Abstract
Ed Lewis introduced the term “transvection” in 1954 to describe mechanisms that can cause the expression of a gene to be sensitive to the proximity of its homologue. Transvection since has been reported at an increasing number of loci in Drosophila, where homologous chromosomes are paired in somatic tissues, as well as at loci in other organisms. At the Drosophila yellow gene, transvection can explain intragenic complementation involving the yellow2 allele (y2). Here, transvection was proposed to occur by enhancers of one allele acting in trans on the promoter of a paired homologue. In this report, we describe two yellow alleles that strengthen this model and reveal an unexpected, second mechanism for transvection. Data suggest that, in addition to enhancer action in trans, transvection can occur by enhancer bypass of a chromatin insulator in cis. We propose that bypass results from the topology of paired genes. Finally, transvection at yellow can occur in genotypes not involving y2, implying that it is a feature of yellow itself and not an attribute of one particular allele.
Studies in a wide variety of organisms have shown that the structure and function of a segment of DNA can be profoundly affected by the presence of homologous sequences (1–13). The impact of homologous sequences can be dramatic, ranging from changes in DNA sequence and methylation to changes in chromatin structure and global chromatin architecture. In many instances, these changes are considered epigenetic. We are interested in understanding homologue interactions and epigenetic forms of regulation. Our approach has been to investigate transvection, a process that can cause genes to be sensitive to the proximity of a homologue (1, 8, 14). Transvection was first defined in Drosophila, where somatic homologue pairing brings homologous sequences into close proximity (1). Our studies have focused on the yellow gene of Drosophila.
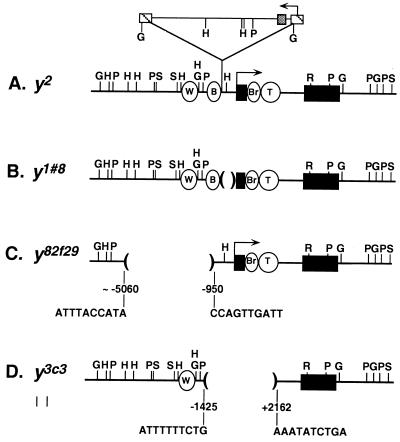
At the yellow gene, transvection is the basis for numerous cases of intragenic complementation (15). The yellow gene is required for cuticle pigmentation (16). The yellow2 allele (y2) reduces pigmentation in the wing and body but does not affect pigmentation of other tissues. It is caused by the insertion of a gypsy retrotransposon between the wing and body enhancers and the promoter (ref. 17; Fig. 1A). Gene expression is disrupted because gypsy is a target for the suppressor of Hairy wing [su(Hw)] protein, which, when bound, establishes a chromatin insulator that prevents the wing and body enhancers from activating transcription (18–20). Several yellow alleles complement y2 (15, 21–24). One case of complementation is illustrated in Fig. 2A. y1#8 is a null allele and causes fully mutant yellow pigmentation of the wing, body, and other cuticular structures. It is a deletion removing the promoter and some transcribed sequences (ref. 15; Fig. 1B). Of interest, although neither y2 nor y1#8 is expressed significantly in wing or body, y2/y1#8 flies show nearly wild-type pigmentation in these tissues. This type of intragenic complementation at yellow was shown to depend on allelic pairing, and, as such, transvection was implicated as the mechanistic basis. Of importance, the paired state of y2 is not sufficient, on its own, to induce transvection. For example, pairing of y2 with the y1 allele, caused by an A to C transition in the translation initiation codon, does not result in complementation. The observation that complementation of y2 is achieved only with a specific subset of alleles indicates that complementing alleles provide input in addition to that of the paired state.
Figure 1.
yellow alleles. y2 is caused by a gypsy insertion (raised triangle) at −700. y1#8, y82f29, and y3c3 are deletions of 0.8, 4.1, and 3.6 kbp, respectively. y1#8 has 17 bp of P-element sequence at its breakpoints (15). Extent of tarsal claw enhancer remaining in y3c3 is unknown. Arrows, promoters; W, wing enhancer, −2873 to −1868; B, body enhancer, −1425 to −700; Br, bristle enhancer; T, tarsal claw enhancer; +1, transcription start; black rectangles, exons; striped square, long terminal repeats; gray square, su(Hw) binding region; G, BglII; H, HindIII; P, PstI; S, SalI; R, EcoRI.
Figure 2.
