Abstract
Vertebrates show diverse sexual characters in sexually attractive and reproductive organs, which are regulated by steroid hormones, particularly androgens. However, the evolutionary history of androgen receptor (AR) gene remains largely unknown on the basis of phylogenic and functional analyses. To elucidate the evolutionary history and functional diversification of AR genes in vertebrates, we cloned the AR cDNAs from a shark, basal ray-finned fishes (Actinopterygii), namely bichir and sturgeon (Acipenseriformes), and teleosts including a basal teleost, arowana (Osteoglossiformes). Molecular phylogenetic analysis revealed that the gene duplication event that gave rise to two different teleost ARs (α and β) likely occurred in the actinopterygian lineage leading to teleosts after the divergence of Acipenseriformes but before the split of Osteoglossiformes, which is compatible with the phylogenetic timing of teleost-specific genome duplication. Searching for AR genes in the medaka genome indicated that the teleost AR gene duplication has been associated with the duplication between chromosomes 10 and 14. Our functional analysis revealed that the shark AR activates the target gene via androgen response element by classical androgens. The teleost ARα showed the unique intracellular localization with a significantly higher transactivating capacity than that by teleost ARβ. These findings indicate that the most ancient type of AR, as activated by the classical androgens as ligands, emerged before the Chondrichthyes-Osteichthyes split, and the AR gene was duplicated during the teleost-specific genome duplication event. We report here for the first time the accurate evolutionary history of AR gene and functional characterization of AR duplicates in teleost lineage.
The common ancestor of all extant-jawed vertebrate contains the most ancient type of androgen receptor (AR), and teleosts have two functionally different ARs duplicated with teleost-specific genome duplication.
Vertebrates exhibit great variety of anatomical structures, physiology, and behaviors for reproduction (1). Androgens are essential for the morphological specification of male type sexual characters that have evolved in each species presumably for survival and/or reproduction. Understanding the mechanisms of androgen-dependent organogenesis underlying the reproductive diversity among species is one of the central problems in evolutional biology. Androgen receptor (AR) belongs to the nuclear receptor (NR) superfamily (2,3,4,5,6) and is the key molecule controlling the expression of such masculine phenotypes.
The six related steroid receptors (SRs), AR, estrogen receptor (ER)-α and -β, progesterone receptor, glucocorticoid receptor, and mineralcorticoid receptor, arose by a series duplications of an ancestral steroid receptor (3) (7,8,9,10,11,12). It has been reported that the first duplication generated an ER and a 3-ketosteroid receptor (9). By the second duplication, the 3-ketosteroid receptor produced a corticoid receptor (CR) and a receptor for 3-ketogonadal steroids (androgens, progestins) before the divergence of lamprey and jawed vertebrates. After the cyclostome-gnathostome divergence, the ER, the CR, and the 3-ketogonadal steroids receptor duplicated again to yield the six steroid receptors, the ER to create ERα and ERβ, the CR to yield the glucocorticoid receptor and the mineralcorticoid receptor, and 3-ketogonadal steroid receptor to create the progesterone receptor and the AR (9). In teleost fishes, two distinct paralogous copies of ARs have been identified from several species including Nile tilapia (Oreochromis niloticus), Japanese eel (Anguilla japonica), and Atlantic croaker (Micropogonias undulatus) (13,14,15). In rainbow trout (Oncorhynchus mykiss), two isoforms of AR, probably derived from salmonid tetraploidy, were cloned (16). Male secondary sexual characters appear as an elongation of the fin ray, kidney hypertrophy, thickened skin, an appearance of breeding colors, and transition of anal fin to copulatory organ (Gonopodium) in teleost fishes (17,18). Thus, AR gene duplication might contribute to the evolutionary divergence of secondary sexual characters in teleost fishes.
For many genes including not only AR but also other NRs and Hox clusters, ray-finned fish have two paralogous copies, whereas one ortholog is present in tetrapods (19). This is mainly due to the teleost-specific genome duplication (TSGD) that occurred after the split of nonteleost actinopterygian lineages (namely, bichir, sturgeon, gar, and bowfin) from the teleost fish lineage but before the divergence of Osteoglossomorpha (20). A recent report of the AR gene evolution indicated that the AR gene duplication is consistent with the TSGD (21). However, it is still unclear when functional AR appeared in the vertebrate lineage and evolutionary analysis and functional characterization is required to discuss the biological importance of the AR gene diversification in vertebrate lineage. In addition to the recent genome sequencing of the elephant shark and several teleosts (22,23), evolutionary analysis of AR function will contribute to the understanding of the origin and diversification of the complex sex differentiation systems of jawed vertebrates.
It has been known that the ligand selectivity of AR is different among species (24). In mammals, testosterone (T) and 5α-dihydrotestosterone (DHT) are considered to be effective ligands for AR (25). 11ketotestosterone (11KT) is known as a potent androgen in teleost fishes (17). Recent research using jawless fish showed that androstenedione, a precursor of testosterone, may act as the androgen-related ligand in the sea lamprey (26). In this work, it is demonstrated that cartilaginous fishes, the most early-branching group of living jawed vertebrates, contain the most ancient type of AR activated by the classical androgens as ligands. To elucidate the evolutionary history and functional diversification of AR genes in vertebrates, we isolated the AR sequences at key lineages for entire vertebrate evolution, which enables us to elucidate when gene duplication of AR occurred and discuss the functional conservation of ARs among the vertebrate species. Based on the current evolutionary and functional characterization of AR genes, it is thus suggested that the most ancient type of AR, as activated by the classical androgens, appeared at the common ancestor of all extant jawed vertebrate chondrichthyans and two functionally different ARs duplicated with TSGD evolved in the teleost lineage.
Materials and Methods
Animals
Species and tissues used for RNA extraction are as follows: Chiloscyllium punctatum, brown-banded bambooshark (testis); Polypterus senegalus, gray bichir (testis); a F2 generation of hybrid sturgeon known as a bester produced by crossing a sterlet Acipenser ruthenus with a beluga Huso huso (testis); Osteoglossum bicirrhosum, silver arowana (testis); HdrR strain of Oryzias latipes, medaka (testis). Brown-banded bambooshark, gray bicher, and silver arowana were obtained from the commercial source (Meito suien). The hybrid sturgeon and HdrR strain of medaka were kindly provided by Miyazaki prefectural fisheries experimental station and Dr. Yuji Ishikawa (National Institute of Radiological Sciences), respectively.
Isolation of AR cDNAs
RT-PCR was carried out using SuperScript One-Step RT-PCR with PLATINUM Taq System (Invitrogen, Tokyo, Japan). To obtain the AR cDNA fragments, oligonucleotide primers were designed based on the amino acid sequences of highly conserved central region of AR genes (listed in Fig. 1A) and then used to amplify shark, bichir, sturgeon, arowana, and medaka cDNAs by RT-PCR, respectively.
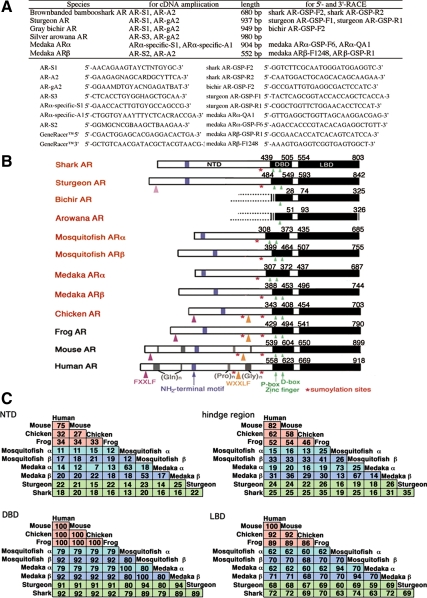
Figure 1.
