Abstract
(Pro)renin receptor (PRR) is present in renal glomeruli, and its expression is up-regulated in diabetes. Similarly, renal inflammation is increased in the presence of hyperglycemia. The linkage between PRR and renal inflammation is not well established. We hypothesized that glucose-induced up-regulation of PRR leads to increased production of the proinflammatory factors IL-1β and cyclooxygenase-2 (COX-2). Studies were conducted in rat mesangial cells (RMCs) exposed to 30 mm d-glucose for 2 wk followed by PRR small interfering RNA knockdown, IL-1 receptor blockade with IL-1 receptor antagonist or angiotensin II type 1 receptor blockade with valsartan. The results showed that d-glucose treatment up-regulates prorenin, renin, angiotensin II, PRR, IL-1β, and COX-2 mRNA and protein expression and increases phosphorylation of ERK1/2, c-Jun N-terminal kinase, c-Jun, and nuclear factor-κB (NF-κB) p65 (serine 276,468 and 536), respectively. PRR small interfering RNA attenuated PRR, IL-1β, and COX-2 mRNA and protein expressions and significantly decreased angiotensin II production and phosphorylation of ERK1/2 and NF-κB p65 associated with high glucose exposure. Similarly, IL-1 receptor antagonist significantly reduced COX-2 mRNA and protein expression induced by high glucose. COX-2 inhibition reduced high-glucose-induced PRR expression. We conclude that glucose induces the up-regulation of PRR and its ligands prorenin and renin, leading to increased IL-1β and COX-2 production via the angiotensin II-dependent pathway. It is also possible that PRR could enhance the production of these inflammatory cytokines through direct stimulation of ERK1/2-NF-κB signaling cascade.
Hyperglycemia and COX2 stimulates (Pro)renin receptor expression and its inflammatory mediators, suggesting a positive feedback loop involving inflammation and this receptor.
In the kidney, hyperglycemia is associated with activation of the renin-angiotensin-system (RAS) (1,2,3,4,5,6,7,8), intracellular signaling cascades such as protein kinase C and MAPK pathways (9,10), and enhanced cytokine production such as IL-1, IL-6, TNFα, and TGFβI (11,12). The interaction between RAS, different intracellular signaling cascades, and cytokine productions compromise a complex network to promote inflammation and disease progress. As a newly discovered member of RAS, (pro)renin receptor (PRR) is up-regulated in the kidney of streptozocin-induced diabetes rat model, especially in glomeruli and renal tubules (8). PRR is a single-pass transmembrane protein, which binds prorenin and renin to mediate the conversion of angiotensinogen to angiotensin I on cell surface and the activation of intracellular MAPK ERK1/2 (13,14). Overexpression of human PRR in transgenic rat model elevates blood pressure and heart rate (15), whereas its blockade improves albuminuria, glomerulosclerosis (16,17,18,19), and inflammation (20). However, the link between PRR and inflammation in diabetic kidney disease is not yet established.
IL-1β is a proinflammatory cytokine that enhances the expression of genes associated with inflammation such as cyclooxygenase (COX)-2, type 2 phospholipase A, and inducible nitric oxide synthase (21). IL-1 level was reported to be increased in diabetic kidney disease (22,23,24,25,26). IL-1β treatment resulted in pronounced biochemical changes associated with cytokine production (27,28,29,30,31), inflammation (27,29,32,33,34,35,36,37,38), and accumulation of extracellular matrix in mesangial cells (28,39,40,41). Hyperglycemia enhances PRR expression (8) and inflammation (11,12). However, the relationship between PRR up-regulation and proinflammatory cytokine production is not yet defined. This study was conducted to evaluate the hypothesis that high glucose promotes the production of inflammatory factors IL-1β and COX-2 via enhanced PRR expression in rat mesangial cells (RMCs).
Materials and Methods
Cell treatment with high glucose, COX-2 inhibitor, and IL-1 receptor antagonist
RMCs were obtained from the American Type Culture Collection (Manassas, VA) and cultured according to American Type Culture Collection-recommended protocol. DMEM containing 5.5 mm d-glucose (Invitrogen, Carlsbad CA) was used in cell culture. To investigate the optimum glucose concentration that could influence PRR expression, RMCs were incubated in culture medium containing 10, 20, 30, or 50 mm l- or d-glucose for 14. The culture medium was refreshed daily. The optimum glucose concentration (30 mm as shown in Results) was used to determine the time course for PRR expression. RMCs were exposed to 30 mm l- or d-glucose (l-glucose as control), and the changes in PRR mRNA and protein were monitored over a 14-d period. As shown in Results, the optimum expression of PRR was observed at 14 d of exposure to 30 mm d-glucose. According to these results, all studies described below used RMCs incubated for 14 d in culture medium containing 30 mm d-glucose (high glucose) for experimental or 30 mm l-glucose for control groups. Before inhibitor treatment, serum starvation was conducted for 12 h with serum-free medium (SFM), Opti-MEM I (Invitrogen) in the presence of 30 mm l- or d-glucose.
To investigate effect of COX-2 inhibition on PRR expression, cells were treated with 10 μm of COX-2 inhibitor NF-398 (Cayman Chemical, Ann Arbor, MI) in SFM for 12 h. In the study of IL-1β receptor inhibition, cells were exposed to recombinant rat IL-1β receptor antagonist (IL-1Ra; R&D Systems, Minneapolis, MN) in SFM at 100 ng/ml for 6 h. For angiotensin II type 1 (AT1) receptor blockade, valsartan (Novartis, East Hanover, NJ) was simultaneously applied to SFM at 1 μm for 12 h when serum starvation started. At the end of the experiments, cells were harvested for the preparation of whole-cell lysate and total RNA extraction.