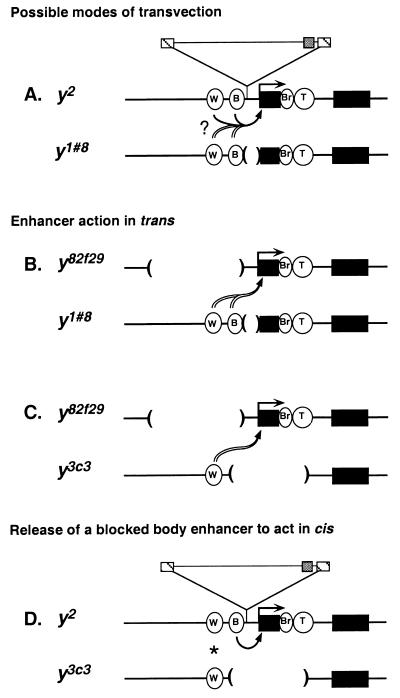
Models. The yellow alleles are drawn only approximately to scale. Symbols are used as in Fig. 1. (A) Transvection involving trans-acting y1#8 enhancers and/or cis-acting y2 enhancers. (B) Transvection involving trans action of the y1#8 wing and body enhancers. (C) Transvection involving trans action of the y3c3 wing enhancer. (D) The y2 body enhancer bypassing the insulator and acting on the y2 promoter. Wing complementation (*) may involve the y3c3 wing enhancer acting in trans and/or the y2 wing enhancer acting in cis.
We are interested in the molecular mechanisms of transvection. One explanation for the complementation of y2 and y1#8 is that, when these two alleles are paired, the wing and body enhancers of y1#8 act in trans on the promoter of y2 (ref. 15; Fig. 2A, double-lined arrow). Such a model is consistent with studies pointing to the ability of regulatory elements to act in trans in other contexts (refs. 1, 25–29, and, most recently, refs. 30–41).
Although the model of trans-acting enhancers at yellow is attractive, it has not been proven. It is formally possible that, because the wing and body enhancers of y2 are intact, y2/y1#8 complementation actually results from the release of the blocked enhancers of y2 to act in cis (Fig. 2A, solid arrow). In fact, as all well documented cases of complementation at yellow have involved y2, no complementing genotype of yellow has demonstrated unambiguously enhancer action in trans. In this report, we address this issue by using two yellow alleles, y82f29 and y3c3, which were identified in a genetic analysis of yellow transvection (J.R.M., J.-l.C., S. T. Filandrinos, R. C. Dunn, R. Fisk, P.K.G., and C.-t.W., unpublished work). These two alleles permitted us to test and confirm the model of trans-acting enhancers at yellow and also led to the suggestion of a second mechanism of transvection.
MATERIALS AND METHODS
Drosophila Stocks.
The y2, y1#8, y82f29, and y3c3 alleles are described in the text. The X chromosome bearing y3c3 also is marked with an allele of echinus. Females hemizygous for a yellow allele were generated by placing the yellow allele in trans to Df(1)y− ac− w1118, a deficiency that removes the entire yellow gene (42). The phenotypes of the echinus allele, ac−, and w1118, described in ref. 16, are not relevant to this study and, therefore, these mutations will not be discussed further.
Culture Condition.
Flies were cultured at 25 ± 1°C on standard Drosophila cornmeal, yeast, sugar, and agar medium with p-hydroxybenzoic acid methyl ester as a mold inhibitor. In general, three females were mated with three or more males in vials and were brooded daily. Temperature and crowding were controlled carefully because both affect pigmentation.
Scoring of Pigmentation.
Pigmentation was scored in 1–3-day-old flies on a scale of 1 to 5. According to this scale, 1 represents the null or nearly null state, and 5 represents the wild-type or nearly wild-type state. The null phenotype is defined by the pigmentation seen in flies that are homozygous or hemizygous for y1 or Df(1)y− ac− w1118, and the wild-type phenotype is defined by the pigmentation seen in our wild-type Canton S strain. Body pigmentation refers to pigmentation in the abdominal stripes. At least two independent crosses were set up for each genotype, and at least 100 females were scored from each cross. Pigmentation scores were determined independently by at least two people.
Analysis of y82f29.
One phage was isolated from a genomic library constructed in the Lambda DASH II vector (Stratagene) and screened with yellow genomic sequences using standard techniques (43). DNA corresponding to the entire phage insert was subcloned as a NotI fragment into Bluescript (Stratagene). Restriction analyses indicated that this fragment contained 4.5 kbp of DNA upstream of the y82f29 deletion breakpoint, the yellow transcription unit, and 2.6 kbp of DNA 3′ to the poly(A) addition signal. Restriction analyses of phage DNA by using HindIII, PstI, and BglII, which cut within gypsy, and Southern analysis (43) with a complete gypsy probe, revealed no gypsy sequences within the cloned region [hybridized in 5× standard saline citrate (SSC), 50% formamide, 5× Denhardt’s solution, 50 mM sodium phosphate (pH 6.8), and 40 μg/ml calf thymus DNA at 42°C for 14 hours and washed in 2× SSC, 0.1% sodium pyrophosphate, and 0.1% SDS). A 1.2-kbp HindIII fragment containing the y82f29 breakpoint was subcloned and sequenced by using the primer 5′TTTCGATTGGGCGTCAC, which begins at −749. This produced ≈400 bp of sequence extending 5′ of the breakpoint. The corresponding wild-type region was cloned from a phage containing y3c3 genomic DNA and was sequenced. Sequence comparison demonstrated a clean deletion in y82f29.
