Abstract
An altered glutamatergic input at corticostriatal synapses has been shown in experimental models of Parkinson’s disease (PD). In the present work, we analyzed the membrane and synaptic responses of striatal neurons to metabotropic glutamate (mGlu) receptor activation in two different mouse models of inherited PD, linked to mutations in PINK1 or Parkin genes.
Both in PINK1 and Parkin knockout (−/−) mice, activation of group I mGlu receptors by 3,5-DHPG caused a membrane depolarization coupled to an increase in firing frequency in striatal cholinergic interneurons that was comparable to the response observed in the respective wild-type (WT) interneurons. The sensitivity to group II and III mGlu receptors was tested on cortically-evoked excitatory postsynaptic potentials (EPSPs) recorded from medium spiny neurons (MSNs). Both LY379268 and L-AP4, agonists for group II and III, respectively, had no effect on intrinsic membrane properties, but dose-dependently reduced the amplitude of corticostriatal EPSPs. However, both in PINK1−/− and Parkin−/− mice, LY379268, but not L-AP4, exhibited a greater potency as compared to WT in depressing EPSP amplitude. Accordingly, the dose–response curve for the response to LY379268 in both knockout mice was shifted leftward. Moreover, consistent with a presynaptic site of action, both LY379268 and L-AP4 increased the paired-pulse ratio either in PINK1−/− and Parkin−/− or in WT mice. Acute pretreatment with L-dopa did not rescue the enhanced sensitivity to LY379268.
Together, these results suggest that the selective increase in sensitivity of striatal group II mGlu receptors represents an adaptive change in mice in which an altered dopamine metabolism has been documented.
Keywords: Striatum, Metabotropic glutamate receptor, Monogenic parkinsonism, Electrophysiology
Introduction
Parkinson’s disease (PD) is a progressive degenerative neurological disorder characterized primarily by motor symptoms that include rigidity, hypokinesia and tremor. Current knowledge on the pathogenesis of idiopathic PD include several potential factors, including neurotransmitter toxicity, mitochondrial metabolism failure, environmental toxins and genetic predisposition (Sherer et al., 2007). Although the occurrence of PD is largely sporadic, monogenic mutations in five distinct genes have been linked to clinical syndromes often indistinguishable from sporadic PD. Mutations in the genes encoding alpha-synuclein and leucine-rich repeat kinase 2 (LRRK2), are responsible for autosomal dominant forms of PD (Polymeropoulos et al., 1997; Paisán-Ruíz et al., 2004; Zimprich et al., 2004), whereas mutations in Parkin, DJ-1 and PINK1 (PTEN induced kinase 1) genes have been found in forms of PD inherited recessively, and include large exonic deletions or frame-shift truncations, suggestive of a “loss of function” mechanism (Kitada et al., 1998; Bonifati et al., 2003; Valente et al., 2004). Parkin and PINK1 are considered the most common causative genes among the autosomal recessive forms of PD (Klein et al., 2007). The loss of nigrostriatal dopaminergic fibers has been shown to induce a complex rearrangement in the functional anatomy of the basal ganglia. As a result, the firing pattern of some of the glutamatergic pathways undergoes profound modifications (Bergman et al.,1990,1994; Bevan et al., 2002; Greenamyre 2001). Accordingly, it has been demonstrated that the corticostriatal glutamatergic activity is increased. This assumption is supported both by in vivo studies showing an elevation in striatal glutamate content in 6-hydroxy-dopamine(6-OHDA)-denervated rats (Meshul et al., 1999) as well as by electrophysiological recordings from striatal projection neurons, showing a relevant rise in glutamate-mediated spontaneous synaptic currents (Calabresi et al., 1993; Picconi et al., 2002).