Structural comparison of AR genes in vertebrates. A, The oligonucleotide primers used to amplify shark, sturgeon, bichir, arowana, and medaka AR cDNAs. B, Structural comparison of brown-banded bambooshark AR with other species ARs. AR is composed of three major functional domains, a hypervariable NTD, a central highly conserved DBD consisting of two zinc finger motifs, and a COOH-terminal LBD. The numbers above each box refer to the position of amino acids in the putative DBD and LBD. We deposited the obtained AR cDNA sequences to GenBank, Chiloscyllium punctatum, brown-banded bambooshark AR: AB213019; Polypterus senegalus, gray bichir AR: AB428795; Acipenser ruthenus × Huso huso (bester), sturgeon AR: AB213020; Osteoglossum bicirrhosum, silver arowana AR: AB428796; Oryzias latipes, medaka ARα: AB252233 and ARβ: AB252679; Gambusia affinis, western mosquitofish ARα: AB174849 and ARβ: AB099303 (18); Gallus gallus, chicken AR: AB193190 (35). We obtained the AR cDNA sequences of Xenopus laevis, African clawed frog AR: U67129, Mus musculus, mouse AR: M37890, Homo sapiens, human AR: M23263 from GenBank. The protein motifs showing the similarities to those of human ARs were indicated. C, Percent similarity of the deduced amino acid sequences of each domain among species. Alignment of the brown-banded bambooshark and sturgeon ARs with other species ARs illustrates high similarities within the putative DBDs and the LBDs. The amino acid sequence of shark AR is highly similar to those of the tetrapod ARs and teleost ARβs but much less to those of teleost ARαs.
Based on the sequence information of obtained PCR products, the 5′ and 3′ ends of AR sequences were amplified using a rapid amplification of cDNA ends (RACE) kit (Gene Racer kit; Invitrogen). Obtained cDNAs were subjected to 5′- and 3′-rapid amplification (RACE) of cDNA ends with Platinum Taq DNA polymerase high fidelity (Invitrogen) using specific primers for shark AR, bichir AR, sturgeon AR, and medaka ARα and ARβ with the supplied adapter-specific primers as listed in Fig. 1A. Amplified products were subcloned and sequenced.
Phylogenetic analysis
Construction of multiple alignments of AR genes and estimation of molecular phylogeny with the neighbor-joining method (27) were carried out using Xced alignment editor (28). Analyses with the maximum-likelihood (ML) method were performed using PhyML (29), assuming JTT+I+Υ4 model. Bootstrap probabilities for the neighbor-joining and ML analyses were calculated based on bootstrap resampling (1000 replicates) (30). Bayesian tree inferences were done using MrBayes 3.1 (31).
In silico genomic analysis
Chromosomal localization of medaka ARα and ARβ were obtained by running BLAST searches (32) against the medaka genomic DNA using cDNA sequences of these ARs as queries on the Ensembl Medaka Genome Browser (http://www.ensembl. org/Oryzias_latipes/index.html) and compared with that of human AR. The genes located in the long arm of human X chromosome were blasted against the medaka genome by running BLAST searches (32) using the Ensembl Genome Browser. The comparative map for putative orthologs between human X chromosome and medaka chromosome 10 and/or 14 were.
Gene transfection assay
The entire protein coding region of AR cDNAs were amplified by RT-PCR using the primes for brown-banded bambooshark, sAR-S2-ATG: 5′-ATGCGCGCCAGCGAGCCGC-3′ and sAR-R2-TGA: 5′-CTGTTCATGAAAAAGAATCGGTTTGGCCATACCAGCG-3′; sturgeon AR, stAR-S1-ATG: 5′-ATGGATATTCAGATTGGATTAGGAGGAC-3′ and stAR-R1-TGA: 5′-CTGCTTGTGAAACAGGATTGGCTTCG-3′; western mosquitofish ARα, wmARα-S1-ATG: 5′-ATGGCCTTTCGCTCCAGGCTG-3′ and wmARα-R1-TGA: 5′-GGCCGTGTTGTGGAACAGGATG-3′; for western mosquitofish ARβ, wmARβ-S1-ATG: 5′-ATGAGCCAAACCAGCCGACAGT-3′ and wmARβ-R1-TGA: 5′-CTTGTGGAACAAGATTGGCTTGG-3′; medaka ARα, medaARα-S1-ATG: 5′-ATGGCCTTTCGCTCCAGCTTGGTG-3′ and medaARα-R1-TGA: 5′-GGCTGTGTTGTGGAAAAGGATGGGCT-3′; medaka ARβ, medaARβ-S1-ATG: 5′-ATGAGCCAAACCAGCCGCCA-3′ and medaARβ-R1-TGA: 5′-CTTGTGAAACAAAATTGGCTTTGCC-3′; and mouse AR, mAR-S1-ATG: 5′-ATGGAGGTGCAGTTAGGGCTGG-3′ and mAR-R1-TGA: 5′-CTGTGTGTGGAAATAGATGGGCTTG-3′. Amplified AR cDNAs were cloned into a CMV expression vector, pCS2+MT (33,34), pCS2+GFP, or pDsRed monomer-N1 (Invitrogen) to generate pCMV-AR series. The mouse and chicken AR cDNAs amplified by RT-PCR were cloned into pCS2+MT, producing the pCMV-mouse AR and pCMV-chicken AR as previously described (35). A reporter plasmid for AR (PGL3 PRE/ARE tk Luc) was provided by Dr. Shigeaki Kato (Institute of Molecular and Cellular Biosciences, University of Tokyo, Tokyo, Japan) (36).
COS-7 cells cultured in 24-well multiwell plates (5.0 × 104 cells/well) were transfected with 400 ng/well of PRE/ARE reporter plasmid, 0.8 ng/well of pRL-SV40 (a Renilla luciferase vector) as the internal control, and 80 ng/well of the expression vector for ARs, using 1.5 μl/well of TrasFast transfection reagent (Promega Corp., Tokyo, Japan). After 6 h of the transfection, the cells were incubated for 12 h in DMEM with 0.2% fetal bovine serum in the presence or absence of DHT (Wako Chemical, Osaka, Japan; 045-26071), T (Wako Chemical; 204-08343), androstenedione (Sigma-Aldrich; Tokyo, Japan; A-9630), 11KT (Sigma; K-8250), ethynyl testosterone (ET; Sigma; E-1001), or flutamide (F; Sigma; F-9397). The reporter gene activities were determined by the dual-luciferase reporter assay system (Promega) with values normalized by pRL-induced activities, i.e. (firefly luciferase activity)/(Renilla luciferase activity). All experiments were repeated more than three times. Statistically significant differences of luciferase activities were tested using the Student’s t test. The data are presented as the means ± sd. Immunocytochemical stainings of mouse and chicken ARs were performed as previously described (35). Localization of green fluorescent protein (GFP) or DsRed fusion ARs and immunostained ARs were visualized under a fluorescence microscope 18 h after transfection. The nuclei were stained with bisbenzimide H33342 (Sigma- Aldrich; B2261) (2 μg/ml).
Cytoplasmic microinjection of DNA (16 ng/μl of PRE/ARE reporter plasmid, 0.06 ng/μl of pRL-SV40, and 5 ng/μl of the expression vector for ARs) into the fertilized medaka eggs was carried out as described by Kinoshita and Ozato (37). The embryos were incubated with or without increasing concentrations of 11KT (10−10 to 10−7 m) for 24 h. The embryos were then solubilized and the reporter gene activities were determined as described above.
Results
Identification of AR genes in brown-banded bambooshark, sturgeon, bichir, arowana, and medaka
We cloned the full-length AR cDNA encoding 803 amino acids from a brown-banded bambooshark (Fig. 1B). Furthermore, we newly isolated the full-length AR cDNA encoding 842 amino acids from sturgeon (Acipenseriformes) and the partial fragments of AR cDNA encoding 325 amino acids from bichir. We identified the partial cDNAs encoding 326 amino acids from arowana and the two distinct subtypes of ARs encoding 687 and 744 amino acids from medaka (Fig. 1B). A neighbor-joining tree based on comparison of the amino acid sequences in DNA binding domain (DBD) and ligand binding domain (LBD) suggested that the obtained medaka ARs were categorized into the two distinct clusters including ARα or ARβ genes of western mosquitofish (Gambusia affinis) and Nile tilapia (Oreochromis niloticus), which are therefore designated as ARα and ARβ, respectively (Fig. 2, A and B).
