Abstract
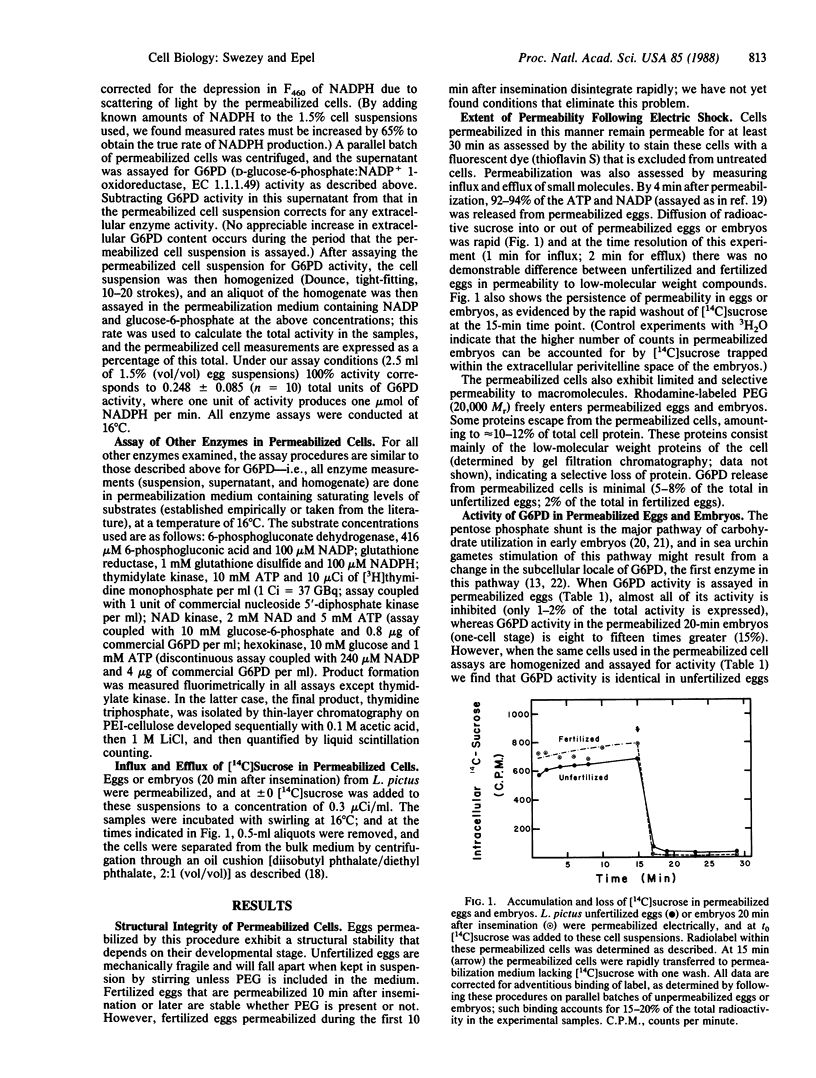
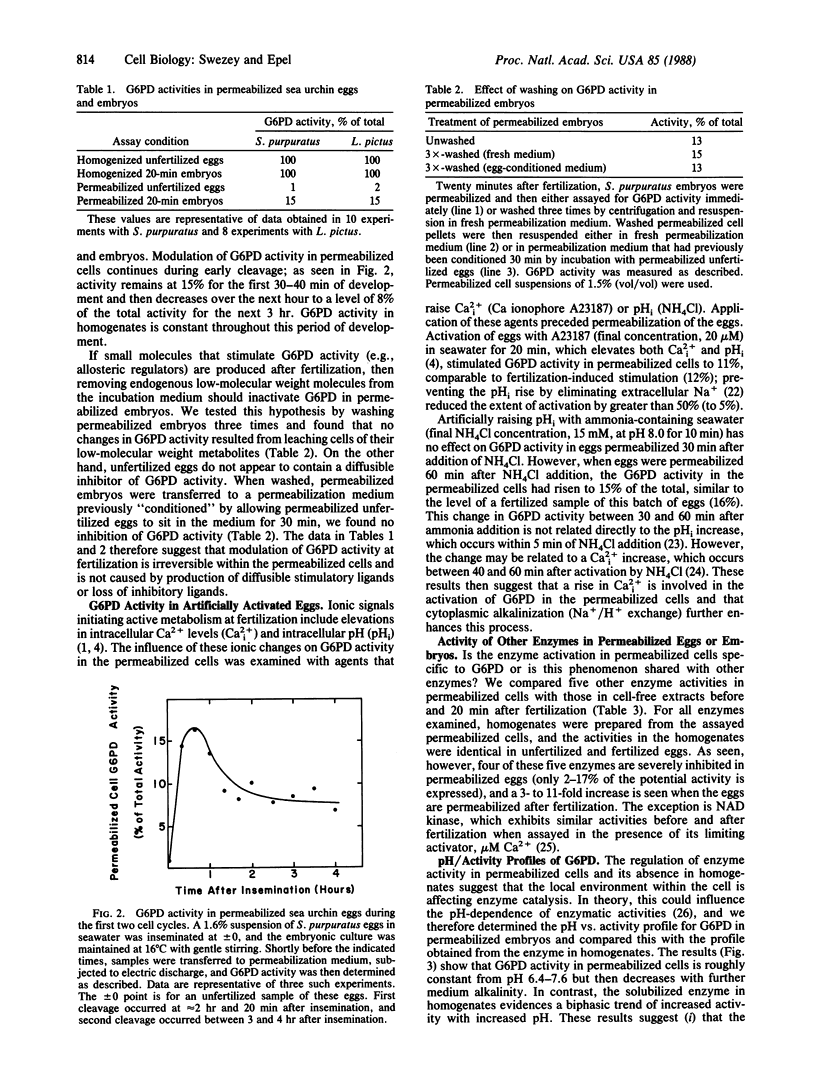
Sea urchin eggs and embryos subjected to high-voltage electric discharge in a medium mimicking the intracellular milieu retain their structural integrity and remain permeable, permitting substrates to enter the cytoplasm and thus assay of enzyme activity. At saturating concentrations of substrates, five of six enzymes assayed for more active (three to fifteen times) in permeabilized embryos than in permeabilized eggs, but no fertilization-related differences are seen in homogenates prepared from these same permeabilized cells. Furthermore, enzyme activity in homogenates always exceeds that in the permeabilized cell suspensions. This difference in enzyme reaction rates between unfertilized eggs and fertilized eggs is not due to differences in the diffusibility of substrates into the permeabilized cells. The activity of glucose-6-phosphate dehydrogenase (D-glucose-6-phosphate:NADP+ 1-oxidoreductase, EC 1.1.1.49) in permeabilized cells was studied in greater detail and has the following characteristics. (i) Regulation of activity persists during early development. (ii) This regulation is not mediated by diffusible allosteric agents. (iii) Stimulation at fertilization is initiated by a rise in intracellular calcium and is further promoted by cytoplasmic alkalinization. (iv) The microenvironment experienced by this enzyme intracellularly differs from that of the enzyme in homogenates as evidenced by markedly different pH vs. activity profiles. These results indicate that the regulatory status of enzymes is preserved in electrically permeabilized cells and suggest that this regulation depends on some cell structural feature(s) that is (are) destroyed upon homogenization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clegg J. S. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am J Physiol. 1984 Feb;246(2 Pt 2):R133–R151. doi: 10.1152/ajpregu.1984.246.2.R133. [DOI] [PubMed] [Google Scholar]
- Cline C. A., Schatten G. Microfilaments during sea urchin fertilization: fluorescence detection with rhodaminyl phalloidin. Gamete Res. 1986;14:277–291. doi: 10.1002/mrd.1120140402. [DOI] [PubMed] [Google Scholar]
