- Maixent J. M., Charlemagne D., de la Chapelle B., Lelievre L. G. Two Na,K-ATPase isoenzymes in canine cardiac myocytes. Molecular basis of inotropic and toxic effects of digitalis. J Biol Chem. 1987 May 15;262(14):6842–6848. [PubMed] [Google Scholar]
- Marks M. J., Seeds N. W. A heterogeneous ouabain-ATPase interaction in mouse brain. Life Sci. 1978 Dec 31;23(27-28):2735–2744. doi: 10.1016/0024-3205(78)90654-9. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Iwata H., Cooper J. R. Specific inactivation of alpha (+) molecular form of (Na+ + K+)-ATPase by pyrithiamin. J Biol Chem. 1984 Mar 25;259(6):3858–3863. [PubMed] [Google Scholar]
- McDonough A., Schmitt C. Comparison of subunits of cardiac, brain, and kidney Na+-K+-ATPase. Am J Physiol. 1985 Mar;248(3 Pt 1):C247–C251. doi: 10.1152/ajpcell.1985.248.3.C247. [DOI] [PubMed] [Google Scholar]
- Mercer R. W., Schneider J. W., Savitz A., Emanuel J., Benz E. J., Jr, Levenson R. Rat-brain Na,K-ATPase beta-chain gene: primary structure, tissue-specific expression, and amplification in ouabain-resistant HeLa C+ cells. Mol Cell Biol. 1986 Nov;6(11):3884–3890. doi: 10.1128/mcb.6.11.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. J., Lai C., Nave K. A., Lenoir D., Ogata J., Sutcliffe J. G. Nucleotide sequences of two mRNAs for rat brain myelin proteolipid protein. Cell. 1985 Oct;42(3):931–939. doi: 10.1016/0092-8674(85)90289-2. [DOI] [PubMed] [Google Scholar]
- Schmitt C. A., McDonough A. A. Developmental and thyroid hormone regulation of two molecular forms of Na+-K+-ATPase in brain. J Biol Chem. 1986 Aug 5;261(22):10439–10444. [PubMed] [Google Scholar]
- Schneider J. W., Mercer R. W., Caplan M., Emanuel J. R., Sweadner K. J., Benz E. J., Jr, Levenson R. Molecular cloning of rat brain Na,K-ATPase alpha-subunit cDNA. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6357–6361. doi: 10.1073/pnas.82.18.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Specht S. C., Sweadner K. J. Two different Na,K-ATPases in the optic nerve: cells of origin and axonal transport. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1234–1238. doi: 10.1073/pnas.81.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling C. E. Radioautographic localization of sodium pump sites in rabbit intestine. J Cell Biol. 1972 Jun;53(3):704–714. doi: 10.1083/jcb.53.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner K. J. Enzymatic properties of separated isozymes of the Na,K-ATPase. Substrate affinities, kinetic cooperativity, and ion transport stoichiometry. J Biol Chem. 1985 Sep 25;260(21):11508–11513. [PubMed] [Google Scholar]
- Sweadner K. J., Gilkeson R. C. Two isozymes of the Na,K-ATPase have distinct antigenic determinants. J Biol Chem. 1985 Jul 25;260(15):9016–9022. [PubMed] [Google Scholar]
- Sweadner K. J. Two molecular forms of (Na+ + K+)-stimulated ATPase in brain. Separation, and difference in affinity for strophanthidin. J Biol Chem. 1979 Jul 10;254(13):6060–6067. [PubMed] [Google Scholar]
- Tamkun M. M., Fambrough D. M. The (Na+ + K+)-ATPase of chick sensory neurons. Studies on biosynthesis and intracellular transport. J Biol Chem. 1986 Jan 25;261(3):1009–1019. [PubMed] [Google Scholar]
- Widdicombe J. H., Basbaum C. B., Highland E. Sodium-pump density of cells from dog tracheal mucosa. Am J Physiol. 1985 May;248(5 Pt 1):C389–C398. doi: 10.1152/ajpcell.1985.248.5.C389. [DOI] [PubMed] [Google Scholar]
- Young R. M., Lingrel J. B. Tissue distribution of mRNAs encoding the alpha isoforms and beta subunit of rat Na+,K+-ATPase. Biochem Biophys Res Commun. 1987 May 29;145(1):52–58. doi: 10.1016/0006-291x(87)91286-1. [DOI] [PubMed] [Google Scholar]
- Young R. M., Shull G. E., Lingrel J. B. Multiple mRNAs from rat kidney and brain encode a single Na+,K+-ATPase beta subunit protein. J Biol Chem. 1987 Apr 5;262(10):4905–4910. [PubMed] [Google Scholar]