Abstract
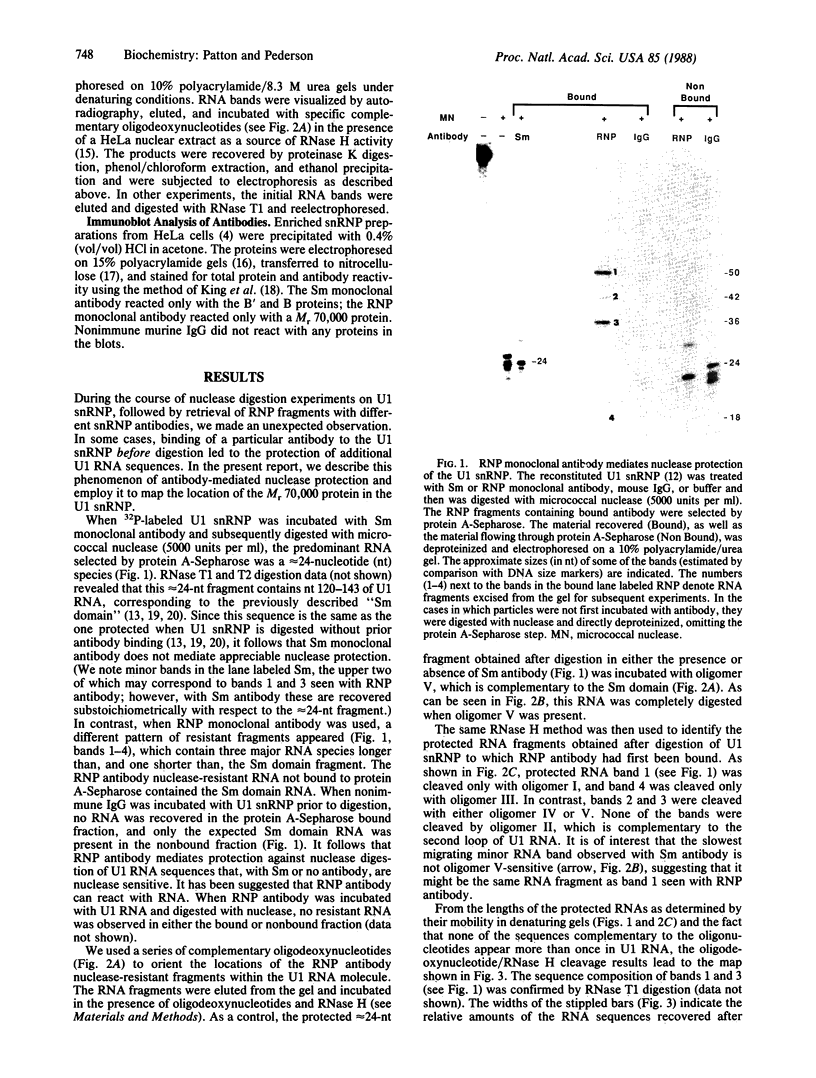
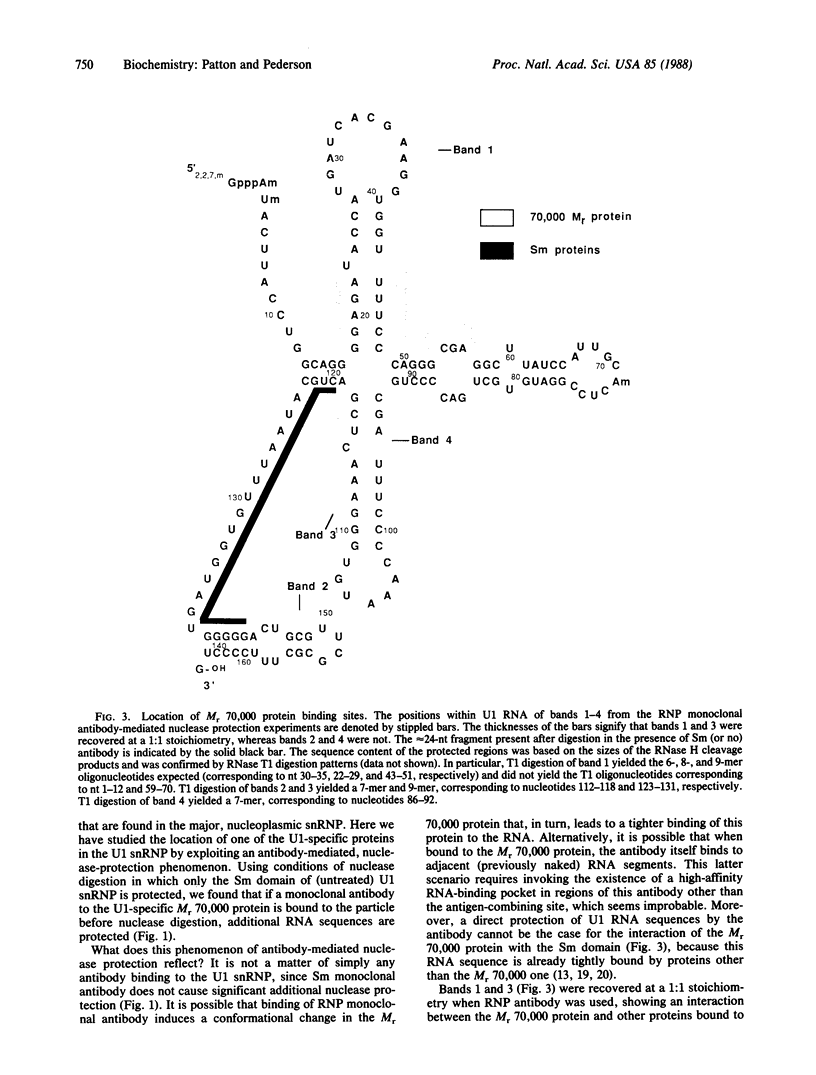
The U1 small nuclear ribonucleoprotein (snRNP) particle, a cofactor in mRNA splicing, contains nine proteins, six of which are also present in other U snRNPs and three of which are specific to the U1 snRNP. Here we have used a reconstituted human U1 snRNP together with snRNP monoclonal antibodies to define the RNA binding sites of one of the U1 snRNP-specific proteins. When Sm monoclonal antibody (specific for the B', B, and D proteins of U snRNPs) was bound to U1 snRNPs prior to micrococcal nuclease digestion, the same approximately equal to 24 nucleotide fragment of U1 RNA (corresponding to nucleotides 120-143 and termed the "Sm domain") was protected as when no antibody was bound prior to digestion. In contrast, when RNP monoclonal antibody, which reacts with the U1 snRNP-specific Mr 70,000 protein, was bound, additional U1 RNA regions were protected against nuclease digestion. This phenomenon, which we term "antibody-mediated nuclease protection," was exploited to map the position of the Mr 70,000 protein to stem-loop I of U1 RNA. However, there were also sites of Mr 70,000 protein interaction with more 3'-ward regions of U1 RNA, particularly the Sm domain. This indicates that in the three-dimensional structure of the U1 snRNP, the RNP and Sm antigens are in contact with each other. The proximity of the Mr 70,000 protein's RNA binding site (stem-loop I) to the functionally important 5' end of U1 RNA suggests that this protein may be involved in the recognition of, or stabilization of base pairing with, pre-mRNA 5' splice sites.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billings P. B., Allen R. W., Jensen F. C., Hoch S. O. Anti-RNP monoclonal antibodies derived from a mouse strain with lupus-like autoimmunity. J Immunol. 1982 Mar;128(3):1176–1180. [PubMed] [Google Scholar]
- Billings P. B., Hoch S. O. Characterization of U small nuclear RNA-associated proteins. J Biol Chem. 1984 Oct 25;259(20):12850–12856. [PubMed] [Google Scholar]
