Abstract
The syndrome called depression may represent the common final pathway at which different aetiopathogenic processes converge. One such atiopathogenic process is innate immune system activation. Some depressed patients increased levels of inflammatory cytokines and other immunologic abnormalities. It is not known whether immune system activation contributes to the pathogenesis of depressive symptoms. Supporting this possibility is the observation that in both rodents and humans, exogenous immune stimuli such as endotoxin can produce symptoms that resemble depression. A new approach to depression research would be to use immune stimuli to elicit depressive symptoms in humans. Here we review each of the symptoms elicited in humans by endotoxin administration, and compare this model to two other immune depression paradigms: interferon-alpha treatment and typhoid vaccine administration, to assess to what degree endotoxin administration represents a valid model of immune depression. We also review corresponding behavioral changes in rodents and the potential molecular pathways through which immune system activation produces each symptom.
Keywords: Depression, Fatigue, Anhedonia, Cognition, Endotoxin, Cytokines, Tumor necrosis factor, Interleukin, Inflammation
1. Depression and inflammation
1.1. Depression
Major Depressive Disorder (“depression”) is a highly prevalent and severely debilitating disorder for which current treatments are inadequate and the pathogenesis of which is poorly understood. Depression is a syndrome that affects various symptom domains (Kennedy 2008), and its diagnosis requires the presence of five or more of the symptoms listed in Table 1(APA 2000). Although its causes are unknown, the current conceptualization of depression posits that it occurs as a combination of certain biological vulnerability factors and exposure to life-stressors such as trauma or loss (Krishnan et al. 2008). One out of six persons will experience at least one episode of depression in their life (Kessler et al. 2005), and in many individuals depression becomes a chronic, disabling illness (Murray et al. 1997). In addition to significant disability, depression is associated with excess mortality (Zheng et al. 1997; Cuijpers et al. 2002), mostly because of co-morbidity with cardiovascular disease, diabetes and obesity. As the pathophysiology of depression has not been fully elucidated, treatments are based on empirical data, not mechanisms of action. Current antidepressants, all of which target monoamines (serotonin, norepinephrine, or dopamine), ameliorate symptoms in about half of patients (Trivedi et al. 2006) and produce remission in only a third (Rush et al. 2006). It remains unclear how these drugs actually work, since their ability to increase synaptic concentrations of monoamines is immediate, while their clinical effects take 2–4 weeks to become apparent (Taylor et al. 2006). Although it is plausible that subtle differences in neurotransmitter profiles could explain differences in efficacy (Millan 2006), differences in efficacy, which are very small and detectable only in large clinical samples, do not correspond to any known combinations of receptor and reuptake effects (Cipriani et al. 2009). This highlights our lack of understanding of the pathophysiology of depression.
Table 1.
Areas of impairment in depression and corresponding diagnostic criteria
| Area of impairment | DSM-IV diagnostic criterion |
|---|---|
| Mood | Depressed mood |
| Motivation | Markedly diminished interest or pleasure |
| Food | Weight loss or gain or decrease or increase in appetite |
| Sleep | Insomnia or hypersomnia |
| Motor | Psychomotor retardation or agitation |
| Energy | Fatigue or loss of energy |
| Self-esteem | Feelings of worthlessness or excessive or inappropriate guilt |
| Cognition | Diminished ability to think or concentrate, or indecisiveness |
| Hope | Recurrent thoughts of death, recurrent suicidal ideation |
1.2. Depression and inflammatory cytokines
One area of research that may shed light on the pathogenesis of depression is brain-immune interactions. Immunologic abnormalities in depression have been described for over two decades (Irwin et al. 2007; Miller et al. 2009), but it is still unclear whether these abnormalities play a role in depression pathogenesis. For readers not familiar with immunology, in Table 2 we describe the main functions of cytokines discussed in this article. The most consistent finding is that patients with depression have elevated levels of inflammatory markers in plasma or serum (Hamer et al. 2009; Howren et al. 2009). Summarized in Table 3 and Table 4 are some of the studies that have found elevated levels of the inflammatory cytokines tumor necrosis factor alpha (TNF) and interleukin-6 ( IL-6) in medically-healthy patients with depression (Maes et al. 1995; Sluzewska et al. 1995; Zorrilla et al. 2001; Hestad et al. 2003; Penninx et al. 2003; Tuglu et al. 2003; Fitzgerald et al. 2006; Bremmer et al. 2007; O'Brien et al. 2007; Sutcigil et al. 2007; Yang et al. 2007; Himmerich et al. 2008; Dinan et al. 2009; Dome et al. 2009). Depression in the context of various “medical”illnesses is also associated with elevated blood levels of inflammatory markers (Elenkov 2007; Irwin et al. 2007; Miller et al. 2008). Although inflammation is believed to play a pathogenic role in many medical illnesses, any causal relationships among medical illness, inflammation and depression remain undetermined. In summary, it has been clearly established that both idiopathic depression in medically healthy individuals and depression that occurs in the context of various medical illnesses, is associated with elevated blood levels of inflammatory mediators.
Table 2.
Cytokine functions
| Cytokine | Main functions |
|---|---|
| Interleukin-1β (IL-1) | Fever HPA axis activation Lymphocyte activation Macrophage and neutrophil activation Prostanoid synthesis Endothelial activation IL-6 synthesis |
| Interleukin-4 (IL-4) | Inhibits production of TNF and IL-1 Stimulates B cells |
| Interleukin-6 (IL-6) | Fever Acute phase protein synthesis T and B cell differentiation and activation |
| Interleukin-8 (IL-8) | Inflammation Neutrophil chemotaxis |
| Interleukin-10 (IL-10) | Inhibits inflammation Inhibits production of IL-1, IL-6, TNF and IFNγ |
| Tumor necrosis factor (TNF) |
Fever Endothelial activation Neutrophil activation Migration of dendritic cells to lymph nodes |
| Interferon-α (IFN-α) | Induction of viral resistance Natural killer cell activation Macrophage activation |
| Interferon-γ (IFN-γ) | Macrophage activation T cell differentiation |
Table 3.
Plasma levels of TNF (pg/ml) in depression
| Study | Subjects | Controls | Currently depressed |
Remitted depressed |
|---|---|---|---|---|
| Dinan et al. (2009) | 24 control 20 depressed 14 remitted |
12.52±2.11 | 20.01±2.52 | 20.96±4.08 |
| Fitzgerald et al. (2006) | 19 control 19 depressed |
12.10±2.56 | 22.02±3.62 | |
| Hestad et al. (2003) | 15 control 23 depressed |
10 | 20 | |
| Himmerich et al. (2008) | 523 control 62 depressed 35 remitted |
12.63±6.71 | 19.83±9.7 | 13.87±14.5 |
| O’Brien et al. (2007) | 24 control 28 depressed 16 remitted |
10.88±1.31 | 19.64±2.44 | 10.77±2.18 |
| Sutcigil et al. (2007) | 25 control 26 depressed |
36.04±12.63 | 77.68±16.21 | |
| Tuglu et al. (2003) | 17 control 30 depressed |
5.19±1.18 | 9.08±3.23 | |
| Yang et al. (2007) | 23 control 33 depressed |
9 | 13 |
Table 4.
Plasma levels of IL-6 (pg/ml) in depression
| Study | Subjects | Controls | Currently depressed |
Remitted depressed |
|---|---|---|---|---|
| Dinan et al. (2009) | 24 control 20 depressed 14 remitted |
1.86±.31 | 3.97±.45 | 5.10±.84 |
| Fitzgerald et al. (2006) | 19 control 19 depressed |
.73±.11 | 1.18±.12 | |
| Maes et al. (1995) | 38 control 77 depressed |
2.5 ± .55 | 5.52 ± 1 | |
| O’Brien et al. (2007) | 24 control 28 depressed 16 remitted |
.74±.08 | 1.25±.19 | .67±.07 |
| Sluzewska et al. (1996) |
15 control 49 depressed |
1.24 ± .8 | 4.28 ± 1.7 | |
| Yang et al. (2007) | 23 control 33 depressed |
5 | 8 |
1.3. Antidepressant and anti-inflammatory agents
Although some studies found elevated inflammatory cytokine levels in remitted depressed patients (O'Brien et al. 2007; Himmerich et al. 2008), other studies found that inflammatory cytokine levels decreased with various antidepressant treatments (Leonard 2001; Kenis et al. 2002; Hestad et al. 2003; Tuglu et al. 2003; Myint et al. 2005), suggesting that antidepressants may reduce inflammation. This is consistent with anti-inflammatory effects of antidepressants described in rodents (Yirmiya et al. 2001). Conversely, anti-inflammatory drugs can have an antidepressant effect. In depressed patients who had not remitted with the antidepressant reboxetine, augmentation with the cyclooxygenase-2 (COX-2) inhibitor celecoxib, which blocks the conversion of arachidonic acid into inflammatory molecules called prostanoids, was superior to placebo (Muller et al. 2006). In patients with psoriasis, the TNF-antagonists infliximab and etanercept reduced depressive symptoms; the latter did so independently of any improvement in joint pain or other psoriatic symptoms (Feldman et al. 2005; Tyring et al. 2006; Bassukas et al. 2008). Such data support the notion that inflammation may in some way contribute to the genesis of depressive symptoms.
1.4. Can inflammatory cytokines “cause” depressive symptoms?
It is well-established that inflammatory cytokines released peripherally have profound effects on mood and behavior. Naturally-occurring infectious illnesses produce symptoms that resemble depression (Bucks et al. 2008). Experimental studies show that activation of the immune system can cause depressive-like symptoms. In experimental exposure of humans to rhinovirus or influenza virus, blood levels of IL-1, TNF and IL-6 predicted reduced positive affect the following day, independent of objective signs of illness (Janicki-Deverts et al. 2007), and various mild immune stimuli, insufficient to cause significant sickness symptoms, can elicit depressive symptoms in humans (Hermann et al. 1998; Reichenberg et al. 2001; Wright et al. 2005; Capuron et al. 2009; Eisenberger et al. 2009). How does immune activation affect the brain?
