Abstract
Upon encounter with antigen, CD4+ T cells differentiate into effector Th subsets with distinctive functions that are related to their unique cytokine profiles and anatomical locations. One of the most important Th functions is to provide signals to developing B cells that induce specific and appropriate antibody responses. The major CD4+ T cell subset that helps B cells is the T follicular helper (TFH) cell, whose expression of the chemokine receptor CXCR5 [chemokine (C–X–C motif) receptor 5] serves to localize this cell to developing germinal centers (GCs) where it provides instructive signals leading to Ig class switching and somatic mutation. TFH cells produce high levels of IL-21, a cytokine that is critical for GC formation and also for the generation of TFH cells. Although TFH cells have been found to produce cytokines characteristic of other Th subsets, they represent a distinct lineage whose development is driven by the transcription factor B-cell CLL lymphoma-6 (BCL6). Consistent with their critical role in the generation of antibody responses, dysregulated TFH function has been associated with the development of systemic autoimmunity. Here, we review the role of IL-21 in the regulation of normal TFH development and function as well as in progression of autoimmune responses.
Keywords: autoimmunity, BCL6, germinal center, Th subsets
Introduction
Specific CD4+ T cell effector responses can be delegated to distinct subsets characterized as Th1, Th2, Th17 or regulatory T cell (Treg) cells, with the development of each of these subsets being driven, respectively, by the master lineage-specific transcription factors T-bet [now also denoted as T-box 21 (TBX21)], GATA binding protein 3 (GATA-3), retinoid-related orphan receptor γt (RORγt) or forkhead box protein P3 (FOXP3) (1). T-cell-dependent antibody production is a critical component of the normal immune response, and Th2 cells were originally believed to be the predominant source of B cell help because of their production of IL-4, a cytokine known to be involved in B cell proliferation as well as Ig class switching (1).
Subsequently, IL-21 was identified as a Th-derived, type I, four-α-helical bundle cytokine that was critical for plasma cell generation as well as isotype switching (2) and normal Ig production (3), consistent with IL-21 being a Th2-specific cytokine (4); however, other data indicated that IL-21 had Th1-like properties as well (5).
More recently it became clear that the CD4+ T cells involved in germinal center (GC) formation and function—denoted T follicular helper cells (TFH cells)—were distinct from any of these previously identified subsets. These TFH cells expressed high levels of the chemokine receptor CXCR5 [chemokine (C–X–C motif) receptor 5], allowing them to home to and be retained by the lymphoid follicle, where contact with antigen-primed B cells led to B cell proliferation, isotype switching and somatic mutation of the Ig repertoire (6, 7). Gene microarray analysis revealed that follicle-localized CXCR5+ Th cells had a very distinctive transcriptional profile that distinguished these cells from Th1 or Th2 cells, with high-level IL-21 and B-cell CLL lymphoma-6 (BCL6) messenger RNAs (mRNAs) (5), both of which are now considered hallmarks of TFH cells (6).
IL-21 is a type I cytokine that signals via a specific receptor protein, IL-21R (8, 9), and the common cytokine receptor γ chain, γc, which is shared by the receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 (10); γc is mutated in humans with X-linked SCID (11). IL-21 signals in part via STAT3 (signal transducer and activator of transcription 3) (12), with actions on a wide range of lineages, including T cells, B cells, NK cells and dendritic cells (10). Specifically, IL-21 can promote the expansion of CD8+ T cells, is critical for normal Ig production by B cells, can inhibit dendritic cell function and, interestingly, can be pro-apoptotic for B cells and NK cells (10). Whereas IL-21 was first identified as a cytokine produced by activated peripheral blood T cells (9), it is now known that it can be produced by a range of differentiated Th populations (10) as well as by NKT cells (13).
In this review, we discuss the role of IL-21 in the generation of TFH cells as well as in the functional interaction of these cells with B cells within the GC. The relationship between TFH and other Th subsets will also be discussed, as more evidence mounts in support of flexibility of cytokine profiles within the TFH cells. In addition, the role played by IL-21 in the interaction between the TFH master transcription factor BCL6 and other lineage-specific transcription factors will be discussed. Finally, elevated levels of IL-21 and TFH-specific surface molecules have been implicated in the development of systemic autoimmune responses, and the mechanisms for disease development will be addressed.