PRR knockdown by small interfering RNA (siRNA)
The single-stranded 19-nt RNA duplexes targeting rat ATP6AP2 mRNA (GenBank access no. XM_217592.4) (5′-CCUACAACCUUGCGUAUAA-3′) for silencing of PRR were purchased from Dharmacon Research Inc. (Boulder, CO). RMCs plated in six-well culture dishes were transfected with siRNA duplexes against rat PRR mRNA by using siPORT NeoFX transfection reagent (Ambion, Austin, TX) according to the manufacturer’s suggested protocol. Pilot experiments demonstrated that 100 nm siRNA duplex results in a maximal suppression of PRR mRNA for 48 h and PRR protein expression for 72 h. Cells for total RNA extraction were harvested at 48 h after transfection. Cells for whole-cell lysate preparation and culture supernatants for ELISA were collected 72 h after transfection, respectively. One micromole of valsartan was applied to SFM Opti-MEM I (Invitrogen) in the presence of 30 mm l- or d-glucose 12 h before the end of 48 (for total RNA extraction) or 72 h (for protein expression studies) PRR siRNA transfection.
Determination of mRNA expression
Quantitative real-time RT-PCR was used to validate changes in the gene expression. RNA was extracted from cultured cells with the RNeasy total RNA isolation kit (QIAGEN, Valencia, CA). The RNA integrity was accessed by 2% formaldehyde agarose gel electrophoresis. Expression levels of PRR mRNA were measured by real-time RT-PCR iCycler according to the manufacturer’s instructions (Bio-Rad, Hercules, CA). Single-stranded cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad). PCR was performed with iQ SYBR green supermix (Bio-Rad) according to the manufacturer’s instructions. Primers sequences are: PRR forward, 5′-TGGCCTATACCAGGAGATCG-3′, reverse, 5′-AAT AGG TTG CCC ACA GCA AG-3′; (pro)renin forward, 5′-GCT TTG GAC GAA TCT TGC TC-3′, reverse, 5′-ATG AAT TCA CCC CAT TCA GC-3′; IL-1β forward, 5′-AGG CTT CCT TGT GCA AGT GT-3′, reverse, 5′-TGA GTG ACA CTG CCT TCC TG-3′; COX-2 forward, 5′-GTG TGA GTG GTA GCC AGC AA-3′, reverse, CCC ACA GGA GGA TCT GAA AA; and β-actin forward, 5′-AGC CAT GTA CGT AGC CAT CC-3′, reverse, 5′-ACC CTC ATA GAT GGG CAC AG-3′. Reactions were performed in triplicate, and threshold cycle numbers were averaged. No-template control was used as negative control. Samples were calculated with normalization to β-actin.
Detection of expression and phosphorylation of proteins
Whole-cell lysates were extracted with lysis buffer [50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 2 mm EDTA, 0.1% sodium dodecyl sulfate, 1% IGEPAL CA-630, 0.5% deoxycholate sodium, 20 μm MG132 (Calbiochem, La Jolla, CA), 50 mm sodium fluoride, 2 mm sodium orthovanadate, 1 mm phenyl methane sulfonyl fluoride, and 1× dilution protease inhibitor cocktail (Roche, Indianapolis, IN)] and quantified by BCA protein assay kit (Pierce Biotechnology, Rockford, IL). A total 20–50 μg of cell lysate was loaded for each sample, separated by SDS-PAGE, and electrotransferred to polyvinylidene fluoride membrane (Immun-Blot 0.2 μm; Bio-Rad). Primary antibodies against ATP6AP2, phosphor-nuclear factor-κB (NF-κB) p65(S276), phosphor-NF-κB p65(S468) (Abcam, Cambridge, MA), NF-κB p65, c-Jun, phosphor-c-Jun(S63) (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-NF-κB p65(S536) (Cell Signaling Technology, Danvers, MA), ERK1/2, phosphor-ERK1/2 (T185/Y187), c-Jun N-terminal kinase (JNK)-1/JNK2, phosphor-JNK (T183/Y185) (Invitrogen), COX-2 (Novus Biologicals, Littleton, CO) were used in this study. Rabbit antirat prorenin/renin antibody was kindly provided by Dr. Tadashi Inagami (Vanderbilt University, Nashville, TN). Signal detection was carried out by using SuperSignal WestPico chemiluminescent subtract (Pierce Biotechnology, Rockford IL). The blot was treated with restore Western blot stripping buffer according to the manufacturer’s recommendation (Pierce Biotechnology) and followed by reprobing with anti-β-actin antibody (Sigma, St. Louis, MO). The band densitometry was preformed by ImageMaster TotalLab version 2.0 (Amersham Pharmacia BioTech, Piscataway, NJ). The band density of target protein was normalized to the corresponding density of β-actin. The arbitrary unit of band densities was represented as the expression level.
Immunoprecipitation of prorenin and renin in culture supernatants
After glucose exposure and serum starvation, culture supernatants were harvested on ice and incubated for 2 h with goat polyclonal antibody to prorenin/renin (Santa Cruz Biotechnology), which was amino linked to antibody coupling gel by using Profound coimmunoprecipitation kit (Pierce Biotechnology) according to the manufacturer’s instructions. The elution buffer was added to the coupling gel in spin cup and was centrifuged. The resultant elute was mixed with loading buffer containing 200 mm dithiothreitol, boiled for 10 min in boiling water, and separated on 4–20% Tris-HCl Criterion precast gel. Blots were incubated with rabbit antirat prorenin/renin antibody for Western immunoblot detection.
