Flap endonuclease 1 from D. amylolyticus was expressed, purified and crystallized by the sitting-drop vapour-diffusion method. X-ray diffraction data were collected to 2.00 Å resolution.
Keywords: FEN1, DNA repair, DNA replication
Abstract
Flap endonuclease 1 (FEN1) is a structure-specific nuclease that removes 5′-overhanging flaps in DNA repair and removes the RNA/DNA primer during maturation of the Okazaki fragment in lagging-strand DNA replication. FEN1 from the hyperthermophilic archaeon Desulfurococcus amylolyticus was expressed in Escherichia coli, purified and crystallized using the sitting-drop vapour-diffusion method with monoammonium dihydrogen phosphate as the precipitant at pH 8.3. X-ray diffraction data were collected to 2.00 Å resolution. The space group of the crystal was determined as the primitive hexagonal space group P321, with unit-cell parameters a = b = 103.76, c = 84.58 Å. The crystal contained one molecule in the asymmetric unit.
1. Introduction
Flap endonuclease 1 (FEN1) is an enzyme that possesses 5′-flap endonuclease and 5′–3′ exonuclease activity (Harrington & Lieber, 1994 ▶). FEN1 plays an important role in DNA replication, long-patch base-excision repair and homologous recombination (Murante et al., 1995 ▶). In DNA repair, it removes the 5′-overhanging flap to eliminate damaged-fragment excision and recombinational mismatch correction (Johnson et al., 1995 ▶; Klungland & Lindahl, 1997 ▶). In lagging-strand DNA replication, FEN1 removes the RNA/DNA primer during maturation of the Okazaki fragment (Goulian et al., 1990 ▶; Liu et al., 2004 ▶). FEN1 specifically recognizes the backbone of a 5′ single-stranded flap strand and tracks down this arm to the cleavage site, which is located at the junction where the two strands of duplex DNA adjoin the single-stranded arm (Hwang et al., 1998 ▶; Murante et al., 1994 ▶; Hosfield et al., 1998 ▶; Lieber, 1997 ▶). Because FEN1 is a key enzyme for maintaining genetic stability, mutations in FEN1 may give rise to a number of genetic diseases, including several ataxias, fragile X syndrome and cancer (Tishkoff et al., 1997 ▶; Freudenreich et al., 1998 ▶; Schweitzer & Livingston, 1998 ▶).
The FEN1 family is conserved in sequence and structure from bacteriophages to humans. In addition to the molecular structures of FEN1 proteins from humans and some archaea (Hwang et al., 1998 ▶; Hosfield et al., 1998 ▶; Chapados et al., 2004 ▶; Sakurai et al., 2005 ▶), the structures of the FEN1-family members T5 exonuclease, T4 RNase H and the exonuclease domain of Taq polymerase have also been reported (Ceska et al., 1996 ▶; Mueser et al., 1996 ▶; Devos et al., 2007 ▶; Kim et al., 1995 ▶). Desulfurococcus amylolyticus (strain Z533) is a hyperthermophilic and obligate anaerobic archaeon. This strain grows within the pH range 5.7–7.5, with an optimum pH of 6.4, and at temperatures of between 341 and 370 K, with an optimum temperature range of 363–365 K (Kil et al., 2000 ▶). The proteins from this organism are suitable for the determination of structures by X-ray crystallography owing to their high thermostability. To develop an understanding of the structural basis of the DNA recognition, binding and cleavage mechanism of FEN1, we have crystallized and performed preliminary X-ray diffraction analysis of FEN1 from D. amylolyticus (DaFEN1).
2. Methods and results
2.1. Expression and purification
The gene fragment of DaFEN1 was amplified from the genomic DNA of D. amylolyticus by PCR using the preliminary results of genomic analysis of this organism. The amplified fragment was cloned into the NdeI/NotI site of pET21a. The plasmid possessing the DaFEN1 gene was transformed into Escherichia coli Rosetta (DE3) for protein expression. The transformants were cultivated at 310 K in LB medium containing 100 µg ml−1 ampicillin. Overexpression was induced by adding 1.0 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) when the optical density at 600 nm reached 0.6 and the culture was continued overnight at 298 K. The cells were harvested by centrifugation at 5000g at 277 K for 10 min and were resuspended in 50 mM Tris–HCl pH 8.0. The cells were disrupted by sonication. After centrifugation at 40 000g at 277 K for 30 min, the supernatant was incubated at 353 K for 30 min and the supernatant after centrifugation at 40 000g at 277 K for 30 min was applied onto DEAE-Sepharose (GE Healthcare). The unbound fraction was collected and concentrated with 3.3 M ammonium sulfate. The precipitate was dissolved in 20 mM Tris–HCl pH 8.0 and dialyzed against the same buffer. As the final purification step, anion-exchange chromatography was performed with a Resource Q 6 ml (GE Healthcare) column equilibrated with 20 mM Tris–HCl pH 8.0 and the protein was eluted with a 0–1 M NaCl gradient. The purified protein was dialyzed against 10 mM Tris–HCl pH 8.0 and concentrated to 10 mg ml−1.