Analysis of y3c3.
Southern analysis (43) of y3c3 indicated this allele to be associated with an intragenic deletion. The deletion was confirmed by PCR amplification and sequencing of a 578-bp fragment spanning the breakpoints. The sequences of the primers were 5′ATGGATCC*TGCAGCGATCGCATCATTAG, where the C* corresponds to position −1629, and 5′GTAGGATCC*GAGTGAGACTGCAACGACCA, where the C* corresponds to position +2533. The 5′ end of both primers contains a short run of nucleotides that is not homologous to yellow sequence.
Plasmid Construction.
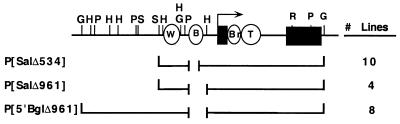
The status of the body enhancer in y3c3 was determined by the analysis of transgenes, each of which contained an internal deletion of the yellow gene. Three constructs were made. P[SalΔ534] and P[SalΔ961] had internal deletions of 534 and 961 bp, respectively. P[5′BglΔ961] differed from P[SalΔ961] by the addition of 3.3 kbp of 5′ sequence.
The internal deletion in P[SalΔ534] was created by digesting pUC8 containing yellow in a 7.7-kbp SalI fragment (42) with DraIII and Eco47III. The 5′ breakpoint of y3c3 was re-created by ligating the digestion product in the presence of the double-stranded oligonucleotide 5′GTGTTTGTTTATTTTTTCTG3′ 3′TGGCACAAACAAATAAAAAAGAC5′. The resulting plasmid was digested with SalI to remove the modified yellow sequences, and these yellow sequences then were cloned into the SalI site of pBSX, a modified Bluescript vector in which the Asp718 site was replaced with an XbaI site. This plasmid then was digested with XbaI, and the yellow sequences were cloned into the XbaI site of pCaSper3 in the reverse orientation relative to white.
The deletion in P[SalΔ961] was created by digesting pUC8 containing the 5′ end of the yellow gene in a 3.1-kbp SalI/ BamHI fragment with DraIII and NsiI. The 5′ breakpoint of y3c3 was re-created by ligating the digestion product in the presence of the double-stranded oligonucleotide 5′GTGTTTGTTTATTTTTTCTGATGCA3′ 3′TGGCACAAACAAATAAAAAAGACT5′. The resulting plasmid was digested with SalI and BamHI to remove the modified yellow sequences, which then were cloned into the SalI and BamHI sites of pBSXyBG. pBSXyBG is a derivative of pBSX that contains the 3′ end of the yellow gene in a 4.6-kbp BamHI/BglII fragment inserted into the BamHI site. Therefore, the insertion of the modified yellow gene into pBSXyBG generated an internally deleted yellow gene. The yellow gene then was cloned into pCaSper3 as described above.
P[5′BglΔ961] differs from P[SalΔ961] by an additional 5′ sequence. P[SalΔ961] was digested with XhoI and BglII and was religated by using the double-stranded oligonucleotide 5′TCGAGATGCTACGCATGACA 3′CTACGATGCGTACTGTCTAG to remove 1 kbp of the 5′ sequence and restore the BglII site. The resulting plasmid was digested with BglII, and a 4.3-kbp BglII fragment containing 3.3 kbp of sequence upstream of the SalI site was inserted in the wild-type orientation. Deletion breakpoints of all constructs were confirmed by sequencing.
Germ-Line Transformation.
P-element mediated germ-line transformation (44) used 0.5 mg/ml construct and 0.1 mg/ml “wings-clipped” helper DNA. The host genotype was Df(1)y− ac− w1118.
RESULTS AND DISCUSSION
The goal of our studies was to understand enhancer action in transvection. We began our studies by asking whether yellow enhancers have the ability to act in trans. We addressed this issue by using the y82f29 allele. y82f29 flies show a tissue-specific alteration in pigmentation similar to that of y2 flies, with mutant pigmentation in wing and body. On a scale of 1 to 5, where 1 represents the null or nearly null state and 5 represents the wild-type or nearly wild-type state, both alleles, when homozygous or hemizygous, give scores of 1 in wing and 1 to 1.5 in body (Table 1). We determined the structure of y82f29 by constructing a genomic library from y82f29 DNA and isolating the yellow gene. Restriction and sequence analysis demonstrated that y82f29 is caused by a 4.1-kbp deletion removing the wing enhancer and much of the region to which the body enhancer had been mapped (refs. 42 and 45; Fig. 1C). This structure, combined with the mutant wing and body phenotype of y82f29, indicates that y82f29 can provide very little, if any, wing and body enhancer activity. We tested the ability of y82f29 to participate in intragenic complementation by placing it in trans to y1#8. As mentioned above, y1#8 is a null allele. It produces pigmentation scores of 1 in wing and body tissue when homozygous or hemizygous (Table 1). Of significance, y82f29 complements y1#8; flies of the genotype y82f29/y1#8 produce pigmentation scores of 3 in both wing and body (Table 1). Although the complementation is not as strong as that seen in y2/y1#8 flies, which produce scores of 4, wing and body pigmentation are notably greater than that seen in the parental y82f29 and y1#8 lines. We conclude that complementation arises from the wing and body enhancers of y1#8 acting in trans on the promoter of y82f29 because y82f29 cannot contribute the complementing levels of wing or body enhancer activity (Fig. 2B). These data demonstrate that transvection at yellow can occur by the action of enhancers in trans.