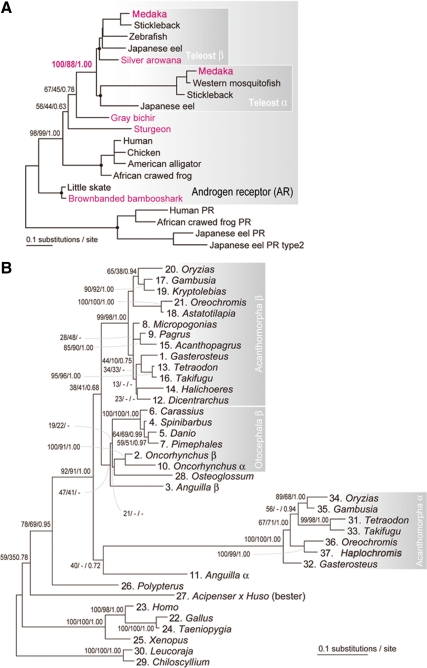
Figure 2.
Phylogenetic analysis of AR genes in vertebrates. A, Molecular phylogenetic tree of AR genes. This tree was estimated as the ML tree with Tree-Puzzle (91) in an exhaustive analysis with all possible tree topologies in the user tree mode, assuming JTT+I+Υ4 model (parameter α = 0.71; 266 amino acid sites). Phylogenetic relationships within the nodes shown with black dots are constrained based on previously proposed species phylogeny (92). Jawed vertebrate PR genes were used as the closest outgroup. Sequences determined in the present study are shown in red. B, Molecular phylogenetic tree of jawed vertebrate AR genes. This tree was estimated as the ML tree with PhyML (29), assuming JTT+I+Υ4 model (parameter α = 0.53; 272 amino acid sites). Chondrichthyan sequences were used as the outgroup. Support values at nodes are in order, bootstrap probabilities in the ML analysis, bootstrap probabilities in the neighbor-joining analysis, and posterior probabilities in the Bayesian analysis. Hyphens are shown when relationship for a particular node is not supported. Names of the species used in these analyses and their accession numbers retrieved from GenBank and Ensembl were shown in supplemental Tables S1 and S2.
The obtained AR cDNA sequences include several domains defined by Krust et al. (38) (Fig. 1B). Alignment of the brown-banded bambooshark and sturgeon ARs with other species ARs illustrates that the high similarities lie within the putative DBDs and the LBDs, sharing 79–94% and 59–74% amino acid identities, respectively (Fig. 1C).
Molecular phylogenetic analyses
Next, the deduced amino acid sequences of DBD and LBD of obtained AR cDNAs, along with other available AR sequences, were used to infer a molecular phylogeny of ARs (Fig. 2, A and B). Our analysis, based on both DBD and LBD, revealed that the brown-banded bambooshark AR identified by the present study tightly clusters with the previously reported chondrichthyan AR (that of the little skate, Leucoraja erinacea; see Fig. 2, A and B), representing the most early-branching lineage within jawed vertebrates. The bichir and sturgeon ARs occupy basal lineages before the split between teleost ARα and ARβ, whereas basal teleost fish arowana AR was included in the ARβ cluster (Fig. 2, A and B). Exclusion of bichir and sturgeon ARs from the teleost AR α/β clade is significantly supported by statistical analysis (e.g. bootstrap probability, 100 in ML analysis, Fig. 2A).
Previously, Douard et al. (21) inferred the molecular phylogeny of AR genes with an alignment of the limited sequences in LBD. By incorporating our current sequence information with their identified sequences, we also conducted molecular phylogenetic analysis for this region (Fig. S1). Based on the phylogenetic analysis with such short alignment from wider range of species, we observed the teleost-specific nature of the additional AR subtype (Fig. S1). However the branching point of the ARα was not unambiguously supported, as seen in the close relationship of the ARα genes to the Otocephala (e.g. zebrafish, catfish) and trout AR genes that are previously identified as ARβ (e.g. bootstrap probability, 23 in ML analysis) (Fig. S1).
The amino acid sequences of each domain of tetrapods ARs were much more similar to those of the teleost ARβs than to those of teleost ARαs. The higher level of divergence in amino acid sequences of teleost ARαs, judged by a long branch in molecular phylogenetic trees (Fig. 2 and supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), indicates that, after the duplication between ARα and ARβ, the coding sequence of ARα accumulated novel mutations at a greater rate than that of ARβ (Fig. 1C).
Chromosomal location in the medaka genome
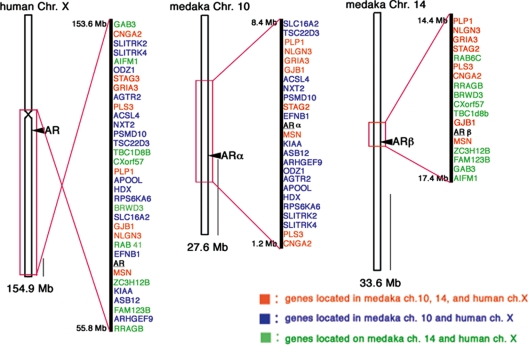
The timing of the gene duplication between ARα and ARβ is closely related to that of the TSGD, raising a question whether the AR gene duplication was involved in a duplication event involving a large chromosomal region or an entire chromosome or a small-scale gene duplication. By identifying the exons of AR genes in the medaka genome using Ensembl Genome Browser (http://www. ensembl.org/), ARα and ARβ were mapped on chromosome 10 and 14, respectively (Fig. 3). The human AR is located on chromosome X. Flanking regions of AR genes on medaka chromosome 10 and 14 contain genes orthologous to those located in syntenic regions on human chromosome X (Fig. 3). The recently reported medaka draft genome sequence indicated that medaka chromosome 10, 13, and 14 are derived from one ancestral chromosome, and one of the duplicated ancestral chromosomes became chromosome 14, whereas the other underwent a fission event, yielding chromosome 10 and 13 (23). This previously proposed observation is consistent with our inference that the teleost AR gene duplication occurred in association with the TSGD.
Figure 3.
Location of ARα and ARβ genes in the medaka genome. Loci of medaka ARα and ARβ genes were mapped on the chromosome 10 and 14, respectively. These chromosomes contain loci present on human chromosome X. Shown in red are genes located on medaka chromosome 10, 14, and human chromosome X. Genes located on medaka chromosome 10 and human X are shown in blue. Genes located on medaka chromosome 14 and human X are marked by green. Scale bar, 10 Mb.
Ligand-dependent transactivating function of brown-banded bambooshark AR in COS-7 cells
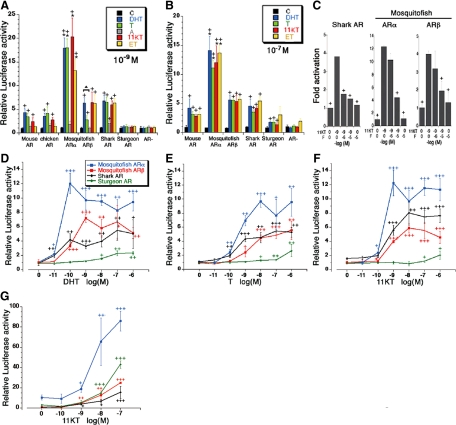
The AR regulates androgen target genes by binding to a specific DNA sequence, the androgen-response element (ARE) (39,40,41). To analyze the evolutionary conservation of AR gene function, we examined the androgen-dependent transactivating function of brown-banded bambooshark AR with COS-7 cells, transiently transfected with shark AR expression vector (pCMV-shark AR) together with the reporter constructs containing AREs (pGL3 PRE/ARE tk Luc) (Fig. 4, A–F). T, DHT, 11KT, and ET induced significant increase of luciferase activity via shark AR (Fig. 4, A and B). The minimum stimulatory dose of DHT, T, and 11KT was 10−11, 10−10, and 10−9 m, respectively (Fig. 4, D–F). The maximum stimulation was found at 10−8 m 11KT (Fig. 4F). 11KT-dependent transactivation of luciferase gene by shark AR was competitively inhibited by flutamide administration (Fig. 4C). The shark AR stimulated the luciferase acitivity at the treatment of 10−11 m DHT (Fig. 4D). These results indicate that the AR activated by the classical androgens as ligands was already possessed by the common ancestor of all extant jawed vertebrates.