Immunofluorescence staining of prorenin and renin protein
After glucose exposure and serum starvation, cells cultured on coverglasses were washed with ice-cold PBS buffer and fixed with cold 2% paraformaldehyde for 20 min. Cells were treated with 50 mg/ml glycine in PBS for 15 min, blocked with 5% serum albumin, and incubated with rabbit antirat-prorenin/renin antibody overnight. After washing, cells were incubated with Alexa Fluor 594 F(ab′)2 fragment of goat-antirabbit IgG (H+L) (Molecular Probes, Eugene, OR) for 1 h. The coverglasses were sealed with mounting medium for fluorescence with 4′,6′-diamino-2-phenylindole (Vector Laboratories, Inc., Burlingame, CA) and then examined by fluorescence microscopy.
Measurement of total cell numbers
Parallel experiments with the same design as described above were conducted simultaneously to determine the total cell number in each sample. The total cell number was determined by quantitatively measuring the release of lactate dehydrogenase of RMCs by CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI). RMCs in serial dilutions of 0, 5,000, 10,000, and 20,000 cells (for plotting standard curve) and cells in experiments were lysed to prepare cell lysates. lactate dehydrogenase levels in lysates were colorimetrically assayed at 490 nm. The total cell number was calculated as the average from triplicates of each sample.
ELISA of rat IL-1β and angiotensin II (Ang II)
Rat IL-1β levels in culture supernatants of RMCs were analyzed by ELISA (R&D Systems) according to the manufacturer’s instructions. Rat IL-1β concentration of each sample was normalized to its corresponding total cell numbers (1 × 106 cells) at the time point of harvesting supernatant.
Rat angiotensin II levels in RMC culture supernatants were assessed by Ang II enzyme immunoassay kit (Cayman Chemical). Rat Ang II concentration of each sample was normalized to its corresponding cell numbers (1 × 106 cells) at the time point of harvesting supernatants.
Statistical analysis
Comparisons among different treatment groups are examined by ANOVA. Data are expressed as mean ± se. P < 0.05 is defined as statistically significant.
Results
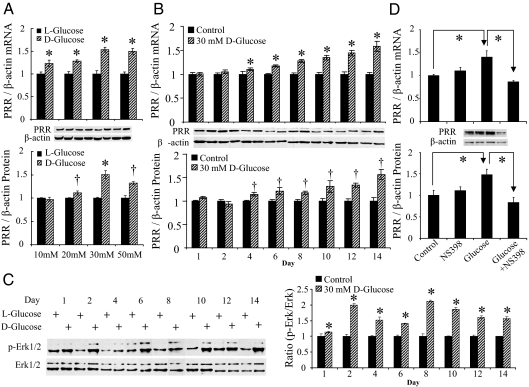
High glucose up-regulated PRR expression in time- and dose-dependent manner and promoted ERK phosphorylation in RMCs
PRR mRNA and protein were constitutively expressed in RMCs. Compared with control cells, the maximum PRR mRNA and protein expression was observed at 14 d of exposure to 30 mm d-glucose (Fig. 1, A and B). There was no significant difference in PRR expression in response to 30 and 50 mm high glucose treatment (Fig. 1A). ERK1/2 phosphorylation was significantly higher in d-glucose-treated groups. Throughout the study, the ratios of phospho-ERK1/2 to total ERK1/2 were significantly higher in d-glucose compared with controls at each day (Fig. 1C).
Figure 1.
PRR mRNA and protein expression and ERK1/2 phosphorylation (p-ERK) induced by high d-glucose and in response to COX-2 inhibitor in RMCs. A, PRR expression in response to different l- or d-glucose concentrations for 14 d. B, Time course for PRR expression in response to 30 mm l- or d-glucose over 14 d. C, ERK1/2 protein phosphorylation over 14 d of 30 mm l- or d-glucose exposure. D, Effect of 12 h treatment with COX-2 inhibitor NS-398 at 10 μm on PRR expression in control and glucose-treated RMCs. Control, 30 mm l-glucose; glucose, 30 mm d-glucose. *, P < 0.01; †, P < 0.05. All the results represent the average of three independent experiments.
COX-2 blockade down-regulated PRR expression in high glucose-treated RMCs
COX-2 blockade by NS-398 did not show significant effects on PRR mRNA and protein expression in the control group (Fig. 1D). In contrast, NS-398 treatment significantly reduced high d-glucose-induced increase of PRR mRNA and protein expression (Fig. 1D).
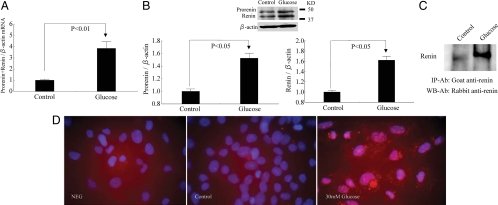
High glucose up-regulated prorenin and renin expression in RMCs
Prorenin and renin were constitutively expressed in RMCs (Fig. 2, A and B). High d-glucose treatment significantly increased the expression of prorenin/renin mRNA and protein (Fig. 2, A and B). In RMC culture supernatants, more renin protein was immunoprecipitated in high-d-glucose-treated group compared with control (Fig. 2C). Immunofluorescence of RMCs showed scarcely distributed fine particles in control and large number of dense and coarse granules in high-d-glucose-treated cells (Fig. 2D).
Figure 2.
Effect of high d-glucose on prorenin and renin expression in RMCs. High-glucose exposure increased prorenin mRNA (A) and prorenin and renin protein (B). Renin protein (C) increased in RMCs culture supernatants in response to high-glucose exposure as demonstrated by immunoprecipitation (IP). Immunofluorescence staining of prorenin and renin (D). NEG, negative control (no antibody control); control, 30 mm l-glucose; glucose, 30 mm d-glucose; KD, knockdown; WB, Western blot; Ab, antibody.