- Bringmann P., Lührmann R. Purification of the individual snRNPs U1, U2, U5 and U4/U6 from HeLa cells and characterization of their protein constituents. EMBO J. 1986 Dec 20;5(13):3509–3516. doi: 10.1002/j.1460-2075.1986.tb04676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P., Reddy R., Busch H. Site-specific cleavage by T1 RNase of U-1 RNA in u-1 ribonucleoprotein particles. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1562–1566. doi: 10.1073/pnas.78.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. E., Conner G. E., Reeves W. H., Wisniewolski R., Blobel G. Small nuclear ribonucleoprotein particle assembly in vivo: demonstration of a 6S RNA-free core precursor and posttranslational modification. Cell. 1985 Oct;42(3):751–758. doi: 10.1016/0092-8674(85)90271-5. [DOI] [PubMed] [Google Scholar]
- Gundelfinger E. D., Krause E., Melli M., Dobberstein B. The organization of the 7SL RNA in the signal recognition particle. Nucleic Acids Res. 1983 Nov 11;11(21):7363–7374. doi: 10.1093/nar/11.21.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J., Kazmaier M., Mattaj I. W. In vitro assembly of U1 snRNPs. EMBO J. 1987 Nov;6(11):3479–3485. doi: 10.1002/j.1460-2075.1987.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberger M., Pettersson I., Steitz J. A. Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J Biol Chem. 1983 Feb 25;258(4):2604–2613. [PubMed] [Google Scholar]
- King S. M., Otter T., Witman G. B. Purification and characterization of Chlamydomonas flagellar dyneins. Methods Enzymol. 1986;134:291–306. doi: 10.1016/0076-6879(86)34097-7. [DOI] [PubMed] [Google Scholar]
- Kinlaw C. S., Dusing-Swartz S. K., Berget S. M. Human U1 and U2 small nuclear ribonucleoproteins contain common and unique polypeptides. Mol Cell Biol. 1982 Oct;2(10):1159–1166. doi: 10.1128/mcb.2.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlaw C. S., Robberson B. L., Berget S. M. Fractionation and characterization of human small nuclear ribonucleoproteins containing U1 and U2 RNAs. J Biol Chem. 1983 Jun 10;258(11):7181–7189. [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987 Jun 19;49(6):763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T. Multiple factors including the small nuclear ribonucleoproteins U1 and U2 are necessary for pre-mRNA splicing in vitro. Cell. 1985 Oct;42(3):725–736. doi: 10.1016/0092-8674(85)90269-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Lin W. L., Pederson T. Ribonucleoprotein organization of eukaryotic RNA. XXXI. Structure of the U1 small nuclear ribonucleoprotein. J Mol Biol. 1984 Dec 25;180(4):947–960. doi: 10.1016/0022-2836(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Otter T., King S. M., Witman G. B. A two-step procedure for efficient electrotransfer of both high-molecular-weight (greater than 400,000) and low-molecular-weight (less than 20,000) proteins. Anal Biochem. 