Figure 4.
Ligand-dependent transcription capacities of ARs. Panels A and B, Ligand-dependent transactivation profiles of vertebrate ARs in COS-7 cells. The COS-7 cells were transfected with pCMV-mouse AR, pCMV-chicken AR, pCMV-western mosquitofish ARα, pCMV-western mosquitofish ARβ, pCMV-shark AR, pCMV-sturgeon AR, or pCS2-MT as control vector and pGL3 PRE/ARE tk Luc as a reporter plasmid. pRL-SV40 was used as an internal control to calculate the transfection efficiency. Transfected cells were treated with the 10−9 or 10−7 m of various androgens [DHT, T, androstenedione (A); 11KT, ET, or without control (C). The relative transcriptional activity of AR was shown as values normalized by the pRL-induced activities. Panel C, Competitive inhibition of androgen-dependent transactivation of shark AR and western mosquitofish ARα and ARβ by F treatment. Transfected cells were treated with the 10−9 m of 11KT and/or AR antagonist F (10−6 or 10−5 m). Panels D–F, Dose-response profiles of shark AR, sturgeon AR, and western mosquitofish ARα and ARβ activated by DHT, T, and 11KT. The transfected cells were incubated with increasing concentrations of androgens (10−11 to 10−6 m) or without ligand for 12 h. Panel G, Dose-response profiles of transactivating functions of ARs in medaka eggs. The embryos injected with pCMV-AR, the reporter plasmid, and pRL-SV40 were incubated with increasing concentrations of 11KT (10−10 to 10−7 m) or without ligand for 24 h. In each experiment, five embryos were used and all experiments were repeated more than three times. Plus signs (+) in panels A and B represent significant differences in responses, compared with controls (+, P < 0.05). A significant difference in AR activity based on the reporter activation was observed between ARα and ARβ. Asterisks on the data of ARα including panels A and B represent a significant difference in responses, compared with each data set of ARβ (*, P < 0.05). ▴ (panel A), Significant differences in responses between DHT and T treatments (P < 0.01); + (panel C), significant differences in responses, compared with the treatment of 10−9 m 11KT (+, P < 0.05). Vertical bars, Mean ± sd. + (D–G), Significant differences in responses, compared with controls. +, P < 0.05; ++, P < 0.01; +++, P < 0.001.
Phylogenetic distribution of protein motifs including AR genes
Multiple-alignment of AR sequences enables us to discuss the structural conservation and evolutionary diversification of the various protein motifs appeared in AR genes. In the DBD of the brown-banded bambooshark and sturgeon ARs, eight cysteine residues constituting the two zinc finger motifs are conserved (Fig. 1B and supplemental Fig. S2C), both of which are known as important for recognition and binding of the hormone-response element of the AR target genes (42). Amino acids that participate in possible interaction with DHT in human AR (N705, Q711, R752 and T877) (43) were conserved in AR LBDs of the brown-banded bambooshark and sturgeon as observed in other species ARs (the shark AR resides N589, Q595, R636, and T761; the sturgeon AR resides N628, Q634, R675, and T798).
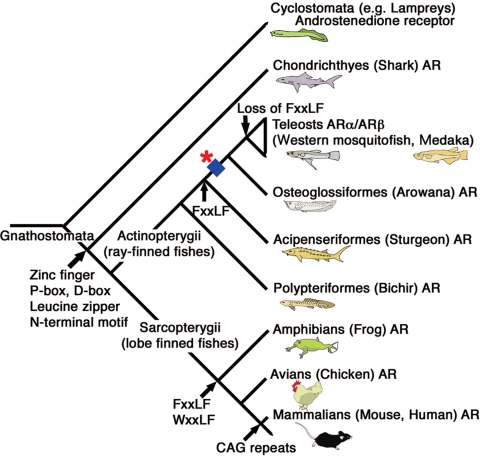
N-terminal domain (NTD) includes the transactivating domain termed AF-1 that is a strong regulator of transcription. However, its sequences are divergent in their length and primary sequences among species (Fig. 1, B and C). The NTD includes several conserved domains. A 14-amino acid N-terminal motif of human AR, which can interact with the heat shock protein-70-interacting protein, are highly conserved in teleost fishes and tetrapods ARs (44). This N-terminal conserved motif is contained in the brown-banded bambooshark AR (supplemental Fig. S2B), suggesting that this motif may have evolved in ancient AR before the divergence between the Actinopterygii and Sarcopterygii lineages. FxxLF motif at human AR N-terminal resides 23–27 is thought to facilitate AR dimerization (45,46,47). The functional significance of AR N/C interaction in vivo is supported by studies of naturally occurring AR mutations that result in androgen insensitivity syndrome (48,49,50). This motif is conserved as (F/Y)Q(N/S)(L/V)F from teleost fishes through primates, although some teleost fishes lack this motif (44). Our analysis also revealed that FxxLF motif was not included in the NTDs of brown-banded bambooshark, western mosquitofish, and medaka ARs, but sturgeon AR contained sequence FENVF at the corresponding region for human FxxLF (supplemental Fig. S2A). These results may indicate that the FxxLF motif was independently acquired in Actinopterygii and Sarcopterygii lineages or it may have evolved after the split of Chondrichthyes from the lineage leading to Sarcopterygii but before the divergence of Actinopterygii, and some teleost fish ARs might have lost these sequences (Fig. 6). Our results suggest that the AR N/C interaction may not be an absolute requirement for Chondrichthyes and teleost AR signaling. The interaction such as LBD dimerization, which has been described for all of the SRs (51,52,53,54,55), might possibly contribute to AR dimerization in Chondrichthyes and Teleostei.
Figure 6.
Composite phylogeny for vertebrates with the hypothesized scenario of AR evolution. The evolutionary tree illustrates that Chondrichthyes (shark) has the most ancient type of AR. The duplication event that gave rise to two different teleost ARs, ARα and ARβ, occurred in the actinopterygian lineage leading to teleosts after the divergence of Acipenseriformes (sturgeon) but before the split of Osteoglossiformes (arowana) (showed by an asterisk), which is associated with the TSGD (indicated by a blue square). Evolutionary appearance of protein motifs including AR genes estimated by the comparison of primary sequences of obtained ARs was designated.
Functional analysis of ray-finned fishes ARs by transient reporter assays
A comparison of the deduced amino acids sequences of DBD and LBD revealed that the current brown-banded bambooshark and sturgeon ARs share high similarities to teleost ARβ and tetrapod ARs, whereas the homologies were comparatively low to teleost ARα (Fig. 1C). To analyze the functional differences of ARα and ARβ, we examined the transactivating function of western mosquitofish ARα and ARβ using COS-7 cells. Both western mosquitofish ARα and ARβ activated the ARE-reporter gene expression by treatment with T, DHT, 11KT, and ET (Fig. 4, A and B). 11KT is known as the potent androgen in teleost fishes (17,56). 11KT stimulated luciferase activity through western mosquitofish ARα and ARβ (Fig. 4, A and B). The minimum stimulatory dose of 11KT was 10−10 m for both western mosquitofish ARα and ARβ (Fig. 4F). 11KT levels in human chorionic gonadotropin-treated and untreated Japanese eel males ranged from 8 × 10−10 to 2.6 × 10−8 m (56). Hence, the physiological dose of 11KT is thought to be sufficient to activate the target gene expression via both ARα and ARβ. Interestingly, the levels of transcriptional activation of the ARE reporter construct by western mosquitofish ARα were significantly higher than those by western mosquitofish ARβ (Fig. 4, A and B).