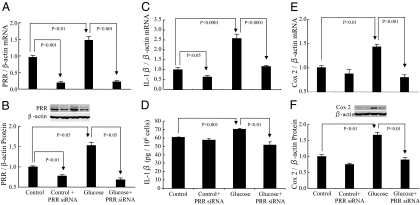
PRR expression in PRR siRNA transfected RMCs
PRR mRNA and protein were detectable in control RMCs (Fig. 3, A and B). High-d-glucose treatment significantly increased the expression of PRR mRNA and protein. PRR siRNA significantly attenuated PRR mRNA and protein expression in control and high-d-glucose-treated cells (Fig. 3, A and B).
Figure 3.
Effects of high d-glucose and PRR siRNA on PRR, IL-1β, and COX-2 mRNA and protein expression in RMCs. A and B, PRR mRNA and protein. C and D, IL-1β mRNA and IL-1β protein in culture supernatants. E and F, COX-2 mRNA and protein. Control, 30 mm l-glucose; glucose, 30 mm d-glucose.
IL-1β production in PRR siRNA transfected RMCs
High-glucose treatment significantly increased IL-1β mRNA (Fig. 3C) and IL-1β protein secretion (Fig. 3D). PRR siRNA significantly reduced IL-1β mRNA but not its protein in control RMCs. In high-glucose-treated cells, PRR siRNA significantly reduced IL-1β mRNA and protein (Fig. 3, C and D).
COX-2 expression in PRR siRNA transfected RMCs
COX-2 mRNA (Fig. 3E) and protein (Fig. 3F) were expressed in very low levels in control RMCs. High- d-glucose treatment significantly increased the expression of COX-2 mRNA and protein (Fig. 3, E and F). COX-2 mRNA and protein expression was not significantly influenced by PRR siRNA treatment in control cells. In high-d-glucose-treated cells, PRR siRNA significantly attenuated COX-2 mRNA and protein (Fig. 3, E and F).
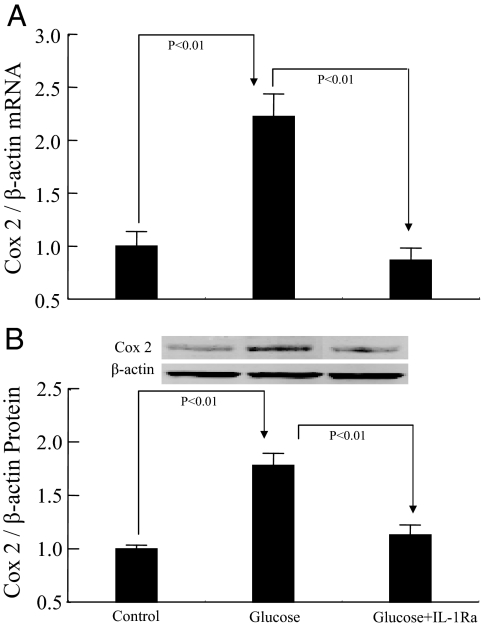
COX-2 expression in response to IL-1Ra blockade
High-d-glucose treatment significantly increased the expression of COX-2 mRNA (Fig. 4A) and protein (Fig. 4B). IL-1Ra treatment significantly attenuated this increase in COX-2 mRNA and protein expression.
Figure 4.
Effects of high d-glucose and IL-1Ra on COX-2 expression in RMCs. A, COX-2 mRNA. B, COX-2 protein. Control, 30 mm l-glucose; glucose, 30 mm d-glucose.
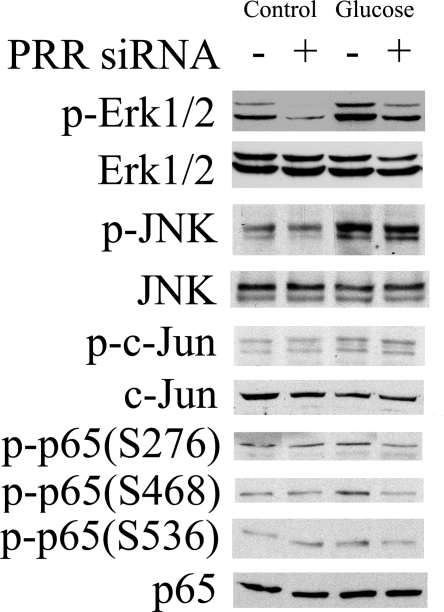
PRR siRNA inhibited ERK1/2 and NF-κB p65 phosphorylation
Basal phosphorylation of ERK1/2, JNK1, JNK2, c-Jun, and NF-κB p65 was detected in control cells (Fig. 5). PRR siRNA significantly suppressed phosphorylation of ERK1/2 but did not show significant influence on the phosphorylation of JNK1 and JNK2, c-Jun, and NF-κB p65 (S276, S468, and S536) in control cells (Fig. 5). Compared with control, high-glucose treatment increased the phosphorylation of ERK1/2, JNK 1/2, c-Jun, and NF-κB p65 (S276, S468, and S536) (Fig. 5). PRR siRNA significantly inhibited high glucose-induced phosphorylation of ERK1/2 and NF-κB p65 (S276, S468, and S536) but did not influence the phosphorylation of JNK1/2 and c-Jun (Fig. 5). There were no changes in total protein expression of ERK1/2, NF-κB p65, JNK, and c-Jun in response to high-d-glucose or PRR siRNA treatments.
Figure 5.
