Abstract
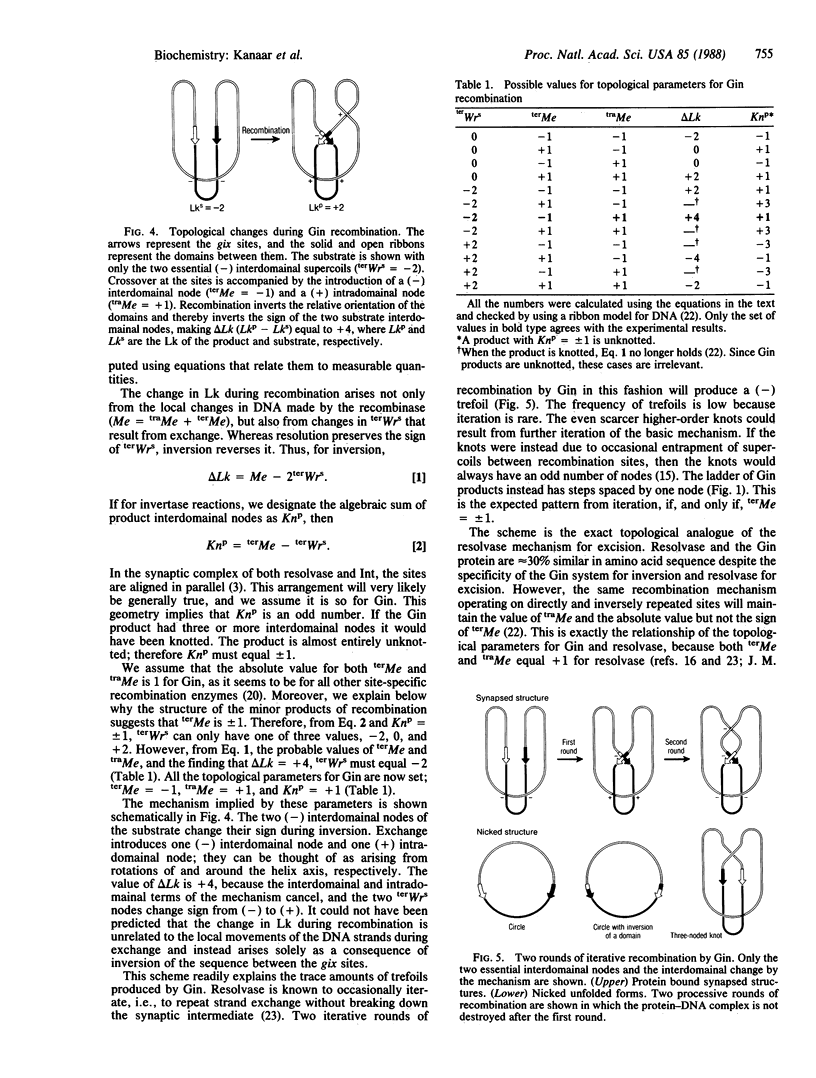
Inversion of the G loop of bacteriophage Mu requires the phage-encoded Gin protein and a host factor. The topological changes in a supercoiled DNA substrate generated by the two purified proteins were analyzed. More than 99% of the inversion products were unknotted rings. This result excludes synapsis by way of a random collision of recombination sites, because the resulting entrapped supercoils would be converted into knots by recombination. Instead, the recombination sites must come together in the synaptic complex in an ordered fashion with a fixed number of supercoils between the sites. The linking number of the substrate DNA increases by four during recombination. Thus, in three successive rounds of inversion, the change in linking number was +4, +8, and +12, respectively. These results lead to a quantitative model for the mechanism of Gin recombination that includes the distribution of supercoils in the synaptic complex, their alteration by strand exchange, and specific roles for the two proteins needed for recombination.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abremski K., Frommer B., Hoess R. H. Linking-number changes in the DNA substrate during Cre-mediated loxP site-specific recombination. J Mol Biol. 1986 Nov 5;192(1):17–26. doi: 10.1016/0022-2836(86)90460-2. [DOI] [PubMed] [Google Scholar]
- Benjamin H. W., Matzuk M. M., Krasnow M. A., Cozzarelli N. R. Recombination site selection by Tn3 resolvase: topological tests of a tracking mechanism. Cell. 1985 Jan;40(1):147–158. doi: 10.1016/0092-8674(85)90318-6. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Krasnow M. A., Gerrard S. P., White J. H. A topological treatment of recombination and topoisomerases. Cold Spring Harb Symp Quant Biol. 1984;49:383–400. doi: 10.1101/sqb.1984.049.01.045. [DOI] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K. Role of DNA topology in Mu transposition: mechanism of sensing the relative orientation of two DNA segments. Cell. 1986 Jun 20;45(6):793–800. doi: 10.1016/0092-8674(86)90554-4. [DOI] [PubMed] [Google Scholar]
- Dean F. B., Stasiak A., Koller T., Cozzarelli N. R. Duplex DNA knots produced by Escherichia coli topoisomerase I. Structure and requirements for formation. J Biol Chem. 1985 Apr 25;260(8):4975–4983. [PubMed] [Google Scholar]
- Grindley N. D., Reed R. R. Transpositional recombination in prokaryotes. Annu Rev Biochem. 1985;54:863–896. doi: 10.1146/annurev.bi.54.070185.004243. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Simon M. I. Hin-mediated site-specific recombination requires two 26 bp recombination sites and a 60 bp recombinational enhancer. Cell. 1985 Jul;41(3):781–791. doi: 10.1016/s0092-8674(85)80059-3. [DOI] [PubMed] [Google Scholar]
