Abstract
A selenium-containing protein, selenoprotein A, is an essential component of the clostridial glycine reductase complex. This enzyme complex catalyzes the reductive deamination of glycine, which is coupled to the esterification of orthophosphate resulting in the formation of ATP. Sequence information was obtained by automated Edman degradation of peptides generated by digesting carboxamidomethylated selenoprotein A with chymotrypsin or trypsin or with endoproteinase Arg-C followed by Staphylococcus aureus V8 protease. The sequence near the selenocysteine (Sec) residue is -Cys-Phe-Val-Sec-Thr-Ala-Ala-Gly-Ala-Met-Asp-Leu-Glu-Asn-Glu-Lys-. Selenium-containing peptides isolated from digests of carboxamidomethylated selenoprotein A with trypsin or endoproteinase Arg-C were found to be blocked at the amino terminus. The sequence of the selenocysteine-containing peptide from selenoprotein A shows no homology with those of two other selenoproteins, glutathione peroxidase and formate dehydrogenase.
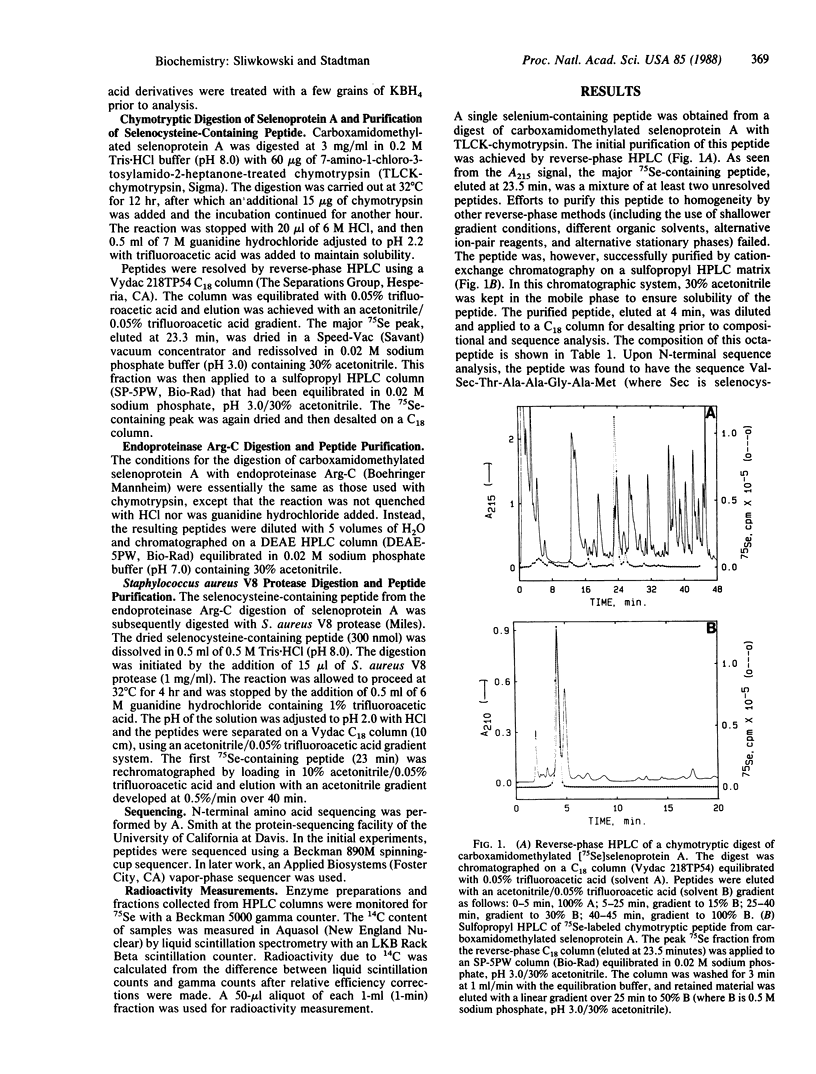
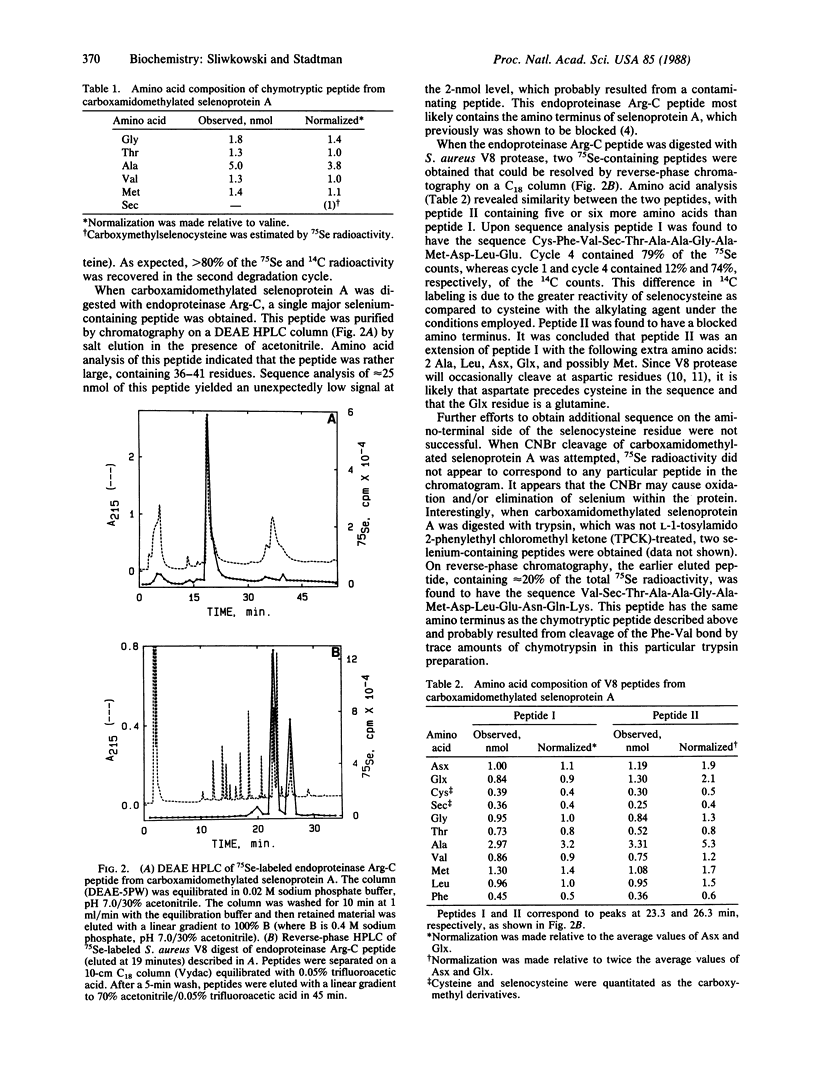
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M., Smith E. L. Action of staphylococcal proteinase on peptides of varying chain length and composition. Biochem Biophys Res Commun. 1976 Sep 20;72(2):411–417. doi: 10.1016/s0006-291x(76)80058-7. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condell R. A., Tappel A. L. Amino acid sequence around the active-site selenocysteine of rat liver glutathione peroxidase. Biochim Biophys Acta. 1982 Dec 20;709(2):304–309. doi: 10.1016/0167-4838(82)90472-1. [DOI] [PubMed] [Google Scholar]
- Cone J. E., Del Río R. M., Davis J. N., Stadtman T. C. Chemical characterization of the selenoprotein component of clostridial glycine reductase: identification of selenocysteine as the organoselenium moiety. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2659–2663. doi: 10.1073/pnas.73.8.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone J. E., del Río R. M., Stadtman T. C. Clostridial glycine reductase complex. Purification and characterization of the selenoprotein component. J Biol Chem. 1977 Aug 10;252(15):5337–5344. [PubMed] [Google Scholar]