Table 1.
Pigmentation scores for control (homozygous and hemizygous) and complementing genotypes
| Genotypes* | Pigmentation wing, body |
|---|---|
| Control | |
| y2/y2 | 1, 1.5 |
| y2/Df | 1, 1 |
| y82f29/y82f29 | 1, 1-1.5 |
| y82f29/Df | 1, 1 |
| y1#8/y1#8 | 1, 1 |
| y1#8/Df | 1, 1 |
| y3c3/y3c3 | 1, 1 |
| y3c3/Df | 1, 1 |
| Complementing | |
| y2/y1#8 | 4, 4 |
| y82f29/y1#8 | 3, 3 |
| y2/y3c3 | 4, 4 |
| y82f29/y3c3 | 3, 1 |
Df is used here to mean Df(1)y−ac−w1118.
Our analysis of y82f29 resolved another issue regarding transvection at yellow. All well documented cases of yellow transvection have involved y2, raising the possibility that y2 and/or gypsy is required. The complementing y82f29/y1#8 genotype demonstrates that transvection at yellow does not require y2 and therefore does not depend on any particular allele. Furthermore, sequence analysis of the breakpoints, followed by restriction and Southern analyses, revealed no gypsy sequences within the cloned y82f29 region (Fig. 1C). Therefore, transvection at yellow also does not require a gypsy element in either participating allele, a finding that is in line with observations at other loci (46, 47). We conclude that transvection at yellow is a feature of the locus and not an attribute of an extraordinary allele.
Although our studies of y82f29 demonstrate that enhancers are capable of acting in trans at yellow, they leave unresolved the question of whether the wing and body enhancers of y2 also can participate in intragenic complementation. Our analysis of y3c3 addressed this issue and led us to propose a second mechanism for transvection. The y3c3 allele is a null; when homozygous or hemizygous, it results in fully mutant pigmentation of wing, body, and other cuticular structures (Table 1). Molecular analysis showed that y3c3 is a 3.6-kbp deletion that removes promoter, 5′ regulatory, and transcribed sequences (Fig. 1D). Like the y1#8 allele, y3c3 complements y2, producing scores of 4 in wing and body (Table 1). For this reason, we expected y3c3 to carry intact wing and body enhancers. The coordinates of the breakpoints confirmed the presence of the wing enhancer. However, the 5′ deletion breakpoint was within the region to which the body enhancer had been mapped in earlier studies (42, 45), making it unclear whether y3c3 carries the body enhancer.
To determine whether y3c3 has a body enhancer, we carried out germ-line transformation studies with three deletion constructs of yellow (Fig. 3). Each construct carried an internal deletion whose 5′ breakpoint was identical to that of y3c3 and whose 3′ breakpoint was upstream of the promoter. Two of the constructs, P[SalΔ534] and P[SalΔ961], carried the same amount of 5′ and 3′ flanking DNA that was shown previously by germ-line transformation to be sufficient to produce wild-type levels of pigmentation in all tissues (42, 45). The third construct, P[5′BglΔ961], differed from the other two by an additional 3.3 kbp of 5′ genomic sequence, placing the 5′ endpoint of P[5′BglΔ961] upstream of the 5′ deletion breakpoint present in y82f29. As y82f29 produces a null or nearly null phenotype in the wing and body, its deletion breakpoints are a good indicator of the boundaries of wing and body enhancer function. Therefore, our constructs should reveal whether y3c3 retains any body enhancer function.
Figure 3.
Transgenic lines. Wild-type yellow gene and constructs are depicted by using symbols as shown in Fig. 1.
The three constructs were used separately to transform a line lacking the endogenous yellow gene. We obtained 22 independent transgenic lines. All except one showed wild-type pigmentation in the wing, indicating that the promoter and wing enhancer were functional. The exceptional line showed reduced wing pigmentation, most likely reflecting a repressive position effect. Of importance, all lines had a low level of body pigmentation, corresponding to scores of 1 to 2, which was considerably less than that seen in wild-type or complementing y2/y3c3 flies. From these data, we conclude that body enhancer function is disrupted severely in y3c3. Therefore, it is unlikely that complementation in the body of y2/y3c3 flies results from a body enhancer of y3c3 acting in trans on the y2 promoter.