Compared to thoroughly investigated roles of ionotropic glutamate receptors, the contribution of metabotropic glutamate (mGlu) receptors in modulating basal ganglia excitability is less elucidated and remains a prime theme in most recent research on glutamate (Conn et al., 2005). To date, eight mGlu receptors have been cloned, and grouped in three classes (groups I–III) according to their sequence homology, biochemical and pharmacological properties (Conn and Pin, 1997). Activation of group I subtypes (mGlu1 and mGlu5 receptors) exerts different effects at postsynaptic level, depending on the striatal neuronal subtype involved (Pisani et al., 2001a,b; Bonsi et al., 2005, 2007). Instead, both group II (mGlu2/3) and group III (mGlu4/7/8) mGlu receptors act presynaptically to modulate corticostriatal inputs (Lovinger and McCool, 1995; Pisani et al., 1997). Of interest, a significant increase in the sensitivity to mGlu2/3 receptor activation has been described in the 6-OHDA rat model of PD (Picconi et al., 2002), supporting the hypothesis that loss of dopaminergic modulation at corticostriatal synapses may alter glutamatergic synaptic activity. In the present work, we analyzed the possible changes in the responses to mGlu receptor activation occurring in the striatum of mice bearing the mutations for two distinct models of inherited parkinsonism. Elucidation of the impact of these gene products on the functional interplay between the dopaminergic system and the glutamatergic pathway at striatal level is crucial for our understanding of the pathogenic mechanisms underlying familial and sporadic PD, and might provide novel targets for therapeutic intervention.
Methods
All experiments were conducted in accord to the EC guidelines (86/609/EEC). Corticostriatal coronal slices (270–300 μm) were prepared from 8- to 11-week-old mice, as described in detail previously (Goldberg et al., 2003; Kitada et al., 2007). For each strain, littermates of the same mixed genetic background were utilized as controls. Mice were killed by cervical dislocation, brains were removed and slices were cut with a vibratome in Krebs solution (in mM: 26 NaCl, 2.5 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 11 glucose, and 25 NaHCO3; 95% O2–5% CO2; 3 °C). For electrophysiological recordings, slices were transferred to a recording chamber mounted on the stage of an upright microscope, completely submerged and continuously super-fused with Krebs solution (32.5 °C; 2–3 ml/min). Intracellular sharp recording electrodes were filled with 2 M KCl (30–60 MΩ). Signals were recorded with an Axoclamp 2B amplifier (Axon Instruments, Foster City CA 94404, USA), displayed on a oscilloscope and stored both on a high-gain chart recorder (Gould RS 3400) and on PC using Digidata 1322A and pClamp 9 (Axon Instruments).
To evoke corticostriatal excitatory postsynaptic potentials (EPSPs), a bipolar electrode was placed in the white matter in close proximity of the recording electrode in order to activate corticostriatal fibers. Test stimuli were delivered at a frequency of 0.1 Hz in the presence of 10 μM bicuculline to block GABAA-mediated responses. The pharmacological effects on EPSPs recorded from knockout mice were calculated as percent of control amplitude in the respective WT neuronal population. Paired-pulse ratio (PPR) for EPSPs was calculated by dividing the amplitude of the second EPSP by the amplitude of the first EPSP in response to a pair of synaptic stimuli (50 ms interstimulus interval). Clampfit 9 (Axon Instruments) was used for off-line analysis. Values given in figures and text are mean±SEM. “n” values refer to the number of cells tested, each cell being recorded from a different slice. One-way and two-ways ANOVA were used to assess statistical significance, set at p<0.05. Since values obtained from the two WT populations (PINK1+/+ and Parkin+/+) were not statistically different, data were pooled.