- Kahmann R., Rudt F., Koch C., Mertens G. G inversion in bacteriophage Mu DNA is stimulated by a site within the invertase gene and a host factor. Cell. 1985 Jul;41(3):771–780. doi: 10.1016/s0092-8674(85)80058-1. [DOI] [PubMed] [Google Scholar]
- Kanaar R., van de Putte P., Cozzarelli N. R. Purification of the Gin recombination protein of Escherichia coli phage Mu and its host factor. Biochim Biophys Acta. 1986 Mar 26;866(2-3):170–177. doi: 10.1016/0167-4781(86)90114-4. [DOI] [PubMed] [Google Scholar]
- Koch C., Kahmann R. Purification and properties of the Escherichia coli host factor required for inversion of the G segment in bacteriophage Mu. J Biol Chem. 1986 Nov 25;261(33):15673–15678. [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Site-specific relaxation and recombination by the Tn3 resolvase: recognition of the DNA path between oriented res sites. Cell. 1983 Apr;32(4):1313–1324. doi: 10.1016/0092-8674(83)90312-4. [DOI] [PubMed] [Google Scholar]
- Nash H. A., Pollock T. J. Site-specific recombination of bacteriophage lambda. The change in topological linking number associated with exchange of DNA strands. J Mol Biol. 1983 Oct 15;170(1):19–38. doi: 10.1016/s0022-2836(83)80225-3. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Ilmer T. A., Van de Putte P. Site-specific recombination by Gin of bacteriophage Mu: inversions and deletions. Virology. 1983 May;127(1):24–36. doi: 10.1016/0042-6822(83)90367-7. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Van de Putte P. Genetic switches by DNA inversions in prokaryotes. Biochim Biophys Acta. 1984 Jun 16;782(2):111–119. doi: 10.1016/0167-4781(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Pollock T. J., Nash H. A. Knotting of DNA caused by a genetic rearrangement. Evidence for a nucleosome-like structure in site-specific recombination of bacteriophage lambda. J Mol Biol. 1983 Oct 15;170(1):1–18. doi: 10.1016/s0022-2836(83)80224-1. [DOI] [PubMed] [Google Scholar]
- Spengler S. J., Stasiak A., Cozzarelli N. R. The stereostructure of knots and catenanes produced by phage lambda integrative recombination: implications for mechanism and DNA structure. Cell. 1985 Aug;42(1):325–334. doi: 10.1016/s0092-8674(85)80128-8. [DOI] [PubMed] [Google Scholar]
- Wasserman S. A., Cozzarelli N. R. Biochemical topology: applications to DNA recombination and replication. Science. 1986 May 23;232(4753):951–960. doi: 10.1126/science.3010458. [DOI] [PubMed] [Google Scholar]
- Wasserman S. A., Dungan J. M., Cozzarelli N. R. Discovery of a predicted DNA knot substantiates a model for site-specific recombination. Science. 1985 Jul 12;229(4709):171–174. doi: 10.1126/science.2990045. [DOI] [PubMed] [Google Scholar]