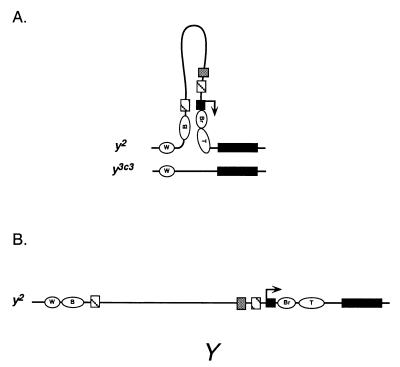
The simplest interpretation of our data is that body pigmentation of y2/y3c3 flies arises from the body enhancer of y2 bypassing the su(Hw) chromatin insulator to act on its own promoter in cis (Fig. 2D). Bypass of the su(Hw) chromatin insulator is surprising considering that this insulator provides a versatile and general block of enhancer-activated transcription (18–20). In fact, current models for insulator function do not predict that the blocked body enhancer can bypass the insulator in the absence of secondary changes in y2 (48–50) or the factors that generate the insulator, such as the su(Hw) protein (18–20). A potential explanation for bypass is suggested by the structure of y3c3 and rests on what we call a pairing-mediated topology effect (TOPE). In this view, bypass results from conformational changes in the gene caused by homologue pairing. The ability of structures arising from homologue pairing to influence gene expression was predicted in 1935 by H. J. Muller (ref. 51; cited by ref. 52) and since has been considered by others (14, 31, 36, 39, 41, 50, 52–62). The body complementation in y2/y3c3 flies advances this concept by providing a compelling example in which the structure of paired alleles can explain the induction of gene expression.
A hypothetical paired structure for the y2/y3c3 genotype is shown in Fig. 4A. In this model, the promoter and body enhancer of y2 are unpaired and looped out because y3c3 lacks these elements. A correlation between the unpaired state and increased accessibility to regulatory factors has been proposed as a contributing factor in transvection-related phenotypes elsewhere (14, 57, 59). In the case of y2/y3c3, it may be that the unpaired state of genetic elements and their presence in a looped structure make them unusually accessible. For example, increased accessibility of the promoter may make it responsive to enhancers that normally are blocked by the insulator or, in an extreme case, cause it to be constitutive. Recently, the unpaired looped state also has been proposed to augment accessibility and facilitate transvection at the Abdominal-B gene (41). Alternatively, inclusion of the promoter and body enhancer of y2within the same loop may lead to the apposition and interaction of these two elements. Finally, the insulator may be compromised by constraints imposed by the looped structure. This interpretation is in line with proposals that binding of su(Hw) protein to gypsy is sensitive to DNA topology (63) and that insulator function entails an increase in DNA flexibility (64). Although our models focus on the promoter, enhancer, and insulator elements and on loop formation, they are also compatible with pairing exerting its effects on other genetic elements or via other topologies, such as the structural alterations of chromatin and DNA that accompany the transcriptionally active state (65). All of these possibilities share the common theme that the structure of paired homologues plays an important role in regulating transvection and gene expression.
Figure 4.
Model for insulator bypass. The yellow alleles are drawn only approximately to scale. Symbols are used as in Fig. 1. (A) Homologue pairing in y2/y3c3 flies generates an unpaired loop in regions of heterogeneity between the alleles. This topology allows for bypass of the su(Hw) chromatin insulator by the y2 body enhancer to act on its own promoter in cis. (B) The mutant phenotype of y2/Y males and hemizygous females (not shown) demonstrates that simple unpairing of yellow sequences is not sufficient to activate the y2 promoter.
Although the most conspicuous aspect of our model is the unpaired state of the y2 promoter and body enhancer, we emphasize that simple unpairing of elements is not sufficient to explain y2 expression in body tissue. Evidence comes from females that carry y2 in trans to a deficiency of the entire yellow gene and from y2/Y males that are hemizygous for yellow, which is present on the X chromosome (Fig. 4B). In both genotypes, the body enhancer and promoter of y2 are unpaired, yet the unpaired state has no effect on the y2 phenotype.
In short, y3c3 plays a key role in inducing y2 transcription, and we suggest that it acts by promoting bypass of the insulator when it is paired with y2. One model for bypass, discussed above, proposes that it results from the topology of paired alleles. We also are considering alternative explanations. For example, y3c3 may potentiate y2 transcription by altering local concentrations of transcription factors. One possibility is that the lack of a body enhancer and promoter in y3c3 causes an increase in the local concentration of transcription factors in the vicinity of the y2 body enhancer. This change may strengthen the y2 body enhancer, making it more difficult to be blocked by the insulator (66). Of importance, regulated transcription in this case still would call for insulator bypass. Alternatively, y3c3 may retain some body enhancer activity. If so, our transgene studies indicate that the level of body pigmentation it directs is significantly below that seen in complementing y2/y3c3 flies. Therefore, should residual body enhancer activity of y3c3 be responsible for complementation, we would need to postulate that it is induced by pairing with y2 to act more strongly in trans than it does in cis. That genetic elements may be strengthened when paired has been proposed elsewhere (14, 36, 55). We also have considered the possibility of a dosage-sensitive trans-acting repressor of y2 transcription contained within the region absent from y3c3. We do not favor this model because it invokes a genetic element for which we have no evidence.