- Dickerman H. W., Steers E., Jr, Redfield B. G., Weissbach H. Methionyl soluble ribonucleic acid transformylase. I. Purification and partial characterization. J Biol Chem. 1967 Apr 10;242(7):1522–1525. [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Grossmann A., Kim S. M., Otting F., Wendel A., Flohé L. The amino-acid sequence of bovine glutathione peroxidase. Hoppe Seylers Z Physiol Chem. 1984 Feb;365(2):195–212. doi: 10.1515/bchm2.1984.365.1.195. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. N., Gilligan J. P. o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J Chromatogr. 1983 Aug 26;266:471–482. doi: 10.1016/s0021-9673(01)90918-5. [DOI] [PubMed] [Google Scholar]
- Marcker K. The formation of N-formyl-methionyl-sRNA. J Mol Biol. 1965 Nov;14(1):63–70. doi: 10.1016/s0022-2836(65)80230-3. [DOI] [PubMed] [Google Scholar]
- STADTMAN T. C., ELLIOTT P., TIEMANN L. Studies on the enzymic reduction of amino acids. III. Phosphate esterification coupled with glycine reduction. J Biol Chem. 1958 Apr;231(2):961–973. [PubMed] [Google Scholar]
- Sliwkowski M. X., Stadtman T. C. Purification and immunological studies of selenoprotein A of the clostridial glycine reductase complex. J Biol Chem. 1987 Apr 5;262(10):4899–4904. [PubMed] [Google Scholar]
- Stadtman T. C. Selenium-dependent enzymes. Annu Rev Biochem. 1980;49:93–110. doi: 10.1146/annurev.bi.49.070180.000521. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Stadtman T. C. Purification of protein components of the clostridial glycine reductase system and characterization of protein A as a selenoprotein. Arch Biochem Biophys. 1973 Jan;154(1):366–381. doi: 10.1016/0003-9861(73)90069-6. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Nirenberg M. Transfer RNA as a cofactor coupling amino acid synthesis with that of protein. Proc Natl Acad Sci U S A. 1968 Sep;61(1):229–236. doi: 10.1073/pnas.61.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]