1987 May 1;162(2):370–377. doi: 10.1016/0003-2697(87)90406-4. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Mount S. M., Steitz J. A., Sharp P. A. Splicing of messenger RNA precursors is inhibited by antisera to small nuclear ribonucleoprotein. Cell. 1983 Nov;35(1):101–107. doi: 10.1016/0092-8674(83)90212-x. [DOI] [PubMed] [Google Scholar]
- Patton J. R., Patterson R. J., Pederson T. Reconstitution of the U1 small nuclear ribonucleoprotein particle. Mol Cell Biol. 1987 Nov;7(11):4030–4037. doi: 10.1128/mcb.7.11.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson I., Hinterberger M., Mimori T., Gottlieb E., Steitz J. A. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984 May 10;259(9):5907–5914. [PubMed] [Google Scholar]
- Reveillaud I., Lelay-Taha M. N., Sri-Widada J., Brunel C., Jeanteur P. Mg2+ induces a sharp and reversible transition in U1 and U2 small nuclear ribonucleoprotein configurations. Mol Cell Biol. 1984 Sep;4(9):1890–1899. doi: 10.1128/mcb.4.9.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyono B., Pederson T. Ribonucleoprotein organization of eukaryotic RNA. XXX. Evidence that U1 small nuclear RNA is a ribonucleoprotein when base-paired with pre-messenger RNA in vivo. J Mol Biol. 1984 Apr 5;174(2):285–295. doi: 10.1016/0022-2836(84)90339-5. [DOI] [PubMed] [Google Scholar]
- Tatei K., Takemura K., Tanaka H., Masaki T., Ohshima Y. Recognition of 5' and 3' splice site sequences in pre-mRNA studied with a filter binding technique. J Biol Chem. 1987 Aug 25;262(24):11667–11674. [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Wieben E. D., Madore S. J., Pederson T. Protein binding sites are conserved in U1 small nuclear RNA from insects and mammals. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1217–1220. doi: 10.1073/pnas.80.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieben E. D., Pederson T. Small nuclear ribonucleoproteins of Drosophila: identification of U1 RNA-associated proteins and their behavior during heat shock. Mol Cell Biol. 1982 Aug;2(8):914–920. doi: 10.1128/mcb.2.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in U1 snRNA suppresses a 5' splice site mutation. Cell. 1986 Sep 12;46(6):827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]