Western blot analysis showing effects of glucose and PRR siRNA on protein phosphorylation (p-) of ERK1/2, JNK, c-Jun, and NF-κB p65. Control, 30 mm l-glucose; glucose, 30 mm d-glucose.
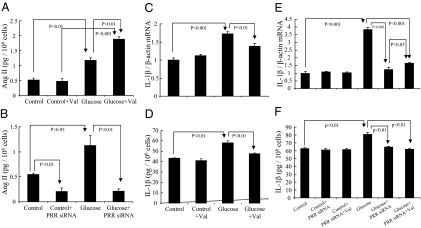
Effect of AT1 receptor blockade and PRR knockdown on Ang II production in RMCs
Compared with control cells, high d-glucose treatment increased Ang II levels in RMCs culture supernatants. Ang II levels increased further with valsartan treatment in high-d-glucose-treated cells (Fig. 6A). In contrast, Ang II levels did not change in response to valsartan treatment in control cells (Fig. 6A). PRR siRNA significantly reduced Ang II levels in culture supernatants of control and high d-glucose-treated cells (Fig. 6B).
Figure 6.
Effect of valsartan and PRR siRNA on Ang II level and IL-1β production in RMCs. A, Ang II level in response to 30 mm d-glucose and valsartan treatment. B, Ang II level and effect of PRR siRNA interference in the absence or presence of 30 mm d-glucose. Effects of valsartan on 30 mm d-glucose-induced IL-1β mRNA (C) and IL-1β protein in culture supernatants (D). Effect of PRR siRNA alone or combined with valsartan on IL-1β mRNA expression (E) and IL-1β protein production in culture supernatants (F). One micromole of valsartan (Val) was used alone and combined with PRR siRNA in SFM containing 30 mm l-glucose (control) or 30 mm d-glucose (glucose) for 12 h of serum-free culture. One hundred nanomoles of PRR siRNA were used to transfect RMCs. In PRR siRNA transfection experiments, serum-free culture and valsartan treatments were conducted simultaneously for 12 h (12 h before end of 48 h for RNA expression or 72 h for protein expression of PRR siRNA transfection). Control, 30 mm l-glucose; glucose, 30 mm d-glucose.
Effect of PRR knockdown alone and combined with AT1 receptor blockade on IL-1β production in the presence of high glucose concentration
Valsartan (Fig. 6C), PRR siRNA, and PRR siRNA plus valsartan (Fig. 6, E and F) did not cause significant changes in the expression of IL-1β mRNA and protein in control cells. High-d-glucose treatment significantly increased the expression of IL-1β mRNA and protein (Fig. 6, C–F). Valsartan (Fig. 6, C and D) and PRR siRNA (Fig. 6, E and F), individually and combined, significantly reduced high-d-glucose-induced increases in IL-1β mRNA and protein. There were no significant differences in IL-1β production between PRR siRNA alone and PRR siRNA combined with valsartan (Fig. 6, E and F).
Discussion
It is well established that renin is mainly produced by renal juxtaglomerular cells (42,43,44). However, renin is also produced by renal glomeruli and tubules (45,46,47). In our study we demonstrated that prorenin mRNA and protein are constitutively expressed in RMCs and their expression is up-regulated by high glucose. Similarly, high glucose increases renin secretion and Ang II formation. These findings are consistent with previous reports of increased prorenin production in diabetes (6,48) and also support the concept for prorenin and renin to function in an autocrine/paracrine fashion in RMCs.
We also documented the presence of PRR in RMCs and their up-regulation by high-glucose treatment in a time- and dose-dependent pattern. This finding is consistent with our recent report (8) of increased PRR expression in the kidney of streptozocin-induced diabetes rat model. We further demonstrated that siRNA targeted to PRR blocks this receptor expression, especially in the presence of high glucose concentration. Interestingly, the knockdown efficiency of PRR is lower under normal conditions compared with cells exposed to high glucose concentration. This finding could be related to the level of this receptor expression under different conditions or the half-life of the PRR protein. PRR is constitutively expressed in RMCs but to lesser quantities under normal conditions. Previous reports of increased renin production (49,50) together with our finding of increased PRR expression in the presence of high glucose level suggests involvement of this receptor in development of kidney disease in diabetes.
Based on the optimal glucose concentration and time-course studies, our results reflect the effects of 2 wk duration of RMC exposure to 30 mm glucose and should be interpreted with caution because most individuals with diabetes do not have this high glucose level. Previous studies used similar high glucose concentrations (51,52). Because diabetes is a chronic disease, it is assumed that exposure to this high glucose level in cell culture may shorten the time course and enhance the development of the pathological processes that otherwise may require weeks to months to occur.
In this study we confirmed that high glucose concentration significantly increases IL-1β production in RMCs. Inhibition of glucose-induced increase in IL-1β production with PRR siRNA suggests involvement of this receptor in inducing inflammation in RMCs. Similarly, our study demonstrated that COX-2 is constitutively expressed in RMCs, although at very limited amounts. This is consistent with previous reports of COX-2 expression in mesangial cells (53,54). In addition, our data demonstrated increased COX-2 production in response to high glucose levels in RMCs. Using PRR siRNA, we confirmed that increased PRR expression is responsible for the increased COX-2 production. Because cytokines are known to enhance COX pathways (53,54), it is plausible that in the presence of high glucose, the observed elevation in IL-1β production mediates the increase of COX-2 synthesis in RMCs. In this study we clearly demonstrated that COX-2 expression is reduced by IL-1 receptor blockade.