- Dubé F., Epel D. The relation between intracellular pH and rate of protein synthesis in sea urchin eggs and the existence of a pH-independent event triggered by ammonia. Exp Cell Res. 1986 Jan;162(1):191–204. doi: 10.1016/0014-4827(86)90438-6. [DOI] [PubMed] [Google Scholar]
- Epel D. Mechanisms of activation of sperm and egg during fertilization of sea urchin gametes. Curr Top Dev Biol. 1978;12:185–246. doi: 10.1016/s0070-2153(08)60597-9. [DOI] [PubMed] [Google Scholar]
- Epel D., Patton C., Wallace R. W., Cheung W. Y. Calmodulin activates NAD kinase of sea urchin eggs: an early event of fertilization. Cell. 1981 Feb;23(2):543–549. doi: 10.1016/0092-8674(81)90150-1. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L., LEVIN Y., KATCHALSKI E. A WATER-INSOLUBLE POLYANIONIC DERIVATIVE OF TRYPSIN. II. EFFECT OF THE POLYELECTROLYTE CARRIER ON THE KINETIC BEHAVIOR OF THE BOUND TRYPSIN. Biochemistry. 1964 Dec;3:1913–1919. doi: 10.1021/bi00900a022. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Kinetic behavior of immobilized enzyme systems. Methods Enzymol. 1976;44:397–443. doi: 10.1016/s0076-6879(76)44031-4. [DOI] [PubMed] [Google Scholar]
- Isono N., Yasumasu I. Pathways of carbohydrate breakdown in sea urchin eggs. Exp Cell Res. 1968 Jun;50(3):616–626. doi: 10.1016/0014-4827(68)90423-0. [DOI] [PubMed] [Google Scholar]
- Johnson C. H., Epel D. Intracellular pH of sea urchin eggs measured by the dimethyloxazolidinedione (DMO) method. J Cell Biol. 1981 May;89(2):284–291. doi: 10.1083/jcb.89.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAHL M. E. Oxidative pathways for glucose in eggs of the sea urchin. Biochim Biophys Acta. 1956 Apr;20(1):27–32. doi: 10.1016/0006-3002(56)90258-x. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Gaining access to the cytosol: the technique and some applications of electropermeabilization. Biochem J. 1986 Mar 15;234(3):497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGwin N. F., Morton R. W., Nishioka D. Increased uptake of thymidine in the activation of sea urchin eggs. II. Cooperativity with phosphorylation, involvement of the cortex, and partial localization of the kinases. Exp Cell Res. 1983 Apr 15;145(1):115–126. doi: 10.1016/s0014-4827(83)80014-7. [DOI] [PubMed] [Google Scholar]