Recent investigations on Atlantic croaker, Micropogonias undulatus, indicate that the actions of different androgens may be mediated by distinct AR subtypes with tissue-specific expression (15). We compared the ligand selectivity of western mosquitofish ARα and ARβ expressed in COS-7 cells treated with two different concentrations of ligands (10−9 and 10−7 m). Both western mosquitofish ARα and ARβ could stimulate the reporter gene expressions by DHT, T, 11KT, and ET (10−9 m in Fig. 4A, 10−7 m in Fig. 4B). At the low ligand concentration (10−9 m), DHT is more effective than T in inducing transcriptional activity of ARβ, whereas similar levels of luciferase expression by T and DHT were observed in the ARα-expressing cells (Fig. 4A).
We isolated the AR cDNA from the hybrid sturgeon known as the bester. The ligand-dependent transactivation mediated by sturgeon AR was observed in the presence of 10−7 m DHT, T and 11KT (Fig. 4B) but not in the presence of 10−9 m of androgens (Fig. 4A). These results indicated that the identified sturgeon AR cDNA encodes a functional AR, but the potency is likely to be lower than other vertebrate ARs in COS-7 cells. Functional analysis of ARs in medaka eggs revealed that the sturgeon AR activates ARE with 10−8 m 11KT (Fig. 4G). It was reported that the level of 11KT concentration in male sturgeon serum increased more than the concentration of 10−7 m during the anadromous migration (57). Taken together, our findings indicated that the sturgeon AR might be activated by classical androgen in vivo.
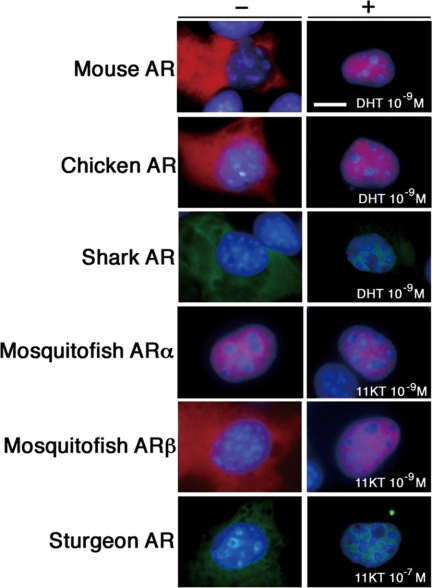
AR is generally known as expressed in the cytoplasm and translocates into the nucleus upon ligand stimulation (58,59,60). Intracellular localization of each species ARs was monitored by transient transfection assays with GFP- or DsRed-tagged AR expression vectors (Fig. 5). The shark, sturgeon, and western mosquitofish ARβ was found as translocating in the nucleus when the transfected COS-7 cells were treated with DHT or 11KT, as observed for the case of tetrapods ARs. In contrast, western mosquitofish ARα was constitutively located in the nucleus irrespective of 11KT stimulation. Similar nuclear localization was observed in the case of medaka ARs (supplemental Fig. S4). The western mosquitofish ARα transiently expressed in medaka eggs also exhibited the constitutive nuclear localization (supplemental Fig. S5)
Figure 5.
Different intracellular localization of teleost ARα compared with the pattern of teleost ARβ and other species ARs. The COS-7 cells were transiently transfected with pCMV-mouse AR, pCMV-chicken AR, pCMV-shark AR-GFP, pCMV-western mosquitofish ARα-DsRed, pCMV-western mosquitofish ARβ-DsRed, or pCMV-sturgeon AR-GFP and treated with or without DHT or 11KT. Shown images represent red fluorescence for immunostaining of mouse AR and chicken AR, DsRed-fused western mosquitofish ARα and ARβ; green fluorescence for GFP-fused sturgeon AR and shark AR; and blue fluorescence for nuclear staining by Hoechst 33342. Scale bar, 10 μm.
The signals involved in the nuclear import and export of SRs had been studied extensively. A nuclear localization signal (NLS) has been identified in the DBD and hinge region of human AR (61,62). This NLS is composed of two clusters of basic amino acids (underlined) separated by 10 amino acid residues: RKCYEAGMTLGARKLKK. It was conserved in the medaka ARβ and western mosquitofish ARβ as KKCFEAGMTLGARKLKK but not in these fishes’ ARα (supplemental Fig. S2C). Amino acid residues KRCFMSGMSLKGRRLKG were found in the corresponding region of western mosquitofish ARα. The NLS located in the LBD of NR is ligand dependent, but their precise location and sequences remain unknown (63,64). The nuclear export signal was identified in human AR DBD (65,66). The intracellular localization of western mosquitofish ARβ was not affected by exchanging its DBD to that of western mosquitofish ARα (supplemental Fig. S8A). Deletion of NTD did not change the intracellular localization of western mosquitofish ARα (supplemental Fig. S8B). The western mosquitofish ARα was located entirely in the cells, when its LBD was exchanged for that of western mosquitofish ARβ (supplemental Fig. S8C). Although further functional analyses are necessary to clarify the nuclear import and/or export mechanisms of the current ARs, highly evolved LBD sequences of western mosquitofish ARα may contribute to such a unique intracellular localization of western mosquitofish ARα.
Discussion
Most ancient type of AR can be activated by the classical androgens at the common ancestor of all extant jawed vertebrates
Evolutionary appearance of functional AR gene in the jawed vertebrate lineage might contribute to the expression of male secondary sexual characters that have diversified through sexual selection in vertebrates. Sharks serve as representatives of the Chondrichthyes, which are the most early-branching group of extant jawed vertebrates appeared approximately 450 million years ago (67). They develop highly differentiated internal and external reproductive organs. The sizes of the claspers become maximal when androgen levels rise (68). The testosterone-binding component of shark resembles physiochemically with the characteristics of classical AR (69). Recent report of the AR gene in sharks requires more structural and functional information on the evolution of ligand responsibility of AR (7,11,21). Structural comparison of the most ancestral type of AR with its homologs of other species enables us to infer the timing of acquisition of the protein motif contained in the AR gene. Amino acids for the possible interaction with DHT in human AR were conserved in the brown-banded bambooshark AR, implying the evolutionary conservation of ligand selectivity throughout all extant jawed vertebrates. The current functional analysis revealed that brown-banded bambooshark AR activates the target gene via ARE by classical androgens, T, DHT, and 11KT, but not by androstenedione. The lamprey androstenedione receptor is substantially different from ARs in other vertebrates because it appears to be attached to the cell membrane (26,70). Based on the currently available information, the most ancient type of AR activated by the classical androgens as ligands appeared at the common ancestor of all extant jawed vertebrates.
TSGD generated the second AR subtype in teleost fishes
In the ray-finned fish lineage, whole genome duplication occurred before the teleost fish radiation (71,72,73,74,75,76,77). Recently the term TSGD was introduced to accurately describe the timing of the additional genome duplication (78). This genome doubling may have facilitated the morphological diversification of teleost fishes (79).