2.2. Crystallization
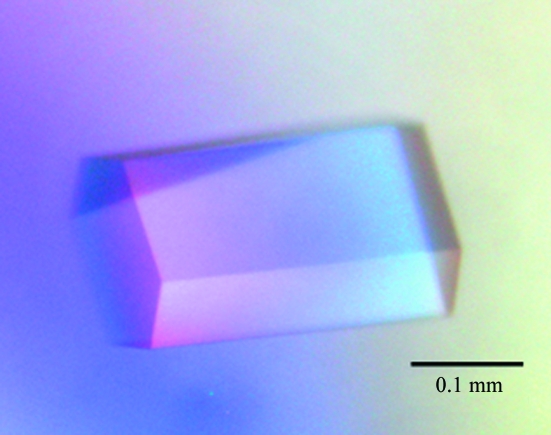
Crystallization experiments were performed at 293 K by the sitting-drop vapour-diffusion method. The drops were formed by mixing 1.5 µl protein solution with 1.5 µl reservoir solution. Initial screening was performed using Crystal Screen HT (Hampton Research). Crystals of the protein appeared in the reservoir solution containing 0.1 M Tris–HCl pH 8.5 and 2.0 M ammonium dihydrogen phosphate. After the crystallization conditions had been refined, crystals suitable for X-ray analysis were obtained with the reservoir solution: 0.1 M Tris–HCl pH 8.3 and 1.98 M ammonium dihydrogen phosphate (Fig. 1 ▶).
Figure 1.
Image of a typical DaFEN1 crystal. The approximate dimensions of the crystal are 0.22 × 0.10 × 0.10 mm.
2.3. X-ray data collection and processing
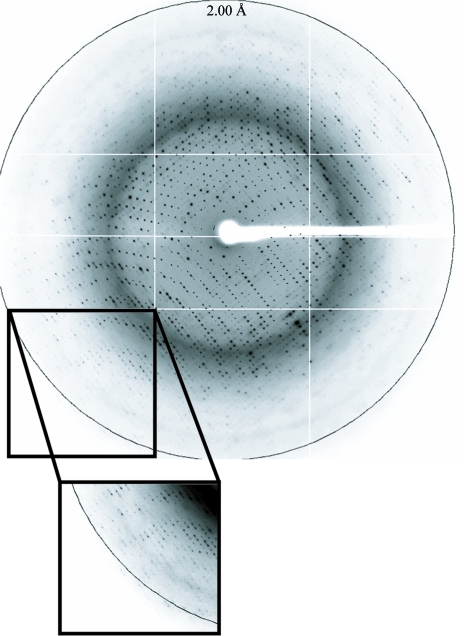
A crystal of DaFEN1 was picked up in a nylon loop (Hampton Research), transferred to a reservoir solution containing 25% ethylene glycol and flash-cooled in a nitrogen stream (95 K). X-ray diffraction data were collected on beamline BL-5A at the Photon Factory, Tsukuba, Japan. The wavelength was set to 1.00000 Å and the distance between the crystal and the detector was 284.3 mm. The crystal diffracted X-rays to 2.00 Å resolution (Fig. 2 ▶). The diffraction data were indexed, integrated and scaled using XDS (Kabsch, 1993 ▶). The crystal belonged to the primitive hexagonal space group P321, with unit-cell parameters a = b = 103.76, c = 84.58 Å. The crystal contained one molecule per asymmetric unit according to the Matthews coefficient (V M = 3.2 Å3 Da−1), which corresponds to a solvent content of 61.8% (Matthews, 1968 ▶). The data-collection statistics are shown in Table 1 ▶. Structure determination by molecular replacement using the coordinates of FEN1 from Pyrococcus horikoshii (PDB code 1mc8; Matsui et al., 2002 ▶) as a search model is under way.
Figure 2.
An X-ray diffraction image of a typical crystal. The edge of the detector corresponds to a resolution of 2.00 Å.
Table 1. Data-collection statistics for DaFEN1.