The data in this paper suggest two mechanisms for transvection: enhancer action in trans and bypass of a chromatin insulator in cis. A good test of the models comes from the placement of y82f29 in trans to y3c3 (Fig. 2C). Our models predict that y82f29/y3c3 flies should show complementation in the wing, because of trans action of the y3c3 wing enhancer on the y82f29 promoter, but not in the body, because neither y3c3 nor y82f29 can provide significant body enhancer activity. This is indeed the case, with wing complementation reaching a level similar to that seen in y82f29/y1#8 flies (Table 1). These results also demonstrate that y3c3 can contribute enhancer action in trans and, combined with the absence of body complementation in y82f29/y3c3 flies, confirm that y3c3 is deficient in body enhancer function. These observations support our proposal that body complementation in y2/y3c3 flies arises from the y2 body enhancer bypassing the insulator. The tissue-specific phenotype of y82f29/y3c3 flies has a further implication. It reinforces the idea that promoter unpairing, by itself, is not sufficient to explain complementation. If unpairing were sufficient, the hypothetical paired structure for y82f29/y3c3, which leaves the y82f29 promoter unpaired, would predict the body to be pigmented. Instead, pigmentation remains minimal in the body. These observations argue that the unpaired state of the promoter is also not sufficient to explain complementation in the y82f29/y1#8 and y2/y3c3 genotypes and that models invoking enhancer action and pairing-mediated topologies are a more likely explanation.
Comparison of the four complementing genotypes shows that complementation is stronger in the wing and, where appropriate, in the body for the two genotypes involving y2 as compared with the two genotypes involving y82f29 (Table 1). The different degrees of complementation may be caused by modifiers extragenic to yellow or the abnormal juxtaposition of sequences at the deletion breakpoints of y82f29. On the other hand, the differences may indicate intricacies of transvection at yellow. For example, the deletion nature of y82f29 may disrupt pairing and compromise transvection in a manner that has been proposed to influence pairing-mediated processes elsewhere (54, 55). Alternatively, if wing and body enhancers are more potent when paired, their absence from y82f29 may compromise the ability of the enhancers of the paired allele to act in trans. It is also possible that, although gypsy sequences are not necessary for transvection, their presence in y2 facilitates transvection. A positive role for gypsy in transvection has been suggested (15, 17, 67), and there is mounting evidence for the ability of the su(Hw) protein to exert its influence in trans on a paired homologue (68–71). Finally, the stronger complementation seen in genotypes involving y2 may arise from the promoter of y2 receiving contribution both from enhancers located in cis, because of insulator bypass, as well as from enhancers located in trans.
In conclusion, we propose that homologue pairing promotes at least two forms of transvection at yellow, the mechanism or mechanisms used being determined by the features of the alleles involved. In one case, gene expression is directed by the trans action of genetic elements. In the other, gene expression is induced by the presence of a structurally dissimilar homologue where an obvious input from the homologue is its extent of homology as translated by the forces of pairing.
Acknowledgments
We thank M. M. Green, affectionately known as Lü Yeh Yeh, for kindly providing the y82f29 flies and years of insight, V. G. Corces and D.A. Gdula for key discussions regarding the location of the body enhancer, and F. Winston, W. Bender, H. Genetti, B. Cohen, T. Enoch, S. Filandrinos, R. Fisk, K. Huisinga, D. Mallin, R. Mollaaghababa, A. Moran, N. Perrimon, G. Ruvkun, K. Scott, and P. Sudarsanam for enlightening discussions, unpublished data, and technical assistance. This research was supported by a National Institutes of Health grant to P.K.G. and a National Science Foundation grant, a National Institutes of Health Shannon Award, a Funds for Discovery Exploratory Award, support from the Monsanto Fellowship Program, and generosity of the Richard and Priscilla Hunt Fellowship to C-t.W.