Identification of a TFH profile
Early molecular profiling of human TFH cells revealed that although a large number of transcripts were shared by TFH, Th1 and Th2 effector cells, a subset of genes were preferentially expressed by TFH cells, including those encoding CXCR5, IL-21, BCL6 and ICOS (inducible co-stimulatory molecule) (5). Although these genes were transiently expressed to some degree by activated Th1 or Th2 cells, the spatial and temporal expression pattern of these proteins in TFH cells was coincident with their functions. Interaction between CXCR5 and chemokine (C–X–C motif) ligand 13 (CXCL13), which is produced in the follicle, was required for the localization of TFH to the GC (14), whereas ICOS co-stimulatory interactions with inducible co-stimulatory molecule ligand (ICOSL) on B cells were found to be important for GC development (15). BCL6 was already known to be highly expressed within the GC and to be required for GC formation (16). Finally, accumulating evidence has revealed that IL-21 is a key determinant of B-cell differentiation (2, 3, 10).
Both in vivo and in vitro evidence demonstrates that IL-21 is a T-cell-derived cytokine that is very important for B-cell proliferation and differentiation (10). Mice that constitutively expressed IL-21 were found to have higher levels of plasma cells as well as higher levels of serum Igs (2). In vitro stimulation of both murine and human B cells with IL-21 induced the plasma-cell-associated transcription factor BLIMP1 (B-lymphocyte-induced maturation protein 1) [now termed PR domain containing 1, with ZNF domain (PRDM1)] and subsequent plasma cell differentiation as well as the accumulation of isotype-switched Igs (2, 17). Interestingly, Ozaki et al. also showed that IL-21 is a potent inducer of BCL6 (2), a transcription factor that negatively regulates expression of BLIMP1, just as BLIMP1 represses BCL6 (18), suggesting that these two transcription factors are induced in distinct populations of cells.
When a population of CXCR5+ tonsillar TFH cells was cultured with naive human B cells, the accumulation of Ig-secreting B cells was decreased dramatically if IL-21 action was blocked, demonstrating that TFH secretion of IL-21 was critical for the differentiation of naive B cells (19). Interestingly, in both murine and human in vitro systems, IL-4 was shown to antagonize the actions of IL-21 on plasma cell differentiation (2, 19), even though analysis of Il21r or Il4 single-knockout (KO) mice and Il21r plus Il4 double-KO mice revealed that cooperative actions of these cytokines were required for Ig production (2). In addition, even though IL-21 is a potent inducer of IL-10 (20), IL-21 was found to be much more potent than IL-10 for the induction of Ig secretion from both naive and memory human B cells in vitro, suggesting that IL-21 plays a major role in both primary and memory responses to T-cell-dependent antigens (19).
Although microarray analysis and hierarchical clustering revealed that the overall transcriptional profile of TFH cells was distinct from that of Th17 cells as well as those of Th1 or Th2 cells (21), both TFH and Th17 subsets share high-level expression of IL-21 (21). IL-21 was found to be required in part for the in vitro differentiation of Th17 cells, but it was not necessary for their in vivo differentiation (22). These data suggested certain similarities but also important differences between TFH cells and Th17 cells, worthy of further investigation.
IL-21 in the GC: B-cell versus TFH-cell action
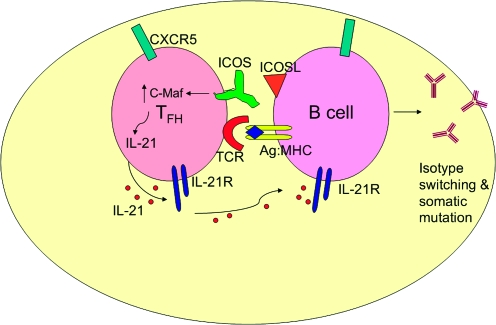
GC development is impaired in mice deficient for IL-21 signaling (3). As producers of high levels of IL-21, TFH cells are important regulators of the GC reaction because of their close association with CXCR5+ B cells localized to the follicle that undergo differentiation in response to both soluble and cell-mediated signals and produce high-affinity Ig (6, 7) (Fig. 1).