2. Immune-to-brain and brain-to-immune pathways
2.1. Immune-depression models in rodents
The literature on immune-induced depressive-like behaviors in rodents is vast. Although a comprehensive review of rodent data is not within the scope of this article, we will briefly outline some studies to illustrate how peripheral cytokines “talk” to the brain. For readers interested in this topic, we recommend other reviews (De La Garza 2005; Dunn et al. 2005; Anisman 2009; Pecchi et al. 2009). The most commonly used immune-depression model in rodents is intraperitoneal (i.p.) administration of endotoxin, a component of the wall of gram-negative bacteria. Activation of the innate immune system with endotoxin leads to a constellation of behaviors similar to depression in humans: anhedonia, decreases in novelty-induced and social behaviors, reduced food intake, and sleep disturbance (Larson et al. 2001). The production of so-called sickness behavior in rodents occurs when peripherally-released cytokines induce expression of inflammatory mediators in brain parenchyma (Pecchi et al. 2009). This has also been demonstrated in primates: Cerebrospinal fluid (CSF) levels of IL-6 increase after i.v. administration of IL-1 in monkeys (Reyes et al. 1996) and after IFN-α treatment in humans (Raison et al. 2008). It is therefore reasonable to assume that immune-to-brain pathways are similar in rodents and humans. Depending on the dose of endotoxin used, depressive-like behaviors in rodents may continue after the acute sickness behavior ends (Frenois et al. 2007). Such delayed effects from immune stimuli may occur because increased brain and peripheral levels of cytokines can last for several weeks (Qin et al. 2007; Moreau et al. 2008).
2.2. Immune-to-brain pathways
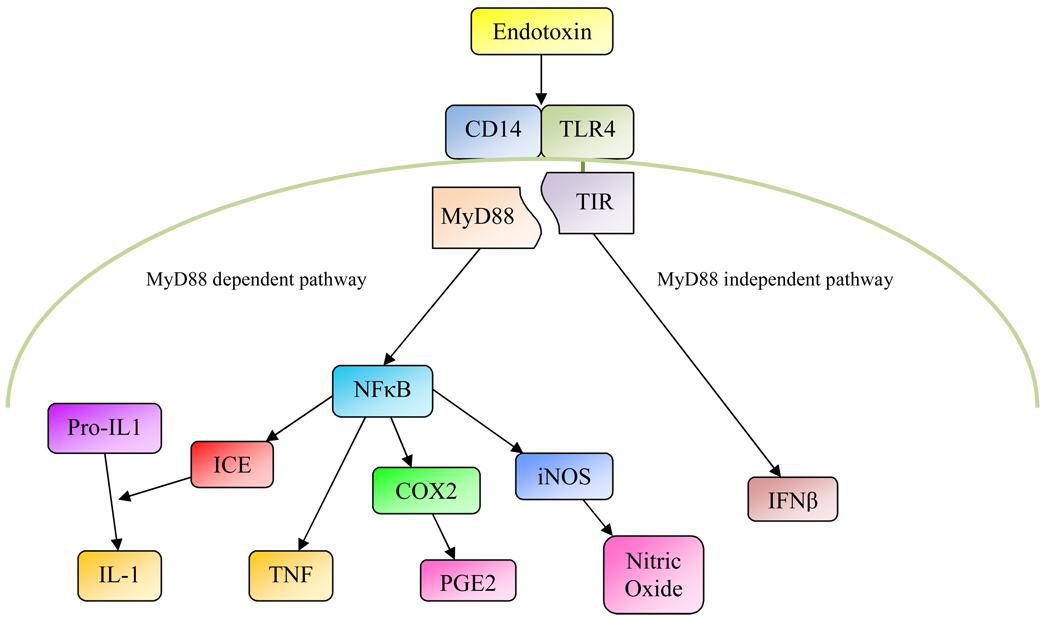
What are the pathways through which endotoxin, when administered peripherally, can cause depressive-like behaviors? Innate immune cells have receptors that recognize endotoxin and other so-called pathogen-associated molecular patterns. As illustrated in Figure 1., endotoxin binds to CD14 which enables interaction with Toll-like receptor 4 (TLR4). This ultimately leads to the activation of the transcription factor NFκB and expression of various genes, including COX-2, TNF, IL-6, IL-1 converting enzyme (ICE) which cleaves pro-IL-1 into bioactive IL-1, and inducible nitric oxide (iNOS) which produces nitric oxide (Gaestel et al. 2009). The NFκB pathway is also activated when IL-1 or TNF bind to their respective receptors. In addition to leukocytes, the NFκB pathway is functional in endothelial cells where it can be activated by endotoxin binding to TLR4/CD14 as well as through TNF and IL-1 binding to their receptors (Dauphinee et al. 2006; Magder et al. 2006). The endothelium and the blood-brain barrier play an essential role in mediating inflammatory signals from both the bloodstream and cerebral ventricles to brain parenchyma: The myeloid differentiation factor 88 (MyD88) is required in endothelial cells to mediate the effects of peripherally administered IL-1 on the brain (Gosselin et al. 2008), and when IL-1 is administered intracerebroventricularly (i.c.v.), the presence of IL-1 receptors on endothelial cells is necessary for activation of hypothalamic neurons and the occurrence of sickness behavior (Ching et al. 2007). In addition to the endothelium and blood-brain barrier, the brain can also detect peripheral inflammatory signals through afferent vagal fibers (Kapas et al. 1998; Opp et al. 1998). Thus, when IL-1 was given i.v., activation of hypothalamic neurons and the occurrence of depressive-like symptoms required the presence of IL-1 receptors in endothelial cells, whereas when IL-1 was injected i.p., endothelial IL-1 receptors were not required (Konsman et al. 2000). Recently, an additional pathway of immuneto- brain communication has been demonstrated, namely the entry of peripheral monocytes into brain parenchyma (D'Mello et al. 2009). In mice with hepatic inflammation, monocytes from the blood enter brain parenchyma, and this process was dependent on TNF-induced expression of monocyte chemoattractant protein-1 in microglia (D'Mello et al. 2009). In summary, peripheral immune stimuli can affect the brain through various pathways, including signaling across the blood-brain barrier, through vagal afferents, and by direct entry of peripheral leukocytes into brain parenchyma.
Figure 1.
Endotoxin binds to CD14 and Toll-like receptor 4 (TLR4) on the surface of monocytes and endothelial cells. The TLR4 associated Toll-IL-1-receptor domain (TIR) binds to myeloid differentiation factor 88 (MyD88), which, through various intermediate steps, leads to the activation of nuclear factor κB (NFκB). This transcription factor initiates the transcription of various inflammatory mediators, including tumor necrosis factor (TNF), interleukin-1 converting enzyme (ICE) which converts pro-IL-1 into bioactive IL-1, cyclooxygenase-2 (COX-2)which converts arachidonic acid into prostanoids such as prostaglandin E2 (PGE2), and inducible nitric oxide synthase (iNOS) which produces nitric oxide. The MyD88-dependent pathway can also be activated by IL-1 and TNF signaling through their cognate receptors. The MyD88-independent pathway leads to the transcription of interferon-β (IFN-β).
2.3. Brain-to-immune pathways
Not only can the immune system affect the brain, the brain also exerts some control over the immune system. One of the most important anti-inflammatory mechanisms is the hypothalamic-pituitary-adrenal (HPA) axis. Activation of the HPA axis by peripherally-released inflammatory cytokines leads to secretion of cortisol, which has potent anti-inflammatory effects (Bierhaus et al. 2003). In addition, the vagus also exerts anti-inflammatory effects through the release of acetylcholine which acts directly on nicotinic receptors on immune cells (Pavlov et al. 2005). Although incompletely understood, the brain also has pro-inflammatory effects: In mice, adenoviral over-expression of IL-1 in the brain led to increased peripheral production of inflammatory cytokines (Campbell et al. 2007), and injection of endotoxin into cerebral ventricles caused increased IL-6 production in the periphery (Huang et al. 2007). When endotoxin or IL-1 is administered directly into the brain, leukocyte adhesion to postcapillary venules depends on the presence of CD14/TLR4 and IL-1 receptors in endothelial cells (Zhou et al. 2006; Ching et al. 2007), demonstrating that the endothelium of the blood-brain barrier is involved in both immune-to-brain and brain-to-immune communication. In rats injected peripherally with endotoxin, prior lidocaine inactivation of neurons in the anterior hypothalamic area, which mediates many of the central effects of inflammation, resulted in reduced peripheral levels of TNF (Yilmaz et al. 2008), demonstrating that the brain is involved in regulating peripheral production of inflammatory cytokines even when the inflammatory stimulus is delivered peripherally. Intriguingly, sickness behavior produced by administration of IL-1 into the anterior hypothalamus can be blocked by peripheral neutralization of TNF (Jiang et al. 2008), suggesting that peripherally-released cytokines play a role in sickness behavior even when the inflammatory stimulus is delivered directly into brain parenchyma. In summary, the brain exerts some inhibition on the innate immune system through anti-inflammatory regulation by the HPA axis and vagus, however, the presence of inflammatory mediators in brain parenchyma can have pro-inflammatory effects peripherally.