Values in parentheses are for the highest resolution shell.
| Beamline | Photon Factory BL-5A |
| Space group | P321 |
| Unit-cell parameters (Å) | a = b = 103.76, c = 84.58 |
| Wavelength (Å) | 1.00000 |
| Resolution (Å) | 50.0–2.00 (2.06–2.00) |
| No. of observations | 389978 (26362) |
| No. of unique reflections | 35852 (2607) |
| Data completeness (%) | 99.8 (100) |
| Redundancy | 10.9 (10.1) |
| Rmerge† | 0.037 (0.393) |
| 〈I〉/〈σ(I)〉 | 41.73 (6.06) |
R
merge = 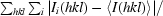
 , where I
i(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and 〈I(hkl)〉 is its average.
, where I
i(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and 〈I(hkl)〉 is its average.
Acknowledgments
The synchrotron-radiation experiment was performed on BL-5A at the Photon Factory, Tsukuba, Japan (Proposal No. 2003S2-002). This work was supported in part by the National Project on Protein Structural and Functional Analyses and the Targeted Proteins Research Program (TPRP) of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Ceska, T., Sayers, J., Stier, G. & Suck, D. (1996). Nature (London), 382, 90–93. [DOI] [PubMed]
- Chapados, B., Hosfield, D., Han, S., Qiu, J., Yelent, B., Shen, B. & Tainer, J. (2004). Cell, 116, 39–50. [DOI] [PubMed]
- Devos, J., Tomanicek, S., Jones, C., Nossal, N. & Mueser, T. (2007). J. Biol. Chem.282, 31713–31724. [DOI] [PubMed]
- Freudenreich, C., Kantrow, S. & Zakian, V. (1998). Science, 279, 853–856. [DOI] [PubMed]
- Goulian, M., Richards, S., Heard, C. & Bigsby, B. (1990). J. Biol. Chem.265, 18461–18471. [PubMed]
- Harrington, J. & Lieber, M. (1994). EMBO J.13, 1235–1246. [DOI] [PMC free article] [PubMed]
- Hosfield, D., Mol, C., Shen, B. & Tainer, J. (1998). Cell, 95, 135–146. [DOI] [PubMed]
- Hwang, K., Baek, K., Kim, H. & Cho, Y. (1998). Nature Struct. Biol.5, 707–713. [DOI] [PubMed]
- Johnson, R., Kovvali, G., Prakash, L. & Prakash, S. (1995). Science, 269, 238–240. [DOI] [PubMed]
- Kabsch, W. (1993). J. Appl. Cryst.26, 795–800.
- Kil, Y., Baitin, D., Masui, R., Bonch-Osmolovskaya, E., Kuramitsu, S. & Lanzov, V. (2000). J. Bacteriol.182, 130–134. [DOI] [PMC free article] [PubMed]
- Kim, Y., Eom, S., Wang, J., Lee, D., Suh, S. & Steitz, T. (1995). Nature (London), 376, 612–616. [DOI] [PubMed]
- Klungland, A. & Lindahl, T. (1997). EMBO J.16, 3341–3348. [DOI] [PMC free article] [PubMed]
- Lieber, M. (1997). Bioessays, 19, 233–240. [DOI] [PubMed]
- Liu, Y., Kao, H. & Bambara, R. (2004). Annu. Rev. Biochem.73, 589–615. [DOI] [PubMed]
- Matsui, E., Musti, K., Abe, J., Yamasaki, K., Matsui, I. & Harata, K. (2002). J. Biol. Chem.277, 37840–37847. [DOI] [PubMed]
- Matthews, B. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Mueser, T., Nossal, N. & Hyde, C. (1996). Cell, 85, 1101–1112. [DOI] [PubMed]
- Murante, R., Huang, L., Turchi, J. & Bambara, R. (1994). J. Biol. Chem.269, 1191–1196. [PubMed]
- Murante, R., Rust, L. & Bambara, R. (1995). J. Biol. Chem.270, 30377–30383. [DOI] [PubMed]
- Sakurai, S., Kitano, K., Yamaguchi, H., Hamada, K., Okada, K., Fukuda, K., Uchida, M., Ohtsuka, E., Morioka, H. & Hakoshima, T. (2005). EMBO J.24, 683–693. [DOI] [PMC free article] [PubMed]
- Schweitzer, J. & Livingston, D. (1998). Hum. Mol. Genet.7, 69–74. [DOI] [PubMed]
- Tishkoff, D., Filosi, N., Gaida, G. & Kolodner, R. (1997). Cell, 88, 253–263. [DOI] [PubMed]