Fig. 1.
The GC reaction is facilitated by the interaction between surface molecules on TFH cells and developing B cells, both of which use CXCR5 to localize there. Interactions include the binding of ICOSL to ICOS, which leads to the up-regulation of c-Maf and subsequent production of high levels of IL-21 by TFH cells. IL-21 then acts on TFH in an autocrine manner to promote its function in GC B-cell development and antibody production. TFH-cell-derived IL-21 can also bind to the IL-21R on B cells.
A recent study investigated whether this IL-21 requirement for the GC reaction reflected the action of IL-21 on either the B cells or the TFH cells (23). Immunization of IL-21-KO mice with a T-cell-dependent antigen showed that CXCR5 surface expression on CD4+ T cells was greatly reduced in the absence of IL-21, suggesting that IL-21 has an autocrine role for proper TFH development. Consistent with this, IL-21R expression was significantly higher on CXCR5+CD4+ than on CXCR5−CD4+ T cells (23). Adoptive transfer of wild-type (WT) CD4+ T cells into Il21r-KO recipients followed by immunization rescued GC formation and partially rescued Ig production. However, the transfer of WT B cells into Il21r-KO mice could not rescue Ig production, indicating that the defect in these mice was intrinsic to the CD4+ T cells (23).
Surface expression of ICOS is required for efficient TFH development, as ICOS-KO mice have reduced numbers of CXCR5+ CD4 T cells (24). In addition, ICOSL-deficient mice had reduced expression of IL-21 (21). Specific deletion of ICOSL in B cells resulted in a significant reduction in both the number of CXCR5+CD4+ T cells as well as the level of IL-21 after immunization, demonstrating that the interaction of ICOS on TFH cells with ICOSL on GC B cells is required for maximal IL-21 production (21).
Analogous to TFH cells, Th17 cells also express ICOS on their cell surface. An investigation of the requirement of ICOS for TFH and Th17 development revealed that although ICOS was required for GC formation, ICOS was not required for Th17 differentiation but instead was required for the maximal expansion of both Th17 cells and TFH cells (25). TFH and Th17 cells were also found to share high expression of the transcription factor c-Maf, and deletion of c-Maf led to reduced numbers of both TFH and Th17 cells. This was related to the direct regulation of IL-21 transcription by c-Maf subsequent to the induction of c-Maf by ICOS–ICOSL interaction (25, 26).
TFH cells producing alternative cytokines
Although it is clear that TFH cells are critical for directing the development of an antibody response by GC B cells, the above studies (21, 23) reported that IL-21 acts primarily in an autocrine manner on the TFH population, even though it is well known that IL-21 receptors are expressed on B cells and B cells can also respond to this cytokine by undergoing differentiation and producing Ig (2, 3, 10). Interestingly, gene microarray analysis suggested that TFH cells lacked expression of most cytokines or transcription factors associated with the Th1, Th2 or Th17 subsets (5, 21), including IL-4 and IFN-γ, making it unclear how and where these cytokines could influence B-cell Ig isotype switching if they were not produced by TFH cells. Importantly, however, in vitro Th activity for B-cell Ig production has also been detected in Th1, Th2 and Th17 polarized populations, indicating that this helper activity is not specific only to TFH populations (27).
Several reports have challenged the notion that TFH cells lack the expression of cytokines associated with these other Th subsets, including three studies that used infection with helminths that elicit strong Th2 responses (28–30).
The first study monitored IL-4 expression by employing a ‘dual-reporter’ mouse model in which IL-4 transcription and protein production could be distinguished. After infection with Schistosoma mansoni, the majority of IL-4-producing cells in lymph node and spleen expressed the TFH markers CXCR5, ICOS and programmed death-1 (PD-1), although the IL-4-producing cells within liver granulomas did not express these markers (28). These IL-4-producing TFH cells expressed amounts of IL-4 and GATA-3 transcripts that were similar to the level found in Th2 cells that did not express TFH markers and also produced the high levels of BCL6 characteristic of TFH cells. Experiments in which CXCR5−PD-1− cells that expressed green fluorescent protein-labeled IL-4 (this marks IL-4 protein in this dual-reporter system) were transferred into naive mice and then antigen challenged demonstrated that 20% of these cells could become CXCR5+PD-1+ in vivo, confirming that at least a minority of Th2 cells can develop into TFH cells (28).