2.4. Immune-brain-immune pathways and depression
In rodents psychogenic stressors can increase expression of inflammatory mediators in brain parenchyma (Anisman et al. 2008), and in humans it has been shown that psychological stressors can increase peripheral inflammation (Bierhaus et al. 2003; Dickerson et al. 2004; Gundersen et al. 2006; Pace et al. 2006). The latter is consistent with the pro-inflammatory brain-to-immune pathways discussed above. It is therefore possible that, rather than peripheral cytokines causing depressive symptoms, the state of depression, a potent psychological stressor, could be the reason why blood levels of TNF and IL-6 are elevated in depression. Given the existence of such complex pathways of communication between the immune system and the brain, it is difficult to establish any causal link between depression and increased blood levels of inflammatory cytokines. Some studies suggest the presence of a neuroinflammatory process in depression, such as increased microglial density (Steiner et al. 2008), and elevated CSF levels of IL-1 and IL-6 in depression and suicide attempters (Levine et al. 1999; Lindqvist et al. 2009). However, in the brains of suicide victims there was an increase in expression of the anti-inflammatory cytokines IL-4 and IL-13 (Tonelli et al. 2008), and in depression TNF levels in CSF were normal (Levine et al. 1999). The absence of a correlation between blood and CSF levels of IL-6 and TNF (Lindqvist et al. 2009), further illustrates the complexity of immune-brain pathways. In summary, in contrast to the robust data on peripheral immune abnormalities in depression, the data on brain immune abnormalities are inconclusive. This may be due to a paucity of post-mortem studies of neuroinflammation in depression. Although the link between depression and immune system abnormalities is complex, it has been clearly established that peripherally-released cytokines have an effect on emotions, cognition and behavior. Based on rodent studies, behavioral effects of peripherally-released inflammatory cytokines involve the immune-to-brain pathways described above and depend on local expression of inflammatory mediators in the brain (Pecchi et al. 2009). It is plausible that depressive symptoms, whether they occur in the context of idiopathic depression or as a consequence of an inflammatory stimulus, may involve the overlapping neural pathways. Because of this, the study of depressive symptoms induced by immune stimuli may deepen our understanding of this serious disorder. To do this we need validated human models of immune depression.
3. Human models of immune depression
3.1. The interferon-alpha model
In patients with hepatitis C treated with interferon-alpha (IFN-α) up to 45% develop depression (Asnis et al. 2006). The symptoms that occur after IFN-α treatment resemble idiopathic depression (Capuron et al. 2009) and respond to treatment with antidepressants (Musselman et al. 2001; Kraus et al. 2007), lending both face and predictive validity to this model. Of note, IFN-α-induced depressive symptoms are associated with increases in blood levels of IL-6 and TNF akin to levels found in depression (Taylor et al. 1998; Wichers et al. 2007), suggesting that the depressogenic effect of IFN-α is mediated by inflammatory cytokines. Studies using the IFN-α model have greatly expanded our knowledge of the role of inflammation in depression, and this model of chronic, low-grade inflammation may mimic the effects of peripheral inflammation on the brain that occur in idiopathic depression. One limitation of this model is that, because the depressogenic effects of IFN-α do not usually occur until 8–12 weeks of treatment (Asnis et al. 2006), it cannot be used as an acute laboratory paradigm of immune depression. Furthermore, the IFN-α model is not double-blind and cannot be applied to healthy volunteers. Therefore it would greatly benefit the field of depression research to develop complimentary models of immune depression.
3.2. The typhoid vaccination model
Administration of the typhoid vaccine is a mild immune stimulus that acutely induces subtle changes in mood (Strike et al. 2004), and slight increases in IL-6 blood levels (see Table 5), without any effect on TNF (Wright et al. 2005). This model also induces fatigue and impairs concentration (Harrison et al. 2009).
Table 5.
Endotoxin-induced TNF and IL-6 plasma levels
| Study | Dose of endotoxin (ng/kg) |
Subjects | Baseline TNF level (pg/ml) |
Peak TNF level (pg/ml) |
Baseline IL-6 level (pg/ml) |
Peak IL-6 level (pg/ml) |
|---|---|---|---|---|---|---|
| Michie et al. (1988) | 4 | 13 Male |
35 | 240 ± 70 | ||
| Suffredini et al. (1999) |
4 | 4 Male |
5 ± 5 | 2,899 ± 970 | 2.4 ± 0.5 | 8.3 ± 0.8 |
| Moller et al. (2002) |
2 | 7 Male 1 Female |
1.7 | 928 | .71 | 1,901 |
| Suffredini et al. (1999) |
2 | 4 Male |
5 ± 5 | 908 ± 126 | 2.4 ± 0.5 | 7.1 ± 0.2 |
| van der Poll et al. (1997) | 2 | 12 Male & Female |
219 ± 42 | |||
| Van Eijk et al. (2007) | 2 | 30 Male & Female |
Females: 965 ± 193 Males: 411 ± 35 |
|||
| Suffredini et al. (1999) |
1 | 4 Male |
5 ± 5 | 122 ± 49 | 2.4 ± 0.5 | 5.4 ± 0.38 |
| Eisenberger et al., 2009 | .8 | ~<5 ± 25* | ~150 ± 25* | |||
| Hannestad unpublished |
.8 | 6 Male |
2 ± 1.2 | 51.3 ± 45.3 | 0.58 ± 0.35 | 243.3 ± 303.1 |
| Reichenberg et al. (2001) | .8 | 20 Male |
~105* | ~100* | ||
| Vedder et al. (2007) | .4 | 24 Male & Female |
~90* | ~80* | ||
| Taudorf et al. (2007) |
.3 | 10 Male |
~1* | ~7* | ~0* | ~20* |
| Hojman et al. (2009) |
.1 | 26 Male |
≈1* | ≈3* | ≈1* | ≈11* |
= figures based on graphs
3.3. The endotoxin model
As described in Section 2.2., endotoxin is a component of the outer membrane of Gram-negative bacteria that, when administered to rodents or humans, leads to activation of the innate immune system. Endotoxin administration in human subjects is a generally-safe experimental procedure that has been used for several decades to study the systemic response to innate immune system activation (Lowry 2005). In humans, endotoxin doses of 2–4 ng/kg body weight cause flu-like symptoms (fever, chills, myalgia, headache, nausea) and increases in blood TNF and IL-6 levels similar to what is seen in sepsis (Michie et al. 1988; van der Poll et al. 1997; van Eijk et al. 2007), while lower doses (0.4–0.8 ng/kg) cause mild depressive symptoms and less pronounced increases in TNF and IL-6 levels (Reichenberg et al. 2001; Vedder et al. 2007; Eisenberger et al. 2009). The inflammatory cytokine blood levels achieved at different doses of endotoxin are summarized in Table 5. Although there is a clear dose-response relationship between the potency of the endotoxic stimulus and blood levels of TNF and IL-6, there are large individual differences in the immune response, rendering the use of endotoxin to experimentally induce depressive symptoms challenging. The purpose of this paper is to review whether endotoxin administration can be used as a model of acute immune depression. We will review the effects of the IFN-α and typhoid vaccination models in parallel as a side-by-side comparison of the three models. Rather than approach depression as a categorical construct, we will review the effect of these immune stimuli on each of the symptoms that constitute the syndrome called depression. We will also include select rodent studies that highlight the potential pathways through which peripherally-released cytokines produce the rodent-equivalent of each of the symptoms discussed.
4. Depressed mood
Depressed mood is one of two core symptoms of depression (Kennedy 2008), and one study showed that acute infection is associated with reduced “contentment” (Bucks et al. 2008). Two studies concluded that low-dose endotoxin administration (0.8 ng/kg) induced depressed mood (Reichenberg et al. 2001; Eisenberger et al. 2009). In the first study (n=20, cross-over), the Depression Adjective Check List (DACL) (Christenfeld et al. 1978) showed a statistically significant difference in depressed mood at 3 hours after endotoxin administration compared to the placebo condition, and DACL scores correlated with TNF plasma levels (Reichenberg et al. 2001). The DACL has been shown to correlate well with the Beck Depression Inventory (BDI) and other more commonly used depression rating scales (Christenfeld et al. 1978).
In the second study (n=39, parallel-group) depression was measured with the Profile of Mood States (POMS), and there was a statistically significant difference in the POMS Depression-Dejection subscale (POMS-D) score between the endotoxin and placebo groups, but no correlation between POMS-D scores and IL-6 plasma levels (Eisenberger et al. 2009). POMS assesses six identified mood factors: Tension-Anxiety, Depression-Dejection, Anger-Hostility, Vigor-Activity, Fatigue-Inertia, and Confusion-Bewilderment, however, only Anger-Hostility, Vigor-Activity, and Fatigue-Inertia have considerable factorial integrity (Norcross et al. 1984). Therefore it is not clear that the POMS-D is a reliable measure of depression or depressed mood. Moreover, since the maximum score on the POMS-D subscale is twenty, the clinical relevance of a mean score of 0.4 is debatable. Consistent with (Eisenberger et al. 2009), in eight healthy subjects who received low-dose endotoxin (0.8 ng/kg) in a placebo-controlled, cross-over study, the mean POMS-D score after endotoxin was 0.3 compared to 0 after placebo (Hannestad unpublished). Moreover, the “Reported Sadness” item of the Montgomery-Ǻsberg Depression Rating Scale (MADRS) did not change in any subject, nor did Beck Depression Inventory items 1 (feeling sad) or 2 (discouraged about the future) (Hannestad unpublished). It has therefore not been clearly demonstrated that endotoxin administration can cause depressed mood. In the IFN-α model “Depressed Mood” on the Hamilton Depression Rating Scale increased in both IFN-α-related depression and idiopathic depression (Capuron et al. 2009), while the typhoid vaccine model has only been evaluated using the POMS (Strike et al. 2004). Using the typhoid vaccination model and an implicit emotional face perception task during functional magnetic resonance imaging, it was demonstrated that inflammation-associated mood deterioration correlated with enhanced activity in the subgenual anterior cingulate cortex (Harrison et al.2009). In summary, more research is needed to assess whether endotoxin administration in humans can induce depressed mood. As rodent studies of depressed mood cannot be performed, we have no data indicating the immune-to-brain pathways that mediate the effects of peripheral inflammation on mood.