Our results demonstrated increased ERK1/2 phosphorylation in response to high-glucose treatment. In contrast to the observed progressive increase in PRR expression with time, the increase in ERK1/2 phosphorylation levels did not change significantly over the 2-wk period of the study. PRR siRNA partially blocked ERK1/2 phosphorylation, suggesting involvement of multiple pathways in this process. The dissociation between high glucose-induced time-dependent increase in PRR expression and time-independent increase in ERK1/2 phosphorylation could be explained by the negative feedback of MAPK phosphatase limiting further phosphorylation of ERK. MAPK phosphatase is expressed in cultured glomerular mesangial cells (55) and is activated with ERK phosphorylation, thus limiting further phosphorylation of ERK (56,57).
In addition to ERK phosphorylation, we also observed that high-glucose concentration results in increased phosphorylation of NF-κB p65 at serine 276,468 and 536, JNK, and c-Jun in RMCs. PRR siRNA significantly inhibited the phosphorylation of these intracellular signal proteins except JNK and c-Jun. Based on these results, we conclude that high glucose promotes the production of IL-1β and COX-2 by enhancing the expression of prorenin/renin and PRR and activating ERK1/2 and NF-κB in mesangial cells.
In this study, we report that high glucose increased Ang II production in RMCs. This production was further increased with AT1 receptor blockade. These results are consistent with previous studies demonstrating increased renal levels of Ang II in diabetes and with valsartan treatment (58). In contrast, PRR knockdown significantly attenuated Ang II production, suggesting that glucose-induced Ang II production in RMCs is PRR dependent. Ang II is known to promote IL-1β production via AT1 receptor in the kidney (59). In this study, both AT1 receptor blockade and PRR knockdown significantly inhibited glucose induced IL-1β production. Interestingly, we observed lack of additional IL-1β inhibition during combined PRR knockdown and AT1 receptor blockade. These results suggest that the effect of PRR on IL-1β production is mainly mediated via a PRR-Ang II-dependent mechanism. However, previous studies demonstrated that the binding of prorenin or renin to PRR could directly activate ERK1/2, which is independent of Ang II (13,14). Thus, it is also possible that the influence of PRR on IL-1β production could partially be mediated via an Ang II-independent mechanism. Our data showing that PRR knockdown reduced phosphorylation of ERK1/2 may also support this alternative mechanism.
In addition to demonstrating that glucose-induced PRR up-regulation promotes IL-1β and COX-2 production, we also demonstrated suppression of high-glucose-induced PRR expression with COX-2 inhibition. To our knowledge, this is the first demonstration that PRR expression is facilitated by inflammation. Further studies are needed to elucidate how inflammation enhances PRR expression.
In conclusion, glucose induces the up-regulation of PRR expression and its ligands prorenin and renin, leading to increased IL-1β and COX-2 production via an Ang II-dependent pathway. It is also possible that PRR could enhance the production of these inflammatory cytokines through direct stimulation of ERK1/2-NF-κB signaling cascade. Interestingly, COX-2 stimulates PRR expression, suggesting a positive feedback loop involving inflammation and this receptor.
Footnotes
This work was supported by Grants DK-078757 and HL091535 from the National Institutes of Health (to H.M.S.).
Disclosure Summary: J.H. and H.M.S. have nothing to declare.
First Published Online October 27, 2009
Abbreviations: Ang II, Angiotensin II; AT1, Ang II type 1; COX, cyclooxygenase; IL-1Ra, IL-1β receptor antagonist; JNK, c-Jun N-terminal kinase; NF-κB, nuclear factor-κB; PRR, (pro)renin receptor; RAS, renin-angiotensin-system; RMC, rat mesangial cell; SFM, serum-free medium; siRNA, small interfering RNA.
References
- Atiyeh BA, Arant Jr BS, Henrich WL, Seikaly MG 1995 In vitro production of angiotensin II by isolated glomeruli. Am J Physiol 268:F266–F272 [DOI] [PubMed] [Google Scholar]
- Becker BN, Yasuda T, Kondo S, Vaikunth S, Homma T, Harris RC 1998 Mechanical stretch/relaxation stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Exp Nephrol 6:57–66 [DOI] [PubMed] [Google Scholar]
- Andrade MC, Quinto BM, Carmona AK, Ribas OS, Boim MA, Schor N, Casarini DE 1998 Purification and characterization of angiotensin I-converting enzymes from mesangial cells in culture. J Hypertens 16:2063–2074 [DOI] [PubMed] [Google Scholar]
- Singh R, Singh AK, Alavi N, Leehey DJ 2003 Mechanism of increased angiotensin II levels in glomerular mesangial cells cultured in high glucose. J Am Soc Nephrol 14:873–880 [DOI] [PubMed] [Google Scholar]
- Anderson S, Jung FF, Ingelfinger JR 1993 Renal renin-angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlations. Am J Physiol 265:F477–F486 [DOI] [PubMed] [Google Scholar]
- Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA 2004 High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 286:F1039–F1045 [DOI] [PubMed] [Google Scholar]
- Singh R, Singh AK, Leehey DJ 2005 A novel mechanism for angiotensin II formation in streptozotocin-diabetic rat glomeruli. Am J Physiol Renal Physiol 288:F1183–F1190 [DOI] [PubMed] [Google Scholar]