Consistent with this, the second study reported that IL-4-producing TFH cells were present in the mesenteric lymph nodes after infection with Heligmosomoides polygyrus. Using the IL-4 transcription/protein dual-reporter mice described above, CD4+ T cells that were committed to the Th2 lineage were found throughout the follicle, but those Th2 cells that produced IL-4 protein were found only near B cells in the GC region (29). These IL-4 protein-producing cells expressed high levels of CXCR5, ICOS, PD-1, IL-21 and BCL6, as is typical of TFH cells.
The third study elegantly addressed the role of TFH cytokine production in isotype switching and somatic mutation. Here, conjugates consisting of B cells plus T cells were isolated from GCs of IL-4/IFNγ dual-reporter mice that had been infected with Leishmania major (30). These T cells expressed high levels of IL-21, BCL6 and CXCR5 characteristic of TFH cells. B-cell–T-cell conjugates with IL-4-expressing cells were shown to contain IgG1 transcripts, whereas conjugates that contained IFNγ-expressing cells contained mainly IgG2a transcripts, demonstrating that the specific cytokine produced by the TFH was critical in the regulation of isotype switching in B cells. Purified IL-4-producing cells were analyzed for IL-21 production by enzyme-linked immunosorbent spot, and IL-4+ TFH cells from lymph nodes produced significantly higher amounts of IL-21 than did IL-4-producing cells from lungs. The IL-4-producing cells from the lungs but not the IL-4-producing TFH from the GC could function as classical Th2 cells in that they could elicit eosinophil recruitment when transferred to IL-4-deficient mice, suggesting important differences between these populations (30).
In addition to evidence supporting the existence of TFH cells expressing either IL-4 or IFNγ, studies in mice prone to autoimmune lupus revealed the existence of TFH cells that produce both IL-17 and IL-21 (31).
The most surprising example of lineage switching involves the conversion of FOXP3+ Treg cells to TFH cells in the Peyer's patches of the gut, where GCs are critical for the production of IgA (32). When FOXP3+ cells were transferred into T-cell-deficient CD3ε-KO mice, 80% of these were found to down-regulate FOXP3 expression, migrate into the B-cell follicles of Peyer's patches and express high levels of IL-21 and BCL6 (32). Although FOXP3 down-regulation was not dependent on B cells as it could occur in B-cell-deficient mice, the acquisition of CXCR5, PD-1 and IL-21 required interaction with B cells in Peyer's patches (32).
IL-21 in combination with transforming growth factor-β1 (TGFβ1) produced by TFH cells can also contribute to the differentiation of IgA-secreting plasmablasts in the gut (33). In this scenario, the combination of TGFβ1 with IL-21 up-regulated chemokine (C–C motif) receptor 10 (CCR10) and down-regulated CXCR5, thus allowing migration of the B cells out of the GC and toward the mucosal surface.
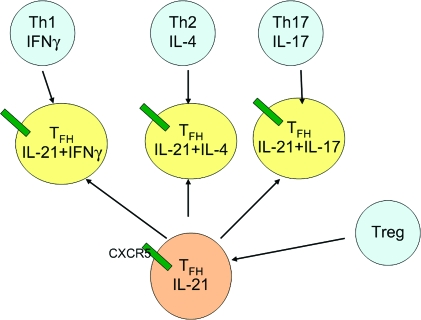
Collectively, the above studies show that TFH cells can produce IL-4, IFNγ or IL-17 and that these cells are potentially derived from a range of lineages, including Th1, Th2, Th17 or Treg cells (Fig. 2).
Fig. 2.