5. Anhedonia
5.1. Immune-induced anhedonia in humans
Anhedonia, the lack of ability to experience pleasure or reward, is the other core symptom of depression (Kennedy 2008). Although anecdotally one could assume that interest would be reduced during acute infections, there are no published studies that have empirically tested this idea. There are also no published studies assessing the effect of endotoxin on anhedonia in humans. In our cohort we found no change in the “Inability to feel” item on the MADRS, however, we did find that when subjects were asked on a visual-analog scale whether they “Want to be alone” or “Want to be with other people”, endotoxin administration reduced the desire for social interactions (Hannestad unpublished). This is consistent with rodent studies (see below). Although not an official DSM-IV diagnostic criterion, one very common symptom in depression is reduced libido (Williams et al. 2006). Reduced libido can be construed as a type of anhedonia, however, there are no data on the effect of endotoxin on libido in humans. Compared to the endotoxin model, in patients who received IFN-α treatment, the Hamilton Depression Rating Scale item “Work/Actvities” was similar in IFN-α and idiopathic depression (Capuron et al. 2009) indicating that the IFN-α model does induce anhedonia. There are no published data on the effect of typhoid vaccination on anhedonia. In summary, there is insufficient data to assess whether endotoxin administration in humans is a useful model of anhedonia.
5.2. Immune-induced anhedonia in rodents: Sucrose preference
The literature on endotoxin-induced anhedonia in rodents is extensive (Anisman et al. 2005; De La Garza 2005). One rodent model of anhedonia is sucrose preference, i.e. increased preference for palatable foods such as sugared water. Sucrose preference is inhibited by endotoxin, IL-1 and TNF (Brebner et al. 2000; De La Garza et al. 2005; Weil et al. 2006), while the data regarding the effects of IFN-α are inconclusive (Sammut et al. 2001; De La Garza et al. 2005; Loftis et al. 2006; Fahey et al. 2007). The inconsistent findings with IFN-α in rodents may be because earlier studies used human recombinant IFN-α which does not induce signal transduction in rodent receptors (Loftis et al. 2006). On the other hand, IFN-α, when overexpressed in the central nervous system, did reduce sucrose preference (Kwant et al. 2004). Pre-treatment with the antidepressants desipramine and fluoxetine blunts IL-1- and IFN-α-induced reductions in sucrose preference (Sammut et al. 2002; Merali et al. 2003), lending predictive validity to this rodent model of anhedonia. Endotoxin-induced decreases in sucrose preference are blocked by non-steroidal anti-inflammatory agents, whereas endotoxin-induced expression of IL-1 and IL-6 in the hypothalamus is not (De La Garza et al. 2005). This indicates that, while prostanoids are involved in mediating the effect of endotoxin on sucrose preference, hypothalamic expression of IL-1 and IL-6 is not. Although sucrose causes release of dopamine in the nucleus accumbens (de Araujo et al. 2008), the region mediating reward (Pizzagalli et al. 2009), it is likely that sucrose preference is a complex behavior that involves not only brain regions that mediate reward, but also brain regions that control appetite and energy homeostasis such as the hypothalamus and brain stem (Ahima et al. 2008).
5.3. Immune-induced anhedonia in rodents: Intracranial self-stimulation
Intracranial self-stimulation (ICSS), a reinforcing electrical stimulation of the forebrain bundle at the level of the posterior lateral hypothalamus, does not involve appetite regulation and is therefore considered a “purer” rodent model of anhedonia (Liebman 1983). Endotoxin reduces ICSS and this is associated with increased dopamine efflux and reduced expression of the exocytotic protein syntaxin in the nucleus accumbens (Borowski et al. 1998; Barr et al. 2003). This indicates that peripheral activation of the innate immune system inhibits reward or pleasure. Studies have failed to show an effect of IL-1 and IL-6 on ICSS anhedonia, although both cause sickness behavior (Anisman et al. 1998) and IL-1 reduces sucrose preference (Brebner et al. 2000), suggesting that 1) the effects of endotoxin on ICSS may involve other cytokines, and 2) that ICSS-anhedonia and sucrose preference are mediated by different brain regions. Of note, stimulation of the ventral tegmental area, which supplies dopamine to the nucleus accumbens, counteracted endotoxin-induced sickness behavior (Kentner et al. 2008), further supporting the notion that endotoxin causes anhedonia.
5.4. Immune-induced anhedonia in rodents: Exploratory and social behaviors
In rodents, both endotoxin and IL-1 decrease exploration of novel environments and engagement in social behaviors (Larson et al. 2001), lending face validity to the notion that immune stimuli cause anhedonia in rodents. Mice pre-treated with endotoxin had lower neuronal activity in brain regions involved in reward when presented with a new environment (Stone et al. 2006). With regards to sexual behaviors, endotoxin and IL-1 disrupt sexual behaviors only in female rodents with no discernable effects in males (Yirmiya et al. 1995; Larson et al. 2001). In summary, endotoxin administration in rodents produces anhedonia as assessed by sucrose preference, ICSS, and exploratory and social behaviors. The specific cytokines involved have yet to be determined. In humans, there are not enough data to determine whether endotoxin administration is good model of anhedonia. Although this symptom is difficult to measure in a laboratory setting, further studies should be conducted needed.
6. Food intake
6.1. Immune-induced suppression of food intake in humans
Lack of appetite, reduced food intake, and weight loss occur frequently in depression, although some patients experience increased appetite (APA 2000). Similarly, during acute infectious illness appetite is reduced (Langhans 2007). In humans, low-dose endotoxin reduced food intake in the first four hours without causing nausea (Reichenberg et al. 2001), and reductions in food intake correlated with plasma levels of TNF and IL-6 (Reichenberg et al. 2002). Treatment with IFN-α also reduces appetite as assessed by the Hamilton Depression Rating Scale, however, this was seen both in patients who developed depressive symptoms and those who did not (Capuron et al. 2009), indicating that IFN-α may reduce appetite independent of other depressive symptoms. There are no published data on the effect of typhoid vaccination on food intake.
6.2. Immune-induced suppression of food intake in rodents
The arcuate nucleus, lateral hypothalamus, dorsal vagal complex, and raphe nuclei are the main regions regulating food intake (Dhillo 2007; Ahima et al. 2008). In rodents, endotoxin and IL-1 induce anorexia (Larson et al. 2001) which involves signaling through the endothelium and the MyD88 pathway (Wisse et al. 2007; Gosselin et al. 2008). Although both endotoxin and IL-1 cause COX-2 expression and prostanoid synthesis in brain endothelium (Dunn et al. 2006; Gosselin et al. 2008), prostanoid synthesis is only required for IL-1-induced anorexia (Elander et al. 2007; Pecchi et al. 2009), indicating that other mediators are involved with endotoxin. When administered i.c.v., IL-1 only produces anorexia if co-administered with IL-6 (Harden et al. 2008), suggesting that IL-6 signaling in the brain may be required to produce anorexia. Both serotonin and orexin have been implicated in the anorexic effects of endotoxin (Asarian et al. 2007; Langhans 2007). Although the role of TNF in mediating immune-induced anorexia has been controversial (Larson et al. 2001), TNF deficiency attenuated anorexia induced by staphylococcal enterotoxin A, which in a similar manner to endotoxin induces release of inflammatory cytokines (Rossi-George et al. 2005), indicating that TNF can also produce anorexia. This is consistent with anorexia produced by i.c.v. administration of TNF in pigs (Warren et al. 1997). One potential pathway through which TNF could reduce food intake is by modulating glutamate release in vagal afferents to the solitary tract nucleus and disrupting autonomic control of the gut (Hermann et al. 2007). Supporting the proposed effect of TNF on glutamate receptors are data showing that endotoxin-induced anorexia is inhibited by metabotropic glutamate receptor antagonism (Weiland et al. 2006). In summary, endotoxin administration suppresses food intake in both humans and rodents and may be an adequate experimental paradigm to mimic the loss of appetite and reduced food intake that occurs in idiopathic depression. As we have described above, one caveat is that reduced food intake in depression may involve both brain regions that mediate reward and brain regions involved in food intake and energy regulation.
7. Sleep
7.1. Immune-induced sleep disturbances in humans
Sleep is almost ubiquitously disturbed in depression. Three out of four patients with depression suffer from insomnia, and among young depressed patients almost half have hypersomnia (Nutt et al. 2008). Sleep is also altered during infections (Imeri et al. 2009). Sleep is divided into rapid eye movement (REM) and non-REM sleep. Non-REM is further classified into stages 1 through 4 according to electroencephalographic characteristics, with stages 3 and 4 defined as slow-wave sleep. Upon falling asleep, non-REM sleep is initiated by gamma aminobutyric acid neurons in the ventrolateral preoptic nucleus of the hypothalamus, which inhibit monoaminergic arousal systems located in the locus caeruleus and raphe nuclei. The switch to REM sleep, which occurs after non-REM sleep has initiated, is mediated by cholinergic neurons in the tegmentum of the pons. The sleep abnormalities found in idiopathic depression include disturbances in sleep continuity, reduced slow wave sleep, shortened REM latency, and increased REM density (Riemann 2007). In humans endotoxin can disrupt sleep continuity at higher doses (Mullington et al. 2000), however slow wave sleep is not affected by endotoxin (Trachsel et al. 1994), REM latency is increased rather than shortened (Korth et al. 1996; Hermann et al. 1998), and REM is suppressed (Trachsel et al. 1994). In other words, the effects of endotoxin on sleep are in many regards opposite of what is seen in idiopathic depression (Riemann 2007). Consistent with this, the direct administration of IL-6 in humans causes decreased REM sleep (Spath-Schwalbe et al. 1998), again opposite of what is seen in idiopathic depression. In patients receiving IFN-α treatment, depression was not associated with significant changes in subjective sleep (Capuron et al. 2009), however there are no published studies of EEG recordings in IFN-α-treated patients. There are no data on the effect of typhoid vaccination on sleep. Interestingly, administration of the TNF antagonist etanercept in abstinent alcoholics decreased the amount of REM sleep (Irwin et al. 2009). As etanercept does not cross the blood-brain barrier (Griffin 2008), this suggests that reducing peripheral TNF activity can inhibit REM sleep. This is consistent with rodent studies showing that blocking IL-1 and TNF also interferes with sleep in the absence of infection or immune system activation, pointing to a physiologic role of these cytokines in sleep regulation (Imeri et al. 2009). In summary, the effects of endotoxin on sleep do not resemble sleep disturbances found in idiopathic depression.