- Minton A. P. The effect of volume occupancy upon the thermodynamic activity of proteins: some biochemical consequences. Mol Cell Biochem. 1983;55(2):119–140. doi: 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- Nonaka M., Terayama H. Compartmentation of thymidine kinase in unfertilized sea urchin eggs and possible release of thymidine kinase from particulates in activated eggs. Dev Biol. 1977 Mar;56(1):68–75. doi: 10.1016/0012-1606(77)90155-5. [DOI] [PubMed] [Google Scholar]
- Osawa T., Tsuji T. Fractionation and structural assessment of oligosaccharides and glycopeptides by use of immobilized lectins. Annu Rev Biochem. 1987;56:21–42. doi: 10.1146/annurev.bi.56.070187.000321. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Reeves R. E., Sols A. Regulation of Escherichia coli phosphofructokinase in situ. Biochem Biophys Res Commun. 1973 Jan 23;50(2):459–466. doi: 10.1016/0006-291x(73)90862-0. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Enzyme-enzyme interactions and the regulation of metabolic reaction pathways. Curr Top Cell Regul. 1986;28:1–68. doi: 10.1016/b978-0-12-152828-7.50003-2. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci U S A. 1974 May;71(5):1915–1919. doi: 10.1073/pnas.71.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprynowicz F. A., Mazia D. Fluctuation of the Ca-sequestering activity of permeabilized sea urchin embryos during the cell cycle. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2389–2393. doi: 10.1073/pnas.82.8.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swezey R. R., Epel D. Regulation of glucose-6-phosphate dehydrogenase activity in sea urchin eggs by reversible association with cell structural elements. J Cell Biol. 1986 Oct;103(4):1509–1515. doi: 10.1083/jcb.103.4.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier V. D. Dynamic changes of the egg cortex. Dev Biol. 1981 May;84(1):1–26. doi: 10.1016/0012-1606(81)90366-3. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D., Hewson J. K. In situ regulation of yeast citrate synthase. Absence of ATP inhibition observed in vitro. FEBS Lett. 1973 Oct 15;36(2):227–231. doi: 10.1016/0014-5793(73)80374-6. [DOI] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Yasumasu I., Fujiwara A., Shoger R. L., Asami K. Distribution of some enzymes concerning carbohydrate metabolism in sea urchin eggs. Exp Cell Res. 1975 May;92(2):444–450. doi: 10.1016/0014-4827(75)90400-0. [DOI] [PubMed] [Google Scholar]