TFH cells are developmentally related to other CD4+ T cell subsets. Although initially characterized as producing IL-21 but not cytokines associated with the Th1, Th2 and Th17 subsets, evidence now indicates that TFH cells localizing to the follicle through their expression of CXCR5 can also express IFNγ, IL-4 and IL-17. Treg cells are capable of undergoing conversion to TFH cells in Peyer's patches. The green bars designate CXCR5.
BCL6 as a master regulator of TFH cells
Although early gene expression analysis established a correlation between BCL6 transcription factor expression and TFH cells, only recently have experimental approaches verified the causal nature of this expression. Consistent with the reduced TFH cell numbers in Il21r-KO mice and with the original demonstration by Ozaki et al. (2) in 2004 that IL-21 is a potent inducer of BCL6, Il21-KO mice showed diminished expression of BCL6, and either IL-6 or IL-21 could induce BCL6 expression in naive CD4+ T cells (34).
While over-expression of BCL6 was shown to up-regulate endogenous BCL6, IL-21R, IL6R and CXCR5 mRNA, it also inhibited IL-17 expression by blocking the functional activity of RORγt without affecting RORγt mRNA expression (34). BCL6 over-expression also can inhibit the expression of Th1-associated genes and Th2-associated genes (34). Experiments employing mixed bone marrow chimeras showed that BCL6 expression was required in both T and B cells for normal GC formation.
BCL6 repression of the development of other Th lineages involves direct binding and suppression of the promoters of the Tbx21 and Rorc genes in TFH cells (34). BCL6 can also repress expression of microRNAs that otherwise suppress expression of TFH-specific genes such as those encoding CXCR5 and PD-1 (35). Thus, over-expression of BCL6 led to a global down-regulation of microRNAs that targeted sites in the 3′ untranslated regions of CXCR5 and PD-1 mRNAs, thereby promoting expression of the TFH program (35).
Constitutive BCL6 expression induced expression of a panel of TFH markers but it also inhibited the expression of BLIMP1 (36), known to be an inhibitor of BCL6 function (18). Over-expression of BLIMP1 in CD4+ T cells significantly reduced the number of TFH cells, without effecting the expression of Th2-specific, Th17-specific or Treg-specific transcription factors (36). Thus, just as BCL6 and BLIMP1 can function as mutually inhibitory cross-regulators of B cell differentiation pathways (18, 37), so can they regulate the CD4 T-cell differentiation pathway, amplifying the TFH responses that will then induce strong BCL6-dependent GC reactions. It is interesting that IL-21 is a potent inducer of both BLIMP1 and BCL6 (2), but because of their cross-inhibition, presumably one of these factors will dominate within a particular cell.
TFH cells and IL-21 in autoimmunity
GCs are a critical site for selection of appropriate antibody specificities and TFH cells play an important role in the regulation of autoantibody production, as shown by the systemic autoimmune responses that result from over-expression of TFH signaling proteins such as ICOS or IL-21.
The first suggestion of a connection of IL-21 to autoimmune disease came from the BXSB-Yaa mouse model of systemic lupus erythematosus, which was found to have elevated levels of IL-21 mRNA and serum protein (2) that temporally correlated with development of disease. In this model, IL-21 signaling was shown to be essential to the development of the disease phenotype, as when the BXSB-Yaa mice were crossed onto the Il21r-KO background, there was no hypergammaglobulinemia, autoantibody production or renal disease (38). Interestingly, these BXSB-Yaa/Il21r-KO mice had greatly reduced numbers of CXCR5+ICOS+CD4+ T cells, and the excessive IL-21 production did not derive from this population of conventional TFH cells, but rather from an extrafollicular population of ICOS+ CD4+ T cells (38).
Mice homozygous for the sanroque allele of Roquin also develop a lupus-like disease accompanied by the accumulation of excessive numbers of both GCs and TFH cells with high levels of ICOS and IL-21 expression (39), and lupus-like symptoms were dependent on enhanced GC formation as they could be reduced by deletion of even one allele of the BCL6 (40). Interestingly, however, TFH formation in this disease may be more dependent on ICOS than IL-21 (40).