7.2. Immune-induced sleep disturbances in rodents
The effects of endotoxin on sleep are mediated by cytokines (Imeri et al. 2006). Consistent with human studies discussed above, in rodents administration of endotoxin, IL-1, TNF, or IFN-γ suppresses REM sleep and enhances the duration of slow-wave sleep (Krueger et al. 1986; Shoham et al. 1987; Krueger et al. 1995). Inhibition of ICE abolished enhancement of non-REM sleep by endotoxin (Imeri et al. 2006), and in IL-6 deficient mice endotoxin-induced increases in non-REM sleep were blunted (Morrow et al. 2005), demonstrating that both IL-1 and IL-6 are involved in mediating the effects of endotoxin on sleep. TNF receptor antagonism did not abolish the effects of endotoxin on sleep (Lancel et al. 1997), whereas TNF administered i.c.v. in pigs led to hypersomnia (Warren et al. 1997). The effects of peripherally-administered endotoxin and IL-1 on sleep are mediated by the vagus (Kapas et al. 1998; Opp et al. 1998), and may involve the hypothalamic suppression of genes that regulate circadian rhythm and activity level (Cavadini et al. 2007). Interestingly, chronic infusion of endotoxin into the lateral hypothalamus of rats for 30 days caused increases in wakefulness after six days which returned to baseline after endotoxin administration was discontinued (Gerashchenko et al. 2004). This highlights that the effects of endotoxin on sleep depend on whether administration is acute or chronic. In summary, immune system activation has profound effects on sleep architecture, causing increased non-REM sleep, interruption of REM sleep and increased REM latency, however, these effects on sleep are in many respects the opposite of those seen in depression. Therefore immune system activation with endotoxin is not an adequate model for sleep disturbances seen in depression. Chronic activation of the immune system may be a better model (Gerashchenko et al. 2004), and the effects of IFN-α on sleep would therefore merit further study.
8. Fatigue and psychomotor slowing
8.1. Immune-induced fatigue in humans
Fatigue is a prominent symptom in depression (Kennedy 2008), one that often does not improve with current treatments (Papakostas et al. 2006). Fatigue occurs commonly during infections and in medical conditions associated with inflammation (Meyers et al. 2005; Huffman et al. 2006; Bower 2007; Davis et al. 2008). Fatigue can also be elicited in humans by administration of endotoxin (Bahador et al. 2007). In our subjects, MADRS Item 7 (Lassitude) changed in most subjects, as did the Vigor-Activity and Fatigue-Inertia clusters of the POMS. In cancer, fatigue has been associated with blood levels of IL-1 (Greenberg et al. 1993), IL-6, and TNF (Meyers et al. 2005), although there have been several negative studies as reviewed in (Miller et al. 2008). A quantitative review of cancer studies found a correlation between fatigue and blood levels of IL-6, but not TNF or IL-1 (Schubert et al. 2007). Fatigue and psychomotor slowing occur frequently during IFN-α treatment (Asnis et al. 2006; Capuron et al. 2009) which leads to increased IL-6 levels both peripherally and centrally (Loftis et al. 2007; Raison et al. 2008). In humans, fatigue can be elicited directly by administration of IL-6 (Spath-Schwalbe et al. 1998), Taken together, these data suggest that IL-6 mediates the effects of illness, endotoxin, and IFN-α on fatigue. Recent imaging studies of immune-induced fatigue implicate the nigrostriatal pathway, a dopamine pathway that facilitates movement and drives motivational behavior (Cools 2008). In IFN-α-induced fatigue there was increased activity in the left nucleus accumbens and putamen (Capuron et al. 2007), and in subjects who received a typhoid vaccine, psychomotor slowing correlated with activity in the substantia nigra and IL-6 levels (Brydon et al. 2008). Although most data indicate a role for IL-6, in patients receiving docetaxel, a chemotherapeutic agent for which fatigue is the dose-limiting side-effect, blocking TNF with etanercept decreased docetaxel-induced fatigue (Monk et al. 2006). Fatigue is also common in autoinflammatory disorders which are characterized by excessive IL-1 activity (Dinarello 2009).
8.2. Immune-induced fatigue in rodents
Fatigue in humans is the feeling that it takes an effort, physical and mental, to engage in activities. This is difficult to model in rodents, however, a reasonable proxy is to measure how long it takes a rodent to tire from physical activity. In rodents endotoxin-induced reductions in wheel-running are blunted by the administration of antibodies against IL-6 (Harden et al. 2006), consistent with the human data on IL-6 discussed above. When administered i.c.v., IL-1, IL-6, and TNF all reduced wheel-running (Netea et al. 2007; Harden et al. 2008), and TNF deficiency or antagonism increases running time (Netea et al. 2007), suggesting that in the brain multiple inflammatory cytokines can induce fatigue. In summary, endotoxin-administration reliably induces fatigue in humans and is therefore a good experimental model of this symptom. Antagonists of IL-6, IL-1, or TNF may prove useful therapies for fatigue in depression and other conditions, and novel compounds could be tested using endotoxin-induced fatigue paradigms.
9. Cognition
9.1. Immune-induced cognitive impairment in humans
Cognitive impairment, including difficulty with memory and concentration, is common in depression (Simons et al. 2009). During acute respiratory tract infection, speed of accessing stored memories is reduced (Bucks et al. 2008). It is well-known that sepsis can cause delirium (Pisani et al. 2007), and in patients with myelogenous leukemia or myelodysplastic syndrome blood levels of IL-6 were associated with poorer executive function (Meyers et al. 2005). In humans low-dose endotoxin administration impaired both immediate and delayed recall, but did not affect attention or executive functions (Reichenberg et al. 2001), while administration of IL-6 impaired concentration (Spath-Schwalbe et al. 1998). Interestingly, during IFN-α treatment no cognitive impairment has been demonstrated (Fontana et al. 2007), while typhoid vaccination does impair concentration (Harrison et al. 2009). It thus appears that endotoxin may be a useful paradigm to experimentally induce mild cognitive deficits.
9.2. Immune-induced cognitive impairment in rodents
In rodents, endotoxin disrupts acquisition of learning, but does not impair performance if task has already been learned (Aubert et al. 1995). Mice deficient in IL-6 are refractory to endotoxin-induced working memory impairment. In these mice increases in plasma IL-1 and TNF levels were the same as in wild-type mice, but IL-1 and TNF levels in hippocampus were attenuated by IL-6 deficiency (Sparkman et al. 2006), indicating that IL-6 is involved in immune-to-brain signaling during endotoxin-induced hippocampal dysfunction. Endotoxin-induced memory deficits were associated with loss of neurons in hippocampus and prefrontal cortex (Semmler et al. 2007), and in rats with endotoxin-induced delirium, gene expression of inflammatory mediators was most pronounced in the hippocampus (Wolff et al. 2009). These data are consistent with the memory functions of this brain region. Endotoxin administered i.c.v. for 28 days impaired spatial memory and caused increases in IL-1 and TNF expression in the basal forebrain and hippocampus (Hauss-Wegrzyniak et al. 1998), indicating that the effect of endotoxin on memory may involve TNF and IL-1 produced locally in the hippocampus, consistent with the ability of endotoxin to induce expression of IL-1 and IL-6 in hippocampal slices (Hellstrom et al. 2005). In neonatal rats, the effect of endotoxin on memory was attenuated by ICE blockade (Bilbo et al. 2005), indicating that IL-1 mediates this. The inhibitory effects of endotoxin on neurogenesis was blunted by indomethacin (Monje et al. 2003), and the COX-2 inhibitor rofecoxib reduces the effects of endotoxin on cognition (Jain et al. 2002), demonstrating a role for prostanoids. For readers interested in further information about the physiologic role of cytokines in cognition we recommend a recent review (McAfoose et al. 2009). In summary, in rodents peripheral inflammatory stimuli have profound effects on cognitive function, and in humans the effect of endotoxin administration on cognition may be an adequate paradigm of depression-like cognitive deficits, while this may not be the case for the IFN-α model. Because neuropsychological measures of various domains of cognition are more “objective” than measures of subjective psychiatric symptoms, endotoxin-induced mild cognitive deficits may be a useful experimental paradigm to test the effect of new medications on cognition.
10. Self-worth, hopelessness, and other symptoms of depression
In our experience with endotoxin administration, subjects do not experience feelings of worthlessness, hopelessness, guilt, or suicidal idation. In the recent study by Eisenberger et al. there was a change in the POMS-D cluster which does include questions about worthlessness and hopelessness, however, as discussed above, the change on this cluster was very small and possibly not clinically significant. The absence of an effect of endotoxin on these symptoms is consistent with the recent study on IFN-α-induced depression, in which the score on the HDRS items on guilt and suicide were not different between IFN-α treated depressed and non-depressed patients, although those items were different in idiopathic depression (Capuron et al. 2009). Therefore, endotoxin administration does not seem an adequate paradigm to mimic depressive symptoms that have to do with negative views of self, past, present and future. Since such symptoms are obviously impossible to model in rodents we do not know whether immune system activation can induce similar mental states in rodents. Symptoms of anxiety are frequently co-morbid with depression, although not considered a diagnostic criterion according to DSM-IV. In human subjects one study found that endotoxin administration induced anxiety as measured by the State Anxiety Inventory (Reichenberg et al. 2001). In rodents, administration of endotoxin or inflammatory cytokines have been shown to decrease open field activity (Larson et al. 2001) and induce other anxiety-like behaviors (Lacosta et al. 1999), while other studies found inconclusive effects of endotoxin on anxiety-like behaviors (Swiergiel et al. 2007).