- Siragy HM, Huang J 2008 Renal (pro)renin receptor up-regulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp Physiol 93:709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside CI, Dlugosz JA 2002 Mesangial cell protein kinase C isozyme activation in the diabetic milieu. Am J Physiol Renal Physiol 282:F975–F980 [DOI] [PubMed] [Google Scholar]
- Wilmer WA, Dixon CL, Hebert C 2001 Chronic exposure of human mesangial cells to high glucose environments activates the p38 MAPK pathway. Kidney Int 60:858–871 [DOI] [PubMed] [Google Scholar]
- Navarro-González JF, Mora-Fernández C 2008 The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19:433–442 [DOI] [PubMed] [Google Scholar]
- King GL 2008 The role of inflammatory cytokines in diabetes and its complications. J Periodontol 79:1527–1534 [DOI] [PubMed] [Google Scholar]
- Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD 1996 Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int 50:1897–1903 [DOI] [PubMed] [Google Scholar]
- Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD 2002 Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109:1417–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcklé CA, Jan Danser AH, Müller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G 2006 Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension 47:552–556 [DOI] [PubMed] [Google Scholar]
- Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H 2007 Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 18:1789–1795 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Ichihara A, Kaneshiro Y, Inomata K, Sakoda M, Takemitsu T, Nishiyama A, Itoh H 2007 Regression of nephropathy developed in diabetes by (pro)renin receptor blockade. J Am Soc Nephrol 18:2054–2061 [DOI] [PubMed] [Google Scholar]
- Ichihara A, Sakoda M, Kurauchi-Mito A, Kaneshiro Y, Itoh H 2008 Involvement of (pro)renin receptor in the glomerular filtration barrier. J Mol Med 86:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T 2004 Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114:1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W 2006 Renin increases mesangial cell transforming growth factor-β1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int 69:105–113 [DOI] [PubMed] [Google Scholar]
- Dinarello CA 2003 Interleukin-1 family [IL-1F1,F2]. In: Thomas AW, Lotze MT, eds. The cytokine handbook. 4th ed. Boston: Elsevier: Academic Press; 644–668 [Google Scholar]
- Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, Kondo M 1991 Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int 40:1007–1012 [DOI] [PubMed] [Google Scholar]
- Navarro JF, Milena FJ, Mora C, León C, García J 2006 Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol 26:562–570 [DOI] [PubMed] [Google Scholar]
- Sassy-Prigent C, Heudes D, Mandet C, Bélair MF, Michel O, Perdereau B, Bariéty J, Bruneval P 2000 Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 49:466–475 [DOI] [PubMed] [Google Scholar]
- Wu Y, Dong J, Yuan L, Liang C, Ren K, Zhang W, Fang F, Shen J 2008 Nephrin and podocin loss is prevented by mycophenolate mofetil in early experimental diabetic nephropathy. Cytokine 44:85–91 [DOI] [PubMed] [Google Scholar]
- Wu Y, Ren K, Liang C, Yuan L, Qi X, Dong J, Shen J, Lin S 2009 Renoprotective effect of total glucosides of paeony (TGP) and its mechanism in experimental diabetes. J Pharmacol Sci 109:78–87 [DOI] [PubMed] [Google Scholar]
- Gauer S, Hauser IA, Obermüller N, Holzmann Y, Geiger H, Goppelt-Struebe M 2008 Synergistic induction of osteopontin by aldosterone and inflammatory cytokines in mesangial cells. J Cell Biochem 103:615–623 [DOI] [PubMed] [Google Scholar]
- Nee L, O'Connell S, Nolan S, Ryan MP, McMorrow T 2008 Nitric oxide involvement in TNF-α and IL-1 β-mediated changes in human mesangial cell MMP-9 and TIMP-1. Nephron Exp Nephrol 110:e59–e66 [DOI] [PubMed] [Google Scholar]
- Xin C, Ren S, Eberhardt W, Pfeilschifter J, Huwiler A 2007 FTY720 suppresses interleukin-1β-induced secretory phospholipase A2 expression in renal mesangial cells by a transcriptional mechanism. Br J Pharmacol 150:943–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovin BH, Wilmer WA, Danne M, Dickerson JA, Dixon CL, Lu L 1999 The mitogen-activated protein kinase p38 is necessary for interleukin 1β-induced monocyte chemoattractant protein 1 expression by human mesangial cells. Cytokine 11:118–126 [DOI] [PubMed] [Google Scholar]
- Lee SK, Park JY, Chung SJ, Yang WS, Kim SB, Park SK, Park JS 1998 Chemokines, osteopontin, ICAM-1 gene expression in cultured rat mesangial cells. J Korean Med Sci 13:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin C, Ren S, Eberhardt W, Pfeilschifter J, Huwiler A 2007 Sphingosylphosphorylcholine acts in an anti-inflammatory manner in renal mesangial cells by reducing interleukin-1β-induced prostaglandin E2 formation. J Lipid Res 48:1985–1996 [DOI] [PubMed] [Google Scholar]
- Solà-Villà D, Camacho M, Solà R, Soler M, Diaz JM, Villa L 2006 IL-1β induces VEGF, independently of PGE2 induction, mainly through the PI3-K/mTOR pathway in renal mesangial cells. Kidney Int 70:1935–1941 [DOI] [PubMed] [Google Scholar]
- Soler M, Camacho M, Solá R, Vila L 2001 Mesangial cells release untransformed prostaglandin H2 as a major prostanoid. Kidney Int 59:1283–1289 [DOI] [PubMed] [Google Scholar]