NRs and Hox clusters have been thought as appropriate indicators of genome duplication (71,80,81,82,83). We estimated the timing of the AR gene duplication with molecular phylogeny including diverse jawed vertebrate lineages. We cloned one single set of AR orthologs shared by brown-banded bambooshark, bichir, sturgeon, and arowana, respectively. The arowana AR was included in the ARβ cluster (Fig. 2, A and B). Thus, it is likely that the arowana genome contains an unidentified ARα or had secondarily lost ARα. Both the ARα and ARβ of rainbow trout were included in the ARβ clade, suggesting that the duplication between rainbow trout ARα and ARβ occurred in the recent salmonid tetraploid event, estimated to have taken place 50 million to 100 million years ago (84). Apart from this lineage-specific gene duplication, our results clearly concluded that the gene duplication between ARα and ARβ occurred after early-branching actinopterygian fishes (bichir and sturgeon) diverged from the future teleost lineage (Fig. 2A). Douard et al. (21) previously explored the phylogenetic relationships among teleost AR genes and suggested that the gene duplication occurred before the radiation of all extant teleost fish lineages. We also analyzed this with our newly identified sequences. Under the probabilistic framework of the ML method, which was not taken into consideration in the previous study, the short alignment of sequences used in the previous study did not confidently support an orthology between Acanthomorpha ARβ and Otocephala ARβ (supplemental Fig. S1). Rather, this analysis suggested an orthology between ARα and Otocephala ARβ. Our phylogenetic analysis with a longer alignment provided more reliable results (Fig. 2B). Here an orthology between Acanthomorpha ARβ and Otocephala ARβ is supported in the ML tree (bootstrap probability; Fig. 2B; Ref. 38). This tree also suggests that the gene duplication between ARα and ARβ occurred before the radiation of all extant teleost fishes including Osteoglossiformes and Elopomorpha. Overall, our phylogenetic analyses revealed that the timing of the split between ARα and ARβ coincided with that of the TSGD. This is strengthened by the following discussion on chromosomal location of AR genes.
We identified the ARα and ARβ on medaka chromosome 10 and 14, respectively. Several duplicated genes at the proximity of ARα/β were present on these two chromosomes, with a conserved synteny relative to a single region in human chromosome X, respectively, indicating that the teleost AR gene duplication had occurred associating with chromosomal duplication. Hence, we concluded that the AR gene duplication that gave rise to the two different teleost AR subtypes probably occurred in the actinopterygian lineage leading to teleost fishes after the divergence of Acipenseriformes (sturgeon) and Actinopterygii but before the split of Osteoglossiformes (arowana) (Fig. 6), which supports its involvement in the TSGD (20,71,72,73,74,75,76,77,78,85,86).
Functionally diversified ARs have been evolved in the teleost lineage
Ohno (87) proposed that gene duplications facilitate the functional diversification of genes and generates the developmental and morphological complexity during evolution. The deduced amino acid sequences of western mosquitofish ARβ and medaka ARβ are highly similar to those of tetrapod ARs but much less to those of western mosquitofish ARα and medaka ARα. These results may indicate that the ARβs retain the original functions, whereas ARαs may acquire a new function. Recently it was suggested that the Otophysi and Salmonides might have lost the ARα gene and two distinct AR duplicates may possess functional differences judged by the comparison of the mutations present in the teleost ARs with those known to be implicated in human androgen insensitivity syndrome (21). However, the proposition of secondary loss of ARα gene was indicated merely by the failure to amplify the cDNA homologs by PCR and the absence of its information in the available expressed sequence tags of salmon and trout. This remains to be verified by whole-genome information of diverse species in this lineage.
Our functional analysis of AR genes revealed that both western mosquitofish ARα and ARβ retain a fundamental function as AR in mammalian cells and medaka embryos. Interestingly, western mosquitofish ARα showed the unique intracellular localization and the significantly higher transactivating capacity via ARE than that by western mosquitofish ARβ. The levels of reporter activation by western mosquitofish ARβ were more similar to those by tetrapod ARs, compared with those by western mosquitofish ARα. The western mosquitofish ARα and ARβ could either homo- or heterodimerize in vitro (supplemental Fig. S6 and S7). The tissue distributions of ARα and ARβ mRNAs were different in western mosquitofish (18). Although further investigation of these AR functions in vivo is necessary to clarify the functional importance of the heterodimerization of ARα and ARβ, there might be a possibility that ARα/β heterodimers might attribute to androgen signaling by combining functional properties of both partners, as observed in the case of ER α/β (88,89).
The contribution of duplicated genes to the origin of evolutionary novelties has been explained by the duplication-degeneration-complementation model (90). On the basis of primary sequences and characterization of ARs, it is estimated that the ARβ has properties similar to those of other vertebrate ARs, and the ARα evolved as different AR subtype in teleost lineage. Therein functionally diversified ARs have been evolved in the teleost lineage through processes of sub- and/or neofunctionalization at the levels of their structures, intracellular localization, and activity as transcription factors.
Supplemental data
Supplemental data include supplemental figure legends, eight figures, and three tables.
Supplementary Material
Acknowledgments
We thank Drs. Shigeru Kuratani, Yuji Ishikawa, Joachim Wittbrodt, Yoshitaka Nagahama, Taisen Iguchi, John A. McLachlan, Shigeaki Kato, Hisayo Nisida, Shinji Fukuda, Keisuke Tanida, Robert Grainger, Hajime Ogino, Masato Kinoshita, Shinichi Miyagawa, Kentaro Suzuki, and Mrs. Miho Matsumoto for their encouragement and help. We acknowledge Miyazaki prefectural fisheries experimental station for providing us sturgeon samples.
Footnotes
This study was supported by Grant-in-Aid for Young Scientists B 19770197 and for Scientific Research on Priority Areas: Mechanisms of Sex Differentiation and General Promotion of Cancer Research in Japan; the Global Centers of Excellence (GCOE) Research Program, Cell Fate Regulation Research and Education Unit; Grant 17-2, 20-3 for Child Health and Development from the Ministry of Health, Labor, and Welfare, Japan. This work was also supported by National Institutes of Health Grant R01-ES016597-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: AR, Androgen receptor; ARE, androgen-response element; CR, corticoid receptor; DBD, DNA binding domain; DHT, 5α-dihydrotestosterone; ER, estrogen receptor; ET, ethynyl T; F, flutamide; GFP, green fluorescent protein; 11KT, 11ketotestosterone; LBD, ligand binding domain; ML, maximum-likelihood; NLS, nuclear localization signal; NR, nuclear receptor; NTD, N-terminal domain; SR, steroid receptor; T, testosterone; TSGD, teleost-specific genome duplication.