11. Summary and Conclusions
Endotoxin-administration in human subjects is a relatively safe procedure that induces specific dose-dependent symptoms: At higher doses (> 1 ng/kg), flu-like symptoms such as fever, chills, myalgias, headache, and nausea predominate, while at lower doses (< 1 ng/kg), symptoms such as loss of fatigue, reduced appetite, and cognitive impairment occur (see Table 6 for a comparison with the IFN-α model). These symptoms are similar to symptoms seen in idiopathic depression. The effects of endotoxin on sleep do not resemble sleep disturbances seen in depression, and endotoxin does not produce other depressive symptoms such as worthlessness, hopelessness, and suicidal ideation. Whether endotoxin administration produces depressed mood or anhedonia, the two core symptoms of depression, needs to be explored further. A validated model of acute immune-depression would be extremely helpful as it could be used for proof-of-concept studies that could lead to the discovery of new antidepressant targets (Gelenberg et al. 2008), to screen orally-available medications that target kinases of the intracellular pathways involved in cytokine signal transduction (Gaestel et al. 2009), and for molecular and functional imaging studies aimed at identifying the brain regions involved in producing each specific symptom induced by innate immune system activation.
Table 6.
Immune-induced depressive symptoms
| Symptom | Endotoxin | Interferon-α | Typhoid vaccine |
|---|---|---|---|
| Depressed mood | +/− | ++ | + |
| Markedly diminished interest or pleasure |
+/− | + | ? |
| Weight loss or decrease in appetite | + | +* | ? |
| Insomnia | − | − | ? |
| Psychomotor retardation | + | ++ | + |
| Fatigue or loss of energy | +++ | ++ | + |
| Feelings of worthlessness or excessive or inappropriate guilt |
− | − | ? |
| Diminished ability to think or concentrate, or indecisiveness |
++ | − | + |
| Recurrent thoughts of death, recurrent suicidal ideation |
− | − | ? |
Independent of the presence of other depressive symptoms
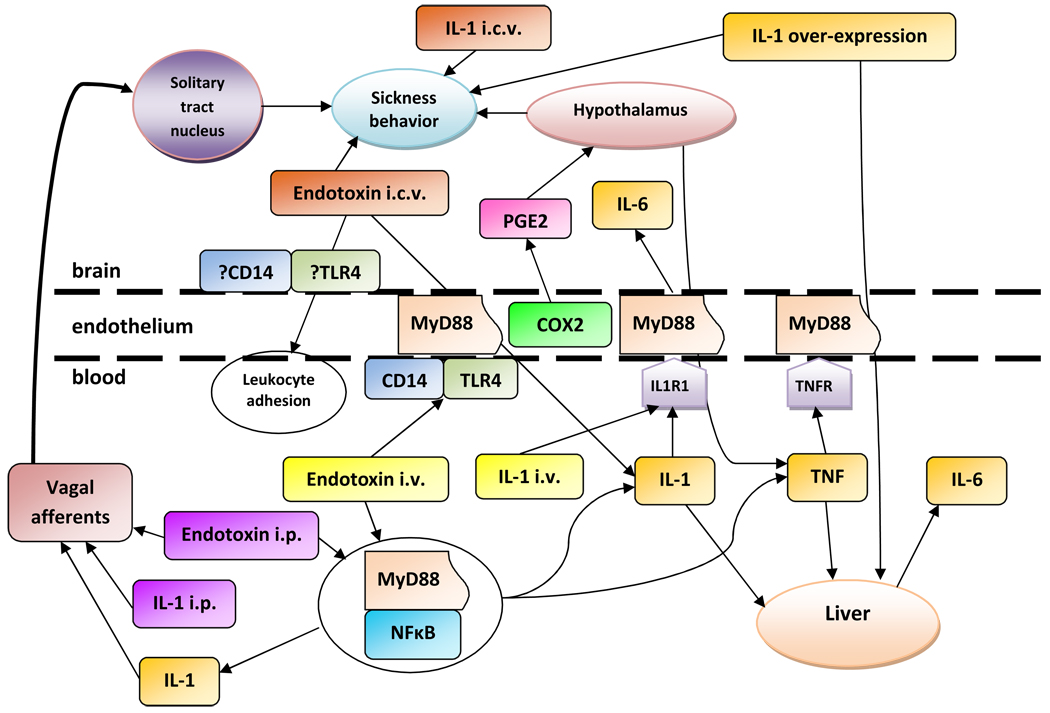
Figure 2.
Pathways through which inflammatory stimuli produce sickness behavior and depressive-like symptoms. Endotoxin administered intraperitoneally (i.p.) or intravenously (i.v.) is detected by leukocytes, causing the release of inflammatory cytokines, including IL-1 and TNF. When endotoxin is administered i.p., inflammatory cytokines released in the abdominal cavity signal to the brain via afferent fibers of the vagus nerve. When endotoxin is administered i.v., inflammatory cytokines released in the blood signal to the brain through receptors on endothelial cells. Endotoxin can also signal directly through endothelial cells when administered i.v. In the brain, peripheral inflammatory signals lead to local production of inflammatory mediators and sickness behavior. Administration of inflammatory signals to the brain, including endotoxin and IL-1, also leads to peripheral release of inflammatory mediators, highlighting the complexity of brain-immune interactions.
Acknowledgments
We would like to thank George Heninger for thoughtful discussions on this topic, the nursing staff of the Yale Clinical Neuroscience Research Unit for their expertise and care during our endotoxin experiments, the research pharmacist Kevin Pohl for preparing endotoxin for our experiments, and Anthony Suffredini at the National Institutes of Health Clinical Center for providing the standard reference endotoxin. This publication was made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Disclosure/Conflict of Interest: Neither N.D. nor J.H. has received any income or financial support from any entity, or has any personal financial holdings, that could be perceived as a potential conflict of interest.
References
- Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008;37:811–823. doi: 10.1016/j.ecl.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Kokkinidis L, Borowski T, Merali Z. Differential effects of interleukin (IL)-1beta, IL-2 and IL-6 on responding for rewarding lateral hypothalamic stimulation. Brain Res. 1998;779:177–187. doi: 10.1016/s0006-8993(97)01114-1. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Publisher; 2000. [Google Scholar]
- Asarian L, Kopf BS, Geary N, Langhans W. Pharmacological, but not genetic, disruptions in 5-HT(2C) receptor function attenuate LPS anorexia in mice. Pharmacol Biochem Behav. 2007;86:493–498. doi: 10.1016/j.pbb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Asnis GM, De La Garza R., 2nd Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40:322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- Aubert A, Vega C, Dantzer R, Goodall G. Pyrogens specifically disrupt the acquisition of a task involving cognitive processing in the rat. Brain Behav Immun. 1995;9:129–148. doi: 10.1006/brbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- Bahador M, Cross AS. From therapy to experimental model: a hundred years of endotoxin administration to human subjects. J Endotoxin Res. 2007;13:251–279. doi: 10.1177/0968051907085986. [DOI] [PubMed] [Google Scholar]
- Barr AM, Song C, Sawada K, Young CE, Honer WG, Phillips AG. Tolerance to the anhedonic effects of lipopolysaccharide is associated with changes in syntaxin immunoreactivity in the nucleus accumbens. Int J Neuropsychopharmacol. 2003;6:23–34. doi: 10.1017/S146114570200319X. [DOI] [PubMed] [Google Scholar]
- Bassukas ID, Hyphantis T, Gamvroulia C, Gaitanis G, Mavreas V. Infliximab for patients with plaque psoriasis and severe psychiatric comorbidity. J Eur Acad Dermatol Venereol. 2008;22:257–258. doi: 10.1111/j.1468-3083.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowski T, Kokkinidis L, Merali Z, Anisman H. Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport. 1998;9:3797–3802. doi: 10.1097/00001756-199812010-00006. [DOI] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun. 21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Hayley S, Zacharko R, Merali Z, Anisman H. Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology. 2000;22:566–580. doi: 10.1016/S0893-133X(99)00166-9. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, Hack CE, et al. Inflammatory markers in late-life depression: Results from a population-based study. J Affect Disord. 2007 doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucks RS, Gidron Y, Harris P, Teeling J, Wesnes KA, Perry VH. Selective effects of upper respiratory tract infection on cognition, mood and emotion processing: a prospective study. Brain Behav Immun. 2008;22:399–407. doi: 10.1016/j.bbi.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Deacon RM, Jiang Y, Ferrari C, Pitossi FJ, Anthony DC. Overexpression of IL-1beta by adenoviral-mediated gene transfer in the rat brain causes a prolonged hepatic chemokine response, axonal injury and the suppression of spontaneous behaviour. Neurobiol Dis. 2007 doi: 10.1016/j.nbd.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009 doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, et al. Basal Ganglia Hypermetabolism and Symptoms of Fatigue during Interferon-alpha Therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, et al. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci U S A. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, et al. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27:10476–10486. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenfeld R, Lubin B, Satin M. Concurrent validity of the Depression Adjective Check List in a normal population. Am J Psychiatry. 1978;135:582–584. doi: 10.1176/ajp.135.5.582. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist. 2008;14:381–395. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72:227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2000;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Younger J, Motivala SJ, Attrep J, Irwin MR. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: implications for fatigue. Brain Behav Immun. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- De La Garza R., 2nd Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Asnis GM, Fabrizio KR, Pedrosa E. Acute diclofenac treatment attenuates lipopolysaccharide-induced alterations to basic reward behavior and HPA axis activation in rats. Psychopharmacology (Berl) 2005;179:356–365. doi: 10.1007/s00213-004-2053-x. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Asnis GM, Pedrosa E, Stearns C, Migdal AL, Reinus JF, et al. Recombinant human interferon-alpha does not alter reward behavior, or neuroimmune and neuroendocrine activation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:781–792. doi: 10.1016/j.pnpbp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dhillo WS. Appetite regulation: an overview. Thyroid. 2007;17:433–445. doi: 10.1089/thy.2007.0018. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosom Med. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Dinan T, Siggins L, Scully P, O'Brien S, Ross P, Stanton C. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. J Psychiatr Res. 2009;43:471–476. doi: 10.1016/j.jpsychires.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Dome P, Teleki Z, Rihmer Z, Peter L, Dobos J, Kenessey I, et al. Circulating endothelial progenitor cells and depression: a possible novel link between heart and soul. Mol Psychiatry. 2009;14:523–531. doi: 10.1038/sj.mp.4002138. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Zhang H, Quan N. Reduced ingestion of sweetened milk induced by interleukin-1 and lipopolysaccharide is associated with induction of cyclooxygenase-2 in brain endothelia. Neuroimmunomodulation. 2006;13:96–104. doi: 10.1159/000096291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elander L, Engstrom L, Hallbeck M, Blomqvist A. IL-1beta and LPS induce anorexia by distinct mechanisms differentially dependent on microsomal prostaglandin E synthase-1. Am J Physiol Regul Integr Comp Physiol. 2007;292:R258–R267. doi: 10.1152/ajpregu.00511.2006. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ. Neurohormonal-cytokine interactions: Implications for inflammation, common human diseases and well-being. Neurochem Int. 2007 doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Fahey B, Hickey B, Kelleher D, O'Dwyer AM, O'Mara SM. The widely-used anti-viral drug interferon-alpha induces depressive- and anxiogenic-like effects in healthy rats. Behav Brain Res. 2007;182:80–87. doi: 10.1016/j.bbr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Feldman SR, Gordon KB, Bala M, Evans R, Li S, Dooley LT, et al. Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: a double-blind placebo-controlled trial. Br J Dermatol. 2005;152:954–960. doi: 10.1111/j.1365-2133.2005.06510.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P, O'Brien SM, Scully P, Rijkers K, Scott LV, Dinan TG. Cutaneous glucocorticoid receptor sensitivity and pro-inflammatory cytokine levels in antidepressant-resistant depression. Psychol Med. 2006;36:37–43. doi: 10.1017/S003329170500632X. [DOI] [PubMed] [Google Scholar]