- Umino T, Kusano E, Muto S, Akimoto T, Yanagiba S, Ono S, Amemiya M, Ando Y, Homma S, Ikeda U, Shimada K, Asano Y 1999 AVP inhibits LPS- and IL-1β-stimulated NO and cGMP via V1 receptor in cultured rat mesangial cells. Am J Physiol 276:F433–F441 [DOI] [PubMed] [Google Scholar]
- Díaz-Cazorla M, Pérez-Sala D, Lamas S 1999 Dual effect of nitric oxide donors on cyclooxygenase-2 expression in human mesangial cells. J Am Soc Nephrol 10:943–952 [DOI] [PubMed] [Google Scholar]
- Rupprecht G, Scholz K, Beck KF, Geiger H, Pfeilschifter J, Kaszkin M 1999 Cross talk between group IIA-phospholipase A2 and inducible NO-synthase in rat renal mesangial cells. Br J Pharmacol 127:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Buckman SY, Miller BW, Springer LD, Morrison AR 1998 Interleukin-1β-induced cyclooxygenase-2 expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J Biol Chem 273:28670–28676 [DOI] [PubMed] [Google Scholar]
- Doller A, Akool el-S, Müller R, Gutwein P, Kurowski C, Pfeilschifter J, Eberhardt W 2007 Molecular mechanisms of cyclosporine A inhibition of the cytokine-induced matrix metalloproteinase-9 in glomerular mesangial cells. J Am Soc Nephrol 18:581–592 [DOI] [PubMed] [Google Scholar]
- Wang R, Wan Q, Zhang Y, Huang F, Yu K, Xu D, Wang Q, Sun J 2007 Emodin suppresses interleukin-1β induced mesangial cells proliferation and extracellular matrix production via inhibiting P38 MAPK. Life Sci 80:2481–2488 [DOI] [PubMed] [Google Scholar]
- Eberhardt W, Beeg T, Beck KF, Walpen S, Gauer S, Böhles H, Pfeilschifter J 2000 Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int 57:59–69 [DOI] [PubMed] [Google Scholar]
- Peters J, Clausmeyer S 2002 Intracellular sorting of renin: cell type specific differences and their consequences. J Mol Cell Cardiol 34:1561–1568 [DOI] [PubMed] [Google Scholar]
- Demopoulos H, Kaley G, Zweifach BW1961 Response of granular juxtaglomerular cells and tissue mast cells in various experimental states. . Circ Res 9:845–850 [DOI] [PubMed] [Google Scholar]
- Hartroft WS, Hartroft PM 1961 New approaches in the study of cardiovascular disease: aldosterone, renin, hypertension and juxtaglomerular cells. Fed Proc 20:845–854 [PubMed] [Google Scholar]
- Rosenberg ME, Correa-Rotter R, Inagami T, Kren SM, Hostetter TH 1991 Glomerular renin synthesis and storage in the remnant kidney in the rat. Kidney Int 40:677–683 [DOI] [PubMed] [Google Scholar]
- Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, Hillas E, Zhang S, Ward K, Bloch-Faure M, Meneton P, Lalouel JM 2003 Renin and kallikrein in connecting tubule of mouse. Kidney Int 64:2155–2162 [DOI] [PubMed] [Google Scholar]
- Siragy HM, Xue C, Abadir P, Carey RM 2005 Angiotensin subtype-2 receptors inhibit renin biosynthesis and angiotensin II formation. Hypertension 45:133–137 [DOI] [PubMed] [Google Scholar]
- Andrade AQ, Casarini DE, Schor N, Boim MA 2002 Characterization of renin mRNA expression and enzyme activity in rat and mouse mesangial cells. Braz J Med Biol Res 35:17–24 [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen X, Wu D, Liu W, Wang J, Feng Z, Cai G, Fu B, Hong Q, Du J 2006 Downregulation of connexin 43 expression by high glucose induces senescence in glomerular mesangial cells. J Am Soc Nephrol 17:1532–1542 [DOI] [PubMed] [Google Scholar]
- Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M 1985 Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med 312:1412–1417 [DOI] [PubMed] [Google Scholar]
- Wu D, Peng F, Zhang B, Ingram AJ, Kelly DJ, Gilbert RE, Gao B, Krepinsky JC 2009 PKC-β1 mediates glucose-induced Akt activation and TGF-β1 up-regulation in mesangial cells. J Am Soc Nephrol 20:554–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Chang CK, Cheng MF, Lin HJ, Cheng JT 2009 The antioxidative effect of bone morphogenetic protein-7 against high glucose-induced oxidative stress in mesangial cells. Biochem Biophys Res Commun 382:292–297 [DOI] [PubMed] [Google Scholar]
- Coyne DW, Nickols M, Bertrand W, Morrison AR 1992 Regulation of mesangial cell cyclooxygenase synthesis by cytokines and glucocorticoids. Am J Physiol 263:F97–F102 [DOI] [PubMed] [Google Scholar]
- Rzymkiewicz D, Leingang K, Baird N, Morrison AR 1994 Regulation of prostaglandin endoperoxide synthase gene expression in rat mesangial cells by interleukin-1β. Am J Physiol 266:F39–F45 [DOI] [PubMed] [Google Scholar]
- Awazu M, Ishikura K, Hida M, Hoshiya M 1999 Mechanisms of mitogen-activated protein kinase activation in experimental diabetes. J Am Soc Nephrol 10:738–745 [DOI] [PubMed] [Google Scholar]
- Li J, Gorospe M, Hutter D, Barnes J, Keyse SM, Liu Y 2001 Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Mol Cell Biol 21:8213–8224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse SM 2008 Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 27:253–261 [DOI] [PubMed] [Google Scholar]
- Siragy HM, Awad A, Abadir P, Webb R 2003 The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor-α in diabetic rats. Endocrinology 144:2229–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, Gelosa P, Guerrini U, Banfi C, Crippa V, Brioschi M, Gianazza E, Nobili E, Gianella A, de Gasparo M, Tremoli E 2004 Anti-inflammatory effects of AT1 receptor blockade provide end-organ protection in stroke-prone rats independently from blood pressure fall. J Pharmacol Exp Ther 311:989–995 [DOI] [PubMed] [Google Scholar]