References
- Behringer RR, Eakin GS, Renfree MB 2006 Mammalian diversity: gametes, embryos and reproduction. Reprod Fertil Dev 18:99–107 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995 The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ME 1997 Steroid receptor phylogeny and vertebrate origins. Mol Cell Endocrinol 135:101–107 [DOI] [PubMed] [Google Scholar]
- Laudet V 1997 Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J Mol Endocrinol 19:207–226 [DOI] [PubMed] [Google Scholar]
- Thornton JW, Kelley DB 1998 Evolution of the androgen receptor: structure-function implications. Bioessays 20:860–869 [DOI] [PubMed] [Google Scholar]
- Escriva H, Delaunay F, Laudet V 2000 Ligand binding and nuclear receptor evolution. Bioessays 22:717–727 [DOI] [PubMed] [Google Scholar]
- Escriva H, Safi R, Hänni C, Langlois MC, Saumitou-Laprade P, Stehelin D, Capron A, Pierce R, Laudet V 1997 Ligand binding was acquired during evolution of nuclear receptors. Proc Natl Acad Sci USA 94:6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ME 2001 Adrenal and sex steroid receptor evolution: environmental implications. J Mol Endocrinol 26:119–125 [DOI] [PubMed] [Google Scholar]
- Thornton JW 2001 Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci USA 98:5671–5676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ME 2002 Albumin, steroid hormones and the origin of vertebrates. J Endocrinol 175:121–127 [DOI] [PubMed] [Google Scholar]
- Bridgham JT, Carroll SM, Thornton JW 2006 Evolution of hormone-receptor complexity by molecular exploitation. Science 312:97–101 [DOI] [PubMed] [Google Scholar]
- Paris M, Pettersson K, Schubert M, Bertrand S, Pongratz I, Escriva H, Laudet V 2008 An amphioxus orthologue of the estrogen receptor that does not bind estradiol: insights into estrogen receptor evolution. BMC Evol Biol 8:219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo T, Ikeuchi T, Kobayashi T, Nagahama Y 1999 Fish androgen receptor: cDNA cloning, steroid activation of transcription in transfected mammalian cells, and tissue mRNA levels. Biochem Biophys Res Commun 254:378–383 [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Todo T, Kobayashi T, Nagahama Y 1999 cDNA cloning of a novel androgen receptor subtype. J Biol Chem 274:25205–25209 [DOI] [PubMed] [Google Scholar]
- Sperry TS, Thomas P 1999 Characterization of two nuclear androgen receptors in Atlantic croaker: comparison of their biochemical properties and binding specificities. Endocrinology 140:1602–1611 [DOI] [PubMed] [Google Scholar]
- Takeo J, Yamashita S 1999 Two distinct isoforms of cDNA encoding rainbow trout androgen receptors. J Biol Chem 274:5674–5680 [DOI] [PubMed] [Google Scholar]
- Borg B 1994 Androgens in teleost fishes. Comp Biochem Physiol 109C:219–245 [Google Scholar]
- Ogino Y, Katoh H, Yamada G 2004 Androgen dependent development of a modified anal fin, gonopodium, as a model to understand the mechanism of secondary sexual character expression in vertebrates. FEBS Lett 575:119–126 [DOI] [PubMed] [Google Scholar]
- Wittbrodt J, Meyer A, Schartl M 1998 More genes in fish? Bioessays 20:511–515 [Google Scholar]
- Hoegg S, Brinkmann H, Taylor JS, Meyer A 2004 Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. J Mol Evol 59:190–203 [DOI] [PubMed] [Google Scholar]
- Douard V, Brunet F, Boussau B, Ahrens-Fath I, Vlaeminck-Guillem V, Haendler B, Laudet V, Guiguen Y 2008 The fate of the duplicated androgen receptor in fishes: a late neofunctionalization event? BMC Evol Biol 8:336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh B, Kirkness EF, Loh YH, Halpern AL, Lee AP, Johnson J, Dandona N, Viswanathan LD, Tay A, Venter JC, Strausberg RL, Brenner S 2007 Survey sequencing and comparative analysis of the elephant shark (Callorhinchus milii) genome. PLoS Biol 5:e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M, Naruse K, Sasaki S, Nakatani Y, Qu W, Ahsan B, Yamada T, Nagayasu Y, Doi K, Kasai Y, Jindo T, Kobayashi D, Shimada A, Toyoda A, Kuroki Y, Fujiyama A, Sasaki T, Shimizu A, Asakawa S, Shimizu N, Hashimoto S, Yang J, Lee Y, Matsushima K, Sugano S, et al. 2007 The medaka draft genome and insights into vertebrate genome evolution. Nature 447:714–719 [DOI] [PubMed] [Google Scholar]
- Leihy MW, Shaw G, Wilson JD, Renfree MB 2004 Penile development is initiated in the tammar wallaby pouch young during the period when 5α-androstane-3α,17β-diol is secreted by the testes. Endocrinology 145:3346–3352 [DOI] [PubMed] [Google Scholar]
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16:271–321 [DOI] [PubMed] [Google Scholar]
- Bryan MB, Scott AP, Li W 2007 The sea lamprey (Petromyzon marinus) has a receptor for androstenedione. Biol Reprod 77:688–696 [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M 1987 The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425 [DOI] [PubMed] [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T 2005 MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O 2003 A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704 [DOI] [PubMed] [Google Scholar]
- Felsenstein J 1985 Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP 2003 MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ 1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Weintraub H 1994 Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev 8:1434–1447 [DOI] [PubMed] [Google Scholar]
- Rupp RA, Snider L, Weintraub H 1994 Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev 8:1311–1323 [DOI] [PubMed] [Google Scholar]
- Katoh H, Ogino Y, Yamada G 2006 Cloning and expression analysis of androgen receptor gene in chicken embryogenesis. FEBS Lett 580:1607–1615 [DOI] [PubMed] [Google Scholar]
- Matsui D, Sakari M, Sato T, Murayama A, Takada I, Kim M, Takeyama K, Kato S 2002 Transcriptional regulation of the mouse steroid 5α-reductase type II gene by progesterone in brain. Nucleic Acids Res 30:1387–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Ozato K 1995 Cytoplasmic microinjection of DNA into fertilized medaka (Oryzias latipes) eggs. Fish Biol J Medaka 7:59–64 [Google Scholar]
- Krust A, Green S, Argos P, Kumar V, Walter P, Bornert JM, Chambon P 1986 The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J 5:891–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C, Westphal HM, Carlson C, Bosshard H, Beato M 1986 Molecular model of the interaction between the glucocorticoid receptor and the regulatory elements of inducible genes. DNA 5:383–391 [DOI] [PubMed] [Google Scholar]
- Strähle U, Klock G, Schütz G 1987 A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc Natl Acad Sci USA 84:7871–7875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham J, Thomson A, Needham M, Webb P, Parker M 1988 Characterization of response elements for androgens, glucocorticoids and progestins in mouse mammary tumour virus. Nucleic Acids Res 16:5263–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K, Evans RM 1989 Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell 57:1139–1146 [DOI] [PubMed] [Google Scholar]
- Sack JS, Kish KF, Wang C, Attar RM, Kiefer SE, An Y, Wu GY, Scheffler JE, Salvati ME, Krystek Jr SR, Weinmann R, Einspahr HM 2001 Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc Natl Acad Sci USA 98:4904–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Bai S, Hnat AT, Kalman RI, Minges JT, Patterson C, Wilson EM 2004 An androgen receptor NH2-terminal conserved motif interacts with the COOH terminus of the Hsp70-interacting protein (CHIP). J Biol Chem 279:30643–30653 [DOI] [PubMed] [Google Scholar]
- Langley E, Kemppainen JA, Wilson EM 1998 Intermolecular NH2-/carboxyl-terminal interactions in androgen receptor dimerization revealed by mutations that cause androgen insensitivity. J Biol Chem 273:92–101 [DOI] [PubMed] [Google Scholar]
- He B, Kemppainen JA, Wilson EM 2000 FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J Biol Chem 275:22986–22994 [DOI] [PubMed] [Google Scholar]
- Hur E, Pfaff SJ, Payne ES, Grøn H, Buehrer BM, Fletterick RJ 2004 Recognition and accommodation at the androgen receptor coactivator binding interface. PLoS Biol 2:E274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Saatcioglu F, Jänne OA, Palvimo JJ 2001 Disrupted amino- and carboxyl-terminal interactions of the androgen receptor are linked to androgen insensitivity. Mol Endocrinol 15:923–935 [DOI] [PubMed] [Google Scholar]
- Ghali SA, Gottlieb B, Lumbroso R, Beitel LK, Elhaji Y, Wu J, Pinsky L, Trifiro MA 2003 The use of androgen receptor amino/carboxyl-terminal interaction assays to investigate androgen receptor gene mutations in subjects with varying degrees of androgen insensitivity. J Clin Endocrinol Metab 88:2185–2193 [DOI] [PubMed] [Google Scholar]