- Fontana RJ, Bieliauskas LA, Lindsay KL, Back-Madruga C, Wright EC, Snow KK, et al. Cognitive function does not worsen during pegylated interferon and ribavirin retreatment of chronic hepatitis C. Hepatology. 2007;45:1154–1163. doi: 10.1002/hep.21633. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov. 2009;8:480–499. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Thase ME, Meyer RE, Goodwin FK, Katz MM, Kraemer HC, et al. The history and current state of antidepressant clinical trial design: a call to action for proof-of-concept studies. J Clin Psychiatry. 2008;69:1513–1528. doi: 10.4088/jcp.v69n1001. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Shiromani PJ. Effects of inflammation produced by chronic lipopolysaccharide administration on the survival of hypocretin neurons and sleep. Brain Res. 2004;1019:162–169. doi: 10.1016/j.brainres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatry. 2008;13:480–497. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- Greenberg DB, Gray JL, Mannix CM, Eisenthal S, Carey M. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J Pain Symptom Manage. 1993;8:196–200. doi: 10.1016/0885-3924(93)90127-h. [DOI] [PubMed] [Google Scholar]
- Griffin WS. Perispinal etanercept: Potential as an Alzheimer therapeutic. J Neuroinflammation. 2008;5:3. doi: 10.1186/1742-2094-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen Y, Opstad PK, Reistad T, Thrane I, Vaagenes P. Seven days' around the clock exhaustive physical exertion combined with energy depletion and sleep deprivation primes circulating leukocytes. Eur J Appl Physiol. 2006;97:151–157. doi: 10.1007/s00421-006-0150-8. [DOI] [PubMed] [Google Scholar]
- Hamer M, Molloy GJ, de Oliveira C, Demakakos P. Persistent depressive symptomatology and inflammation: To what extent do health behaviours and weight control mediate this relationship? Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin-6 and leptin mediate lipopolysaccharide-induced fever and sickness behavior. Physiol Behav. 2006;89:146–155. doi: 10.1016/j.physbeh.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin (IL)-6 and IL-1 beta act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav Immun. 2008;22:838–849. doi: 10.1016/j.bbi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation Causes Mood Changes Through Alterations in Subgenual Cingulate Activity and Mesolimbic Connectivity. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, et al. Neural Origins of Human Sickness in Interoceptive Responses to Inflammation. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer's disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hellstrom IC, Danik M, Luheshi GN, Williams S. Chronic LPS exposure produces changes in intrinsic membrane properties and a sustained IL-beta-dependent increase in GABAergic inhibition in hippocampal CA1 pyramidal neurons. Hippocampus. 2005;15:656–664. doi: 10.1002/hipo.20086. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Mullington J, Hinze-Selch D, Schreiber W, Galanos C, Pollmacher T. Endotoxin-induced changes in sleep and sleepiness during the day. Psychoneuroendocrinology. 1998;23:427–437. doi: 10.1016/s0306-4530(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. TNF{alpha}:A Trigger of Autonomic Dysfunction. Neuroscientist. 2007 doi: 10.1177/1073858407305725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestad KA, Tonseth S, Stoen CD, Ueland T, Aukrust P. Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. J Ect. 2003;19:183–188. doi: 10.1097/00124509-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, et al. Depression, comorbidities and the TNF-alpha system. Eur Psychiatry. 2008 doi: 10.1016/j.eurpsy.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, Smith FA, Quinn DK, Fricchione GL. Post-MI psychiatric syndromes: six unanswered questions. Harv Rev Psychiatry. 2006;14:305–318. doi: 10.1080/10673220601070013. [DOI] [PubMed] [Google Scholar]
- Imeri L, Bianchi S, Opp MR. Inhibition of caspase-1 in rat brain reduces spontaneous nonrapid eye movement sleep and nonrapid eye movement sleep enhancement induced by lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2006;291:R197–R204. doi: 10.1152/ajpregu.00828.2005. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007 doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor Necrosis Factor Antagonism Normalizes Rapid Eye Movement Sleep in Alcohol Dependence. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain NK, Patil CS, Kulkarni SK, Singh A. Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav Brain Res. 2002;133:369–376. doi: 10.1016/s0166-4328(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Doyle WJ, Turner RB, Treanor JJ. Infection-induced proinflammatory cytokines are associated with decreases in positive affect, but not increases in negative affect. Brain Behav Immun. 2007;21:301–307. doi: 10.1016/j.bbi.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Deacon R, Anthony DC, Campbell SJ. Inhibition of peripheral TNF can block the malaise associated with CNS inflammatory diseases. Neurobiol Dis. 2008 doi: 10.1016/j.nbd.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Kapas L, Hansen MK, Chang HY, Krueger JM. Vagotomy attenuates but does not prevent the somnogenic and febrile effects of lipopolysaccharide in rats. Am J Physiol. 1998;274:R406–R411. doi: 10.1152/ajpregu.1998.274.2.R406. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kennedy SH. Core symptoms of major depressive disorder: relevance to diagnosis and treatment. Dialogues Clin Neurosci. 2008;10:271–277. doi: 10.31887/DCNS.2008.10.3/shkennedy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner AC, Takeuchi A, James JS, Miki T, Seino S, Hayley S, et al. The effects of rewarding ventral tegmental area stimulation and environmental enrichment on lipopolysaccharide-induced sickness behavior and cytokine expression in female rats. Brain Res. 2008;1217:50–61. doi: 10.1016/j.brainres.2008.04.041. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, Bluthe RM, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci. 2000;12:4434–4446. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- Korth C, Mullington J, Schreiber W, Pollmacher T. Influence of endotoxin on daytime sleep in humans. Infect Immun. 1996;64:1110–1115. doi: 10.1128/iai.64.4.1110-1115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MR, Schafer A, Schottker K, Keicher C, Weissbrich B, Hofbauer I, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: A randomized, double-blind, placebo-controlled study. Gut. 2007 doi: 10.1136/gut.2007.131607. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Kubillus S, Shoham S, Davenne D. Enhancement of slow-wave sleep by endotoxin and lipid A. Am J Physiol. 1986;251:R591–R597. doi: 10.1152/ajpregu.1986.251.3.R591. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Majde JA. Cytokines and sleep. Int Arch Allergy Immunol. 1995;106:97–100. doi: 10.1159/000236827. [DOI] [PubMed] [Google Scholar]
- Kwant A, Sakic B. Behavioral effects of infection with interferon-gamma adenovector. Behav Brain Res. 2004;151:73–82. doi: 10.1016/j.bbr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 1999;818:291–303. doi: 10.1016/s0006-8993(98)01288-8. [DOI] [PubMed] [Google Scholar]
- Lancel M, Mathias S, Schiffelholz T, Behl C, Holsboer F. Soluble tumor necrosis factor receptor (p75) does not attenuate the sleep changes induced by lipopolysaccharide in the rat during the dark period. Brain Res. 1997;770:184–191. doi: 10.1016/s0006-8993(97)00783-x. [DOI] [PubMed] [Google Scholar]
- Langhans W. Signals generating anorexia during acute illness. Proc Nutr Soc. 2007;66:321–330. doi: 10.1017/S0029665107005587. [DOI] [PubMed] [Google Scholar]
- Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain Behav Immun. 2001;15:371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The immune system, depression and the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:767–780. doi: 10.1016/s0278-5846(01)00155-5. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Liebman JM. Discriminating between reward and performance: a critical review of intracranial self-stimulation methodology. Neurosci Biobehav Rev. 1983;7:45–72. doi: 10.1016/0149-7634(83)90007-6. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 Is Elevated in the Cerebrospinal Fluid of Suicide Attempters and Related to Symptom Severity. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Hauser P, Macey TA, Lowe JD. Can rodents be used to model interferonalpha-induced depressive symptoms? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1364–1365. doi: 10.1016/j.pnpbp.2006.04.004. author reply 1366. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. Neurosci Lett. 2007 doi: 10.1016/j.neulet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Wall JM, Pagel RL, Hauser P. Administration of pegylated interferonalpha-2a or -2b does not induce sickness behavior in Lewis rats. Psychoneuroendocrinology. 2006;31:1289–1294. doi: 10.1016/j.psyneuen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Lowry SF. Human endotoxemia: a model for mechanistic insight and therapeutic targeting. Shock. 2005;24 Suppl 1:94–100. doi: 10.1097/01.shk.0000191340.23907.a1. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- Magder S, Neculcea J, Neculcea V, Sladek R. Lipopolysaccharide and TNF-alpha produce very similar changes in gene expression in human endothelial cells. J Vasc Res. 2006;43:447–461. doi: 10.1159/000095162. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Merali Z, Brennan K, Brau P, Anisman H. Dissociating anorexia and anhedonia elicited by interleukin-1beta: antidepressant and gender effects on responding for "free chow" and "earned" sucrose intake. Psychopharmacology (Berl) 2003;165:413–418. doi: 10.1007/s00213-002-1273-1. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- Michie HR, Manogue KR, Spriggs DR, Revhaug A, O'Dwyer S, Dinarello CA, et al. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Multi-target strategies for the improved treatment of depressive states: Conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther. 2006;110:135–370. doi: 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24:1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- Moreau M, Andre C, O'Connor JC, Dumich SA, Woods JA, Kelley KW, et al. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain Behav Immun. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Mullington J, Korth C, Hermann DM, Orth A, Galanos C, Holsboer F, et al. Dose-dependent effects of endotoxin on human sleep. Am J Physiol Regul Integr Comp Physiol. 2000;278:R947–R955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Myint AM, Leonard BE, Steinbusch HW, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J Affect Disord. 2005;88:167–173. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Netea MG, Kullberg BJ, Vonk AG, Verschueren I, Joosten LA, van der Meer JW. Increased voluntary exercise in mice deficient for tumour necrosis factor-alpha and lymphotoxin-alpha. Eur J Clin Invest. 2007;37:737–741. doi: 10.1111/j.1365-2362.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Norcross JC, Guadagnoli E, Prochaska JO. Factor structure of the Profile of Mood States (POMS): two partial replications. J Clin Psychol. 1984;40:1270–1277. doi: 10.1002/1097-4679(198409)40:5<1270::aid-jclp2270400526>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10:329–336. doi: 10.31887/DCNS.2008.10.3/dnutt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. 2007;41:326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Opp MR, Toth LA. Somnogenic and pyrogenic effects of interleukin-1beta and lipopolysaccharide in intact and vagotomized rats. Life Sci. 1998;62:923–936. doi: 10.1016/s0024-3205(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M. Resolution of sleepiness and fatigue in major depressive disorder: A comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60:1350–1355. doi: 10.1016/j.biopsych.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pecchi E, Dallaporta M, Jean A, Thirion S, Troadec JD. Prostaglandins and sickness behavior: Old story, new insights. Physiol Behav. 2009 doi: 10.1016/j.physbeh.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167:1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals With Major Depressive Disorder. Am J Psychiatry. 2009 doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of Central Nervous System Inflammatory Pathways by Interferon-Alpha: Relationship to Monoamines and Depression. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Kraus T, Haack M, Schuld A, Pollmacher T, Yirmiya R. Endotoxin-induced changes in food consumption in healthy volunteers are associated with TNF-alpha and IL-6 secretion. Psychoneuroendocrinology. 2002;27:945–956. doi: 10.1016/s0306-4530(01)00101-9. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Coe CL. Interleukin-1 beta differentially affects interleukin-6 and soluble interleukin-6 receptor in the blood and central nervous system of the monkey. J Neuroimmunol. 1996;66:135–141. doi: 10.1016/0165-5728(96)00038-0. [DOI] [PubMed] [Google Scholar]
- Riemann D. Insomnia and comorbid psychiatric disorders. Sleep Med. 2007;8 Suppl 4:S15–S20. doi: 10.1016/S1389-9457(08)70004-2. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, Urbach D, Colas D, Goldfarb Y, Kusnecov AW. Neuronal, endocrine, and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: dependence on tumor necrosis factor-alpha. J Neurosci. 2005;25:5314–5322. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sammut S, Bethus I, Goodall G, Muscat R. Antidepressant reversal of interferon-alpha-induced anhedonia. Physiol Behav. 2002;75:765–772. doi: 10.1016/s0031-9384(02)00677-7. [DOI] [PubMed] [Google Scholar]
- Sammut S, Goodall G, Muscat R. Acute interferon-alpha administration modulates sucrose consumption in the rat. Psychoneuroendocrinology. 2001;26:261–272. doi: 10.1016/s0306-4530(00)00051-2. [DOI] [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, et al. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204:733–740. doi: 10.1016/j.expneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987;253:R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- Simons CJ, Jacobs N, Derom C, Thiery E, Jolles J, van Os J, et al. Cognition as predictor of current and follow-up depressive symptoms in the general population. Acta Psychiatr Scand. 2009 doi: 10.1111/j.1600-0447.2008.01339.x. [DOI] [PubMed] [Google Scholar]
- Sluzewska A, Rybakowski JK, Laciak M, Mackiewicz A, Sobieska M, Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann N Y Acad Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D. Depressive behavior in mice due to immune stimulation is accompanied by reduced neural activity in brain regions involved in positively motivated behavior. Biol Psychiatry. 2006;60:803–811. doi: 10.1016/j.biopsych.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Strike PC, Wardle J, Steptoe A. Mild acute inflammatory stimulation induces transient negative mood. J Psychosom Res. 2004;57:189–194. doi: 10.1016/S0022-3999(03)00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, et al. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86:651–659. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Grossberg SE. The effects of interferon-alpha on the production and action of other cytokines. Semin Oncol. 1998;25:23–9. [PubMed] [Google Scholar]
- Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry. 2006;63:1217–1223. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel L, Holsboer F, Pollmacher T. Endotoxin enhances EEG alpha and beta power in human sleep. Sleep. 1994;17:132–139. doi: 10.1093/sleep/17.2.132. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Coyle SM, Levi M, Jansen PM, Dentener M, Barbosa K, et al. Effect of recombinant dimeric tumor necrosis factor receptor on inflammatory responses to intravenous endotoxin in normal humans. Blood. 1997;89:3727–3734. [PubMed] [Google Scholar]
- van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- Vedder H, Schreiber W, Schuld A, Kainz M, Lauer CJ, Krieg JC, et al. Immune-endocrine host response to endotoxin in major depression. J Psychiatr Res. 2007;41:280–289. doi: 10.1016/j.jpsychires.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Warren EJ, Finck BN, Arkins S, Kelley KW, Scamurra RW, Murtaugh MP, et al. Coincidental changes in behavior and plasma cortisol in unrestrained pigs after intracerebroventricular injection of tumor necrosis factor-alpha. Endocrinology. 1997;138:2365–2371. doi: 10.1210/endo.138.6.5180. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Bowers SL, Pyter LM, Nelson RJ. Social interactions alter proinflammatory cytokine gene expression and behavior following endotoxin administration. Brain Behav Immun. 2006;20:72–79. doi: 10.1016/j.bbi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Weiland TJ, Anthony-Harvey-Beavis D, Voudouris NJ, Kent S. Metabotropic glutamate receptors mediate lipopolysaccharide-induced fever and sickness behavior. Brain Behav Immun. 2006;20:233–245. doi: 10.1016/j.bbi.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferonalpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62:207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Williams K, Reynolds MF. Sexual dysfunction in major depression. CNS Spectr. 2006;11:19–23. doi: 10.1017/s1092852900026729. [DOI] [PubMed] [Google Scholar]
- Wisse BE, Ogimoto K, Tang J, Harris MK, Jr, Raines EW, Schwartz MW. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology. 2007;148:5230–5237. doi: 10.1210/en.2007-0394. [DOI] [PubMed] [Google Scholar]
- Wolff S, Klatt S, Wolff JC, Wilhelm J, Fink L, Kaps M, et al. Endotoxin-induced gene expression differences in the brain and effects of iNOS inhibition and norepinephrine. Intensive Care Med. 2009;35:730–739. doi: 10.1007/s00134-009-1394-7. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yang K, Xie G, Zhang Z, Wang C, Li W, Zhou W, et al. Levels of serum interleukin (IL)-6, IL-1beta, tumour necrosis factor-alpha and leptin and their correlation in depression. Aust N Z J Psychiatry. 2007;41:266–273. doi: 10.1080/00048670601057759. [DOI] [PubMed] [Google Scholar]
- Yilmaz MS, Millington WR, Feleder C. The preoptic anterior hypothalamic area mediates initiation of the hypotensive response induced by LPS in male rats. Shock. 2008;29:232–237. doi: 10.1097/shk.0b013e3180caac7e. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Avitsur R, Donchin O, Cohen E. Interleukin-1 inhibits sexual behavior in female but not in male rats. Brain Behav Immun. 1995;9:220–223. doi: 10.1006/brbi.1995.1021. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, et al. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24:531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Zheng D, Macera CA, Croft JB, Giles WH, Davis D, Scott WK. Major depression and all-cause mortality among white adults in the United States. Ann Epidemiol. 1997;7:213–218. doi: 10.1016/s1047-2797(97)00014-8. [DOI] [PubMed] [Google Scholar]
- Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177:8103–8110. doi: 10.4049/jimmunol.177.11.8103. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]