Footnotes
References
- 1.Lewis E B. Am Nat. 1954;88:225–239. [Google Scholar]
- 2.Dorer D R, Henikoff S. Cell. 1994;77:993–1002. doi: 10.1016/0092-8674(94)90439-1. [DOI] [PubMed] [Google Scholar]
- 3.Aramayo R, Metzenberg R L. Cell. 1996;86:103–113. doi: 10.1016/s0092-8674(00)80081-1. [DOI] [PubMed] [Google Scholar]
- 4.Cline T W, Meyer B J. Annu Rev Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- 5.Meyer P, Saedler H. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:23–48. doi: 10.1146/annurev.arplant.47.1.23. [DOI] [PubMed] [Google Scholar]
- 6.Bartolomei M S, Tilghman S M. Annu Rev Genet. 1997;31:493–525. doi: 10.1146/annurev.genet.31.1.493. [DOI] [PubMed] [Google Scholar]
- 7.Heard E, Clerc P, Avner P. Annu Rev Genet. 1997;31:571–610. doi: 10.1146/annurev.genet.31.1.571. [DOI] [PubMed] [Google Scholar]
- 8.Henikoff S. Curr Opin Cell Biol. 1997;9:388–395. doi: 10.1016/s0955-0674(97)80012-9. [DOI] [PubMed] [Google Scholar]
- 9.Hollick J B, Dorweiler J E, Chandler V L. Trends Genet. 1997;13:302–308. doi: 10.1016/s0168-9525(97)01184-0. [DOI] [PubMed] [Google Scholar]
- 10.Pal-Bhadra M, Bhadra U, Birchler J A. Cell. 1997;90:479–490. doi: 10.1016/s0092-8674(00)80508-5. [DOI] [PubMed] [Google Scholar]
- 11.Selker E U. Trends Genet. 1997;13:296–301. doi: 10.1016/s0168-9525(97)01201-8. [DOI] [PubMed] [Google Scholar]
- 12.Garrick D, Fiering S, Martin D I K, Whitelaw E. Nature Genet. 1998;18:56–59. doi: 10.1038/ng0198-56. [DOI] [PubMed] [Google Scholar]
- 13.Matzke M A, Matzke A J M. Cell Mol Life Sci. 1998;54:94–103. doi: 10.1007/s000180050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C-t. J Cell Biol. 1993;120:587–590. doi: 10.1083/jcb.120.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geyer P K, Green M M, Corces V G. EMBO J. 1990;9:2247–2256. doi: 10.1002/j.1460-2075.1990.tb07395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsley D L, Zimm G G. The Genome of Drosophila melanogaster. New York: Academic; 1992. [Google Scholar]
- 17.Corces V G, Geyer P K. Trends Genet. 1991;7:86–90. doi: 10.1016/0168-9525(91)90277-W. [DOI] [PubMed] [Google Scholar]
- 18.Gdula D A, Gerasimova T I, Corces V G. Proc Natl Acad Sci USA. 1996;93:9378–9383. doi: 10.1073/pnas.93.18.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorsett D. Mol Cells. 1996;6:381–387. [Google Scholar]
- 20.Geyer P K. Curr Opin Genet Dev. 1997;7:242–248. doi: 10.1016/s0959-437x(97)80134-7. [DOI] [PubMed] [Google Scholar]
- 21.Stone W. Dros Inf Serv. 1935;4:62–63. [Google Scholar]
- 22.Frye S H. Dros Inf Serv. 1960;34:49. [Google Scholar]
- 23.Green M M. Genetics. 1961;46:1385–1388. doi: 10.1093/genetics/46.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nash W G. Dev Biol. 1976;48:336–343. doi: 10.1016/0012-1606(76)90095-6. [DOI] [PubMed] [Google Scholar]
- 25.Lewis E B. Am Nat. 1955;89:73–89. [Google Scholar]
- 26.Davison D, Chapman C H, Wedeen C, Bingham P M. Genetics. 1985;110:479–494. doi: 10.1093/genetics/110.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirrotta V, Stellar H, Bozzetti M P. EMBO J. 1985;4:3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zachar Z, Chapman C H, Bingham P M. Cold Spring Harbor Symp Quant Biol. 1985;50:337–346. doi: 10.1101/sqb.1985.050.01.043. [DOI] [PubMed] [Google Scholar]
- 29.Müller H-P, Schaffner W. Trends Genet. 1990;6:300–304. doi: 10.1016/0168-9525(90)90236-y. [DOI] [PubMed] [Google Scholar]
- 30.Castelli-Gair J E, Capdevila M-P, Micol J-L, García-Bellido A. Mol Gen Genet. 1992;234:177–184. doi: 10.1007/BF00283837. [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Laborda A, González-Reyes A, Morata G. EMBO J. 1992;11:3645–3652. doi: 10.1002/j.1460-2075.1992.tb05449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scholz H, Deatrick J, Klaes A, Klämbt C. Genetics. 1993;135:455–468. doi: 10.1093/genetics/135.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leiserson W M, Bonini N M, Benzer S. Genetics. 1994;138:1171–1179. doi: 10.1093/genetics/138.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendrickson J E, Sakonju S. Genetics. 1995;139:835–848. doi: 10.1093/genetics/139.2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopmann R, Duncan D, Duncan I. Genetics. 1995;139:815–833. doi: 10.1093/genetics/139.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldsborough A S, Kornberg T B. Nature (London) 1996;381:807–810. doi: 10.1038/381807a0. [DOI] [PubMed] [Google Scholar]