- Jääskelainen J, Deeb A, Schwabe JW, Mongan NP, Martin H, Hughes IA 2006 Human androgen receptor gene ligand-binding-domain mutations leading to disrupted interaction between the N- and C-terminal domains. J Mol Endocrinol 36:361–368 [DOI] [PubMed] [Google Scholar]
- Savory JG, Préfontaine GG, Lamprecht C, Liao M, Walther RF, Lefebvre YA, Haché RJ 2001 Glucocorticoid receptor homodimers and glucocorticoid-mineralocorticoid receptor heterodimers form in the cytoplasm through alternative dimerization interfaces. Mol Cell Biol 21:781–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell SE, Lees JA, White R, Parker MG 1990 Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell 60:953–962 [DOI] [PubMed] [Google Scholar]
- Bledsoe RK, Montana VG, Stanley TB, Delves CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson TM, Lambert MH, Moore JT, Pearce KH, Xu HE 2002 Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110:93–105 [DOI] [PubMed] [Google Scholar]
- Williams SP, Sigler PB 1998 Atomic structure of progesterone complexed with its receptor. Nature 393:392–396 [DOI] [PubMed] [Google Scholar]
- Nemoto T, Ohara-Nemoto Y, Shimazaki S, Ota M 1994 Dimerization characteristics of the DNA- and steroid-binding domains of the androgen receptor. J Steroid Biochem Mol Biol 50:225–233 [DOI] [PubMed] [Google Scholar]
- Miura T, Yamauchi K, Takahashi H, Nagahama Y 1991 Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). Proc Natl Acad Sci USA 88:5774–5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barannikova IA, Dyubin VP, Bayunova LV, Semenkova TB 2002 Steroids in control of reproductive function in fish. Neurosci Behav Physiol 32:141–148 [DOI] [PubMed] [Google Scholar]
- Georget V, Lobaccaro JM, Terouanne B, Mangeat P, Nicolas JC, Sultan C 1997 Trafficking of the androgen receptor in living cells with fused green fluorescent protein-androgen receptor. Mol Cell Endocrinol 129:17–26 [DOI] [PubMed] [Google Scholar]
- Simental JA, Sar M, Lane MV, French FS, Wilson EM 1991 Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem 266:510–518 [PubMed] [Google Scholar]
- Roy AK, Tyagi RK, Song CS, Lavrovsky Y, Ahn SC, Oh TS, Chatterjee B 2001 Androgen receptor: structural domains and functional dynamics after ligand-receptor interaction. Ann NY Acad Sci 949:44–57 [DOI] [PubMed] [Google Scholar]
- Jenster G, Trapman J, Brinkmann AO 1993 Nuclear import of the human androgen receptor. Biochem J 293(Pt 3):761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZX, Sar M, Simental JA, Lane MV, Wilson EM 1994 A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J Biol Chem 269:13115–13123 [PubMed] [Google Scholar]
- Picard D, Yamamoto KR 1987 Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J 6:3333–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin-Delannoy S, Thénot S, Delaunay F, Buisine E, Begue A, Duterque-Coquillaud M, Laudet V 2003 A specific and unusual nuclear localization signal in the DNA binding domain of the Rev-erb orphan receptors. J Mol Endocrinol 30:197–211 [DOI] [PubMed] [Google Scholar]
- Black BE, Holaska JM, Rastinejad F, Paschal BM 2001 DNA binding domains in diverse nuclear receptors function as nuclear export signals. Curr Biol 11:1749–1758 [DOI] [PubMed] [Google Scholar]
- Saporita AJ, Zhang Q, Navai N, Dincer Z, Hahn J, Cai X, Wang Z 2003 Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J Biol Chem 278:41998–42005 [DOI] [PubMed] [Google Scholar]
- Kumar S, Hedges SB 1998 A molecular timescale for vertebrate evolution. Nature 392:917–920 [DOI] [PubMed] [Google Scholar]
- Garnier DH, Sourdaine P, Jégou B 1999 Seasonal variations in sex steroids and male sexual characteristics in Scyliorhinus canicula. Gen Comp Endocrinol 116:281–290 [DOI] [PubMed] [Google Scholar]
- Cuevas ME, Callard G 1992 Androgen and progesterone receptors in shark (Squalus) testis: characteristics and stage-related distribution. Endocrinology 130:2173–2182 [DOI] [PubMed] [Google Scholar]
- Bryan MB, Scott AP, Li W 2008 Sex steroids and their receptors in lampreys. Steroids 73:1–12 [DOI] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH 1998 Zebrafish hox clusters and vertebrate genome evolution. Science 282:1711–1714 [DOI] [PubMed] [Google Scholar]
- Amores A, Suzuki T, Yan YL, Pomeroy J, Singer A, Amemiya C, Postlethwait JH 2004 Developmental roles of pufferfish Hox clusters and genome evolution in ray-fin fish. Genome Res 14:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Schartl M 1999 Gene and genome duplications in vertebrates: the one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr Opin Cell Biol 11:699–704 [DOI] [PubMed] [Google Scholar]
- Taylor JS, Van de Peer Y, Braasch I, Meyer A 2001 Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos Trans R Soc Lond B Biol Sci 356:1661–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JS, Van de Peer Y, Meyer A 2001 Revisiting recent challenges to the ancient fish-specific genome duplication hypothesis. Curr Biol 11:R1005–R1008 [DOI] [PubMed] [Google Scholar]
- Taylor JS, Braasch I, Frickey T, Meyer A, Van de Peer Y 2003 Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res 13:382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Peer Y, Frickey T, Taylor J, Meyer A 2002 Dealing with saturation at the amino acid level: a case study based on anciently duplicated zebrafish genes. Gene 295:205–211 [DOI] [PubMed] [Google Scholar]
- Kuraku S, Meyer A 2009 The evolution and maintenance of Hox gene clusters in vertebrates and the teleost-specific genome duplication. Int J Dev Biol 53:765–773 [DOI] [PubMed] [Google Scholar]
- Volff JN 2005 Genome evolution and biodiversity in teleost fish. Heredity 94:280–294 [DOI] [PubMed] [Google Scholar]
- Escrivá García H, Laudet V, Robinson-Rechavi M 2003 Nuclear receptors are markers of animal genome evolution. J Struct Funct Genomics 3:177–184 [PubMed] [Google Scholar]
- Postlethwait JH, Woods IG, Ngo-Hazelett P, Yan YL, Kelly PD, Chu F, Huang H, Hill-Force A, Talbot WS 2000 Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res 10:1890–1902 [DOI] [PubMed] [Google Scholar]
- Graham A, Papalopulu N, Krumlauf R 1989 The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell 57:367–378 [DOI] [PubMed] [Google Scholar]
- Garcia-Fernández J, Holland PW 1994 Archetypal organization of the amphioxus Hox gene cluster. Nature 370:563–566 [DOI] [PubMed] [Google Scholar]
- Allendorf FW, Thorgaard GH 1984 Tetraploidy and the evolution of Salmonid fishes. Evolutionary genetics of fishes. New York: Plenum Publishing Corp.; 1–53 [Google Scholar]
- Cresko WA, Yan YL, Baltrus DA, Amores A, Singer A, Rodríguez-Marí A, Postlethwait JH 2003 Genome duplication, subfunction partitioning, and lineage divergence: Sox9 in stickleback and zebrafish. Dev Dyn 228:480–489 [DOI] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Brunet F, Petit JL, Stange-Thomann N, Mauceli E, Bouneau L, Fischer C, Ozouf-Costaz C, Bernot A, Nicaud S, Jaffe D, Fisher S, Lutfalla G, Dossat C, Segurens B, Dasilva C, Salanoubat M, Levy M, Boudet N, Castellano S, Anthouard V, Jubin C, Castelli V, Katinka M, Vacherie B, Biémont C, Skalli Z, Cattolico L, Poulain J, De Berardinis V, Cruaud C, Duprat S, Brottier P, Coutanceau JP, Gouzy J, Parra G, Lardier G, Chapple C, McKernan KJ, McEwan P, Bosak S, Kellis M, Volff JN, Guigó R, Zody MC, Mesirov J, Lindblad-Toh K, Birren B, Nusbaum C, Kahn D, Robinson-Rechavi M, Laudet V, Schachter V, Quétier F, Saurin W, Scarpelli C, Wincker P, Lander ES, Weissenbach J, Roest Crollius H 2004 Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 431:946–957 [DOI] [PubMed] [Google Scholar]
- Ohno S 1970 Evolution of gene duplication. New York: Springer Verlag [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG, Gustafsson JA 1997 Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Mol Endocrinol 11:1486–1496 [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Labrie F, Giguère V 1999 Dominant activity of activation function 1 (AF-1) and differential stoichiometric requirements for AF-1 and -2 in the estrogen receptor α-β heterodimeric complex. Mol Cell Biol 19:1919–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J 1999 Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151:1531–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HA, Strimmer K, Vingron M, von Haeseler A 2002 TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18:502–504 [DOI] [PubMed] [Google Scholar]
- Azuma Y, Kumazawa Y, Miya M, Mabuchi K, Nishida M 2008 Mitogenomic evaluation of the historical biogeography of cichlids toward reliable dating of teleostean divergences. BMC Evol Biol 8:215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.