In the MRLlpr mouse, which also develops a lupus-like disease, an extrafollicular population of ICOShiCD4+ T cells that have down-regulated P-selectin glycoprotein ligand 1 (PSGL-1) were the primary source for IL-21 production and subsequent extrafollicular development of IgG+ plasmablasts (41). These extrafollicular PSGL-1lo CD4+ cells also occurred at a high frequency in several other autoimmune models. In contrast to the originally defined TFH population, these PSGL-1lo CD4+ T cells also secreted IL-4 and IFNγ, which could instruct appropriate isotype switching in this extrafollicular setting (41). The relationship between these extrafollicular CD4+ T cells and the TFH population remains to be determined.
It is important to recognize that IL-21 can contribute to other autoimmune diseases as well, ostensibly by B-cell-independent mechanisms. For example, in the non-obese diabetic (NOD) mouse model of type I diabetes, disease no longer develops when the NOD mouse is crossed to the Il21r-KO background (42, 43). Thus, whereas the relationship of IL-21 to TFH is very important, one must be cognizant that it may not dominate all types of autoimmune responses.
Conclusions
TFH cells are now recognized as a distinct helper subset whose differentiation requires the transcription factor BCL6, leads to a GC-localized population of cells that are critical in the development of a strong Ig response. Naive TFH cells produce abundant IL-21, which then acts as an autocrine factor for the functional activity of these cells. In addition, it is now clear that TFH also have the capacity to produce cytokines characteristic of Th1, Th2 and Th17 cells, emphasizing the developmental and functional plasticity of all these subsets. Future studies are needed to further unravel mechanisms for controlling the production and activation of TFH cells, as these cells and the IL-21 they produce play important roles in a number of systemic autoimmune diseases.
Funding
Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health.
Acknowledgments
We thank Drs Irina Rochman, Edwin Wan and Jian-Xin Lin, National Heart, Lung and Blood Institute, National Institutes of Health for critical comments.
Conflict of Interest: The authors are inventors on patents and patent applications related to IL-21.
References
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozaki K, Spolski R, Ettinger R, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 2004;173:5361. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 3.Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 4.Wurster AL, Rodgers VL, Satoskar AR, et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J. Exp. Med. 2002;196:969. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chtanova T, Tangye SG, Newton R, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 2004;173:68. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 6.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol. 2008;26:741. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 7.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc. Natl Acad. Sci. USA. 2000;97:11439. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 10.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 2008;26:57. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 12.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coquet JM, Kyparissoudis K, Pellicci DG, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol. 2007;178:2827. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 14.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000;192:1553. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bossaller L, Burger J, Draeger R, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 2006;177:4927. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 16.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 17.Ettinger R, Sims GP, Fairhurst AM, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 2005;175:7867. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 18.Shaffer AL, Lin KI, Kuo TC, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 19.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 2007;179:8180. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 20.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J. Immunol. 2009;182:2859. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spolski R, Leonard WJ. Cytokine mediators of Th17 function. Eur. J. Immunol. 2009;39:658. doi: 10.1002/eji.200839066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Akiba H, Takeda K, Kojima Y, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 2005;175:2340. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 25.Bauquet AT, Jin H, Paterson AM, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009;10:167. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pot C, Jin H, Awasthi A, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 2009;183:797. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eddahri F, Denanglaire S, Bureau F, et al. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113:2426. doi: 10.1182/blood-2008-04-154682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med. 2009;206:991. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J. Exp. Med. 2009;206:1001. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 2009;10:385. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J. Immunol. 2008;181:6038. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuji M, Komatsu N, Kawamoto S, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 33.Dullaers M, Li D, Xue Y, et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu. Rev. Immunol. 2008;26:133. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- 38.Bubier JA, Sproule TJ, Foreman O, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl Acad. Sci. USA. 2009;106:1518. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinuesa CG, Cook MC, Angelucci C, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 40.Linterman MA, Rigby RJ, Wong RK, et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 2009;206:561. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odegard JM, Marks BR, DiPlacido LD, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J. Exp. Med. 2008;205:2873. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc. Natl Acad. Sci. USA. 2008;105:14028. doi: 10.1073/pnas.0804358105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland AP, Van Belle T, Wurster AL, et al. Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes. 2009;58:1144. doi: 10.2337/db08-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]