- 37.Neumann C J, Cohen S M. Genetics. 1996;142:1147–1155. doi: 10.1093/genetics/142.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buratovich M A, Phillips R G, Whittle J R S. Dev Biol. 1997;185:244–260. doi: 10.1006/dbio.1997.8562. [DOI] [PubMed] [Google Scholar]
- 39.Casares F, Bender W, Merriam J, Sánchez-Herrero E. Genetics. 1997;145:123–137. doi: 10.1093/genetics/145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krebs J E, Dunaway M. Mol Cell. 1998;1:301–308. doi: 10.1016/s1097-2765(00)80030-1. [DOI] [PubMed] [Google Scholar]
- 41.Sipos L, Mihály J, Karch F, Schedl P, Gausz J, Gyurkovics H. Genetics. 1998;149:1031–1050. doi: 10.1093/genetics/149.2.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geyer P K, Corces V G. Genes Dev. 1987;1:996–1004. doi: 10.1101/gad.1.9.996. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 44.Rubin G M, Spradling A C. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 45.Martin M, Meng Y B, Chia W. Mol Gen Genet. 1989;218:118–126. doi: 10.1007/BF00330574. [DOI] [PubMed] [Google Scholar]
- 46.Hazelrigg T, Levis R, Rubin G M. Cell. 1984;36:469–481. doi: 10.1016/0092-8674(84)90240-x. [DOI] [PubMed] [Google Scholar]
- 47.Lewis E B. Cold Spring Harbor Symp Quant Biol. 1985;50:155–172. doi: 10.1101/sqb.1985.050.01.021. [DOI] [PubMed] [Google Scholar]
- 48.Geyer P K, Green M M, Corces V G. Proc Natl Acad Sci USA. 1988;85:3938–3942. doi: 10.1073/pnas.85.11.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith P A, Corces V G. Mol Gen Genet. 1992;233:65–70. doi: 10.1007/BF00587562. [DOI] [PubMed] [Google Scholar]
- 50.Georgiev P, Tikhomirova T, Yelagin V, Belenkaya T, Gracheva E, Parshikov A, Evgen’ev M B, Samarina O P, Corces V G. Genetics. 1997;146:583–594. doi: 10.1093/genetics/146.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller H J. 15th Int. Physiol. Congr., Summaries of Communications. Moscow–Leningrad: State Publishing House for Biological and Medical Literature; 1935. pp. 286–289. [Google Scholar]
- 52.Ephrussi B, Sutton E. Proc Natl Acad Sci USA. 1944;30:183–197. doi: 10.1073/pnas.30.8.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gubb D, Ashburner M, Roote J, Davis T. Genetics. 1990;126:167–176. doi: 10.1093/genetics/126.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dreesen T D, Henikoff S, Loughney K. Genes Dev. 1991;5:331–340. doi: 10.1101/gad.5.3.331. [DOI] [PubMed] [Google Scholar]
- 55.Hazelrigg T, Petersen S. Genetics. 1992;130:125–138. doi: 10.1093/genetics/130.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Assaad F F, Tucker K L, Signer E R. Plant Mol Biol. 1993;22:1067–1085. doi: 10.1007/BF00028978. [DOI] [PubMed] [Google Scholar]
- 57.Peterson K M, Davis P S, Judd B H. Mol Gen Genet. 1994;242:717–726. doi: 10.1007/BF00283427. [DOI] [PubMed] [Google Scholar]
- 58.Henikoff S, Jackson J M, Talbert P B. Genetics. 1995;140:1007–1017. doi: 10.1093/genetics/140.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu C-t, Howe M. Genetics. 1995;140:139–181. doi: 10.1093/genetics/140.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keeney S, Kleckner N. Genes Cells. 1996;1:475–489. doi: 10.1046/j.1365-2443.1996.d01-257.x. [DOI] [PubMed] [Google Scholar]
- 61.Ye F, Signer E R. Proc Natl Acad Sci USA. 1996;93:10881–10886. doi: 10.1073/pnas.93.20.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gubb D, Roote J, Trenear J, Coulson D, Ashburner M. Genetics. 1997;146:919–937. doi: 10.1093/genetics/146.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spana C, Corces V G. Genes Dev. 1990;4:1505–1515. doi: 10.1101/gad.4.9.1505. [DOI] [PubMed] [Google Scholar]
- 64.Shen B, Kim J, Dorsett D. Mol Cell Biol. 1994;14:5645–5652. doi: 10.1128/mcb.14.9.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werner M H, Burley S K. Cell. 1997;88:733–736. doi: 10.1016/s0092-8674(00)81917-0. [DOI] [PubMed] [Google Scholar]
- 66.Scott K C. Dissertation. Iowa City: Univ. of Iowa; 1998. [Google Scholar]
- 67.Henikoff S. Genetics. 1994;138:1–5. doi: 10.1093/genetics/138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Georgiev P, Corces V G. Proc Natl Acad Sci USA. 1995;92:5184–5188. doi: 10.1073/pnas.92.11.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Georgiev P, Kozycina M. Genetics. 1996;142:425–436. doi: 10.1093/genetics/142.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morcillo P, Rosen C, Dorsett D. Genetics. 1996;144:1143–1154. doi: 10.1093/genetics/144.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sigrist C J A, Pirrotta V. Genetics. 1997;147:209–221. doi: 10.1093/genetics/147.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]