Whole-cell recordings were obtained from individual cells visualized using infrared differential interference contrast (IR-DIC) video-microscopy in the striatum (Kitada et al., 2007) by utilizing borosilicate glass pipettes (1.5 mm outer diameter, 0.86 inner diameter) pulled on a P-97 Puller (Sutter Instruments). Pipette resistances ranged from 2.5 to 5 MΩ. Membrane currents were continuously monitored and access resistance measured in voltage-clamp was in the range of 5–30 MΩ prior to electronic compensation (60–80% was routinely used). Signals were recorded with an Axopatch 200B amplifier and stored on PC using pClamp 9 (Axon Instruments, Foster City, CA, USA). Whole-cell access resistance was 10–20 MΩ. Neurons in which access resistance changed by more than 15% were discarded from statistics. To study glutamate-mediated spontaneous (sEPSCs) and miniature excitatory postsynaptic currents (mEPSCs), recording pipettes were filled with internal solution containing (mM): 125 K+-gluconate,10 NaCl, 1 CaCl2, 2 MgCl2,1 BAPTA, 19 Hepes, 0.3 GTP and 2 Mg-ATP, adjusted to pH 7.3 with KOH. Bicuculline (10 μM) was added to the perfusing solution to block GABAA-mediated transmission. Tetrodotoxin (TTX, 1 μM) was added to the bicuculline-containing solution to obtain mEPSCs. Cells were clamped at the holding potential (HP) of −80 mV. Drugs (bicuculline-methiodide from Sigma, Italy; baclofen, 3,5-DHPG, LY379268, LY341495, L-AP4, TTX from Tocris, UK) were added to the bathing solution and applied by switching the perfusion from control to drug-containing solution.
Results
Membrane and synaptic properties of medium spiny neurons
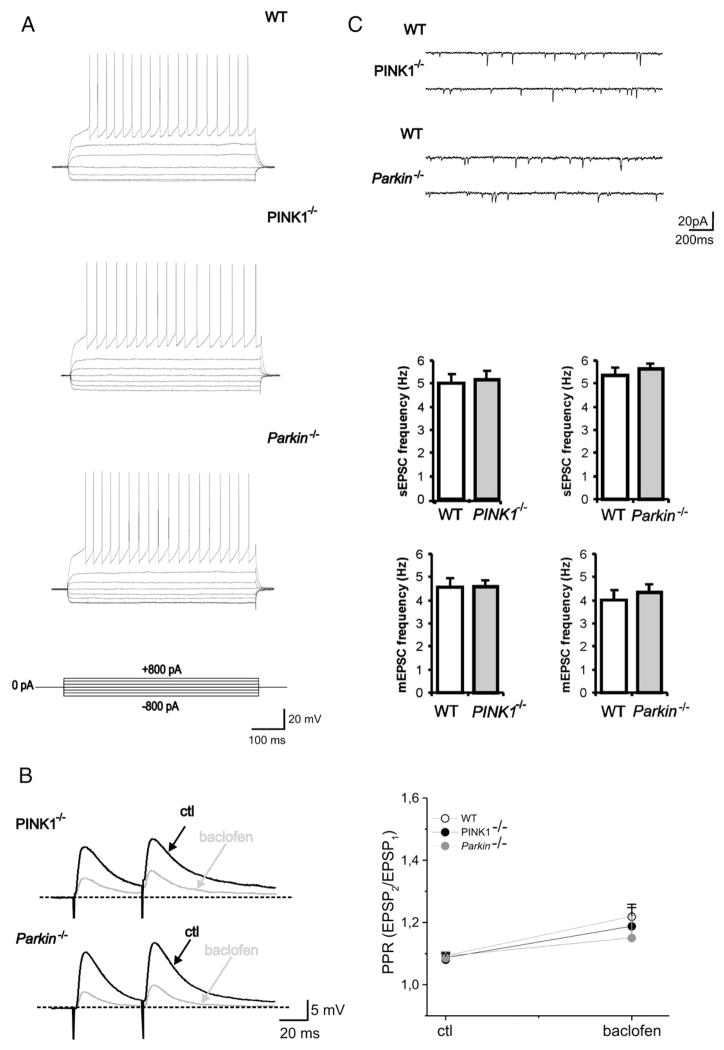
In agreement with previous reports (Goldberg et al., 2003; Kitada et al., 2007), medium spiny neurons (MSNs) recorded from both PINK1−/−, Parkin−/− mice as well as from their respective WT littermates exhibited no significant changes in their intrinsic membrane properties. MSNs from both WT and mutant mice had similar resting membrane potential (−83±3 mV for WT, n=32; −82±4 mV for PINK1−/−, n=38; −85±2.9 mV for Parkin−/−, n=35; p>0.05) were silent at rest and, upon depolarizing current pulses showed membrane rectification and tonic action potential discharge (Fig. 1A). The current–voltage relationship did not show any relevant change among groups (data not shown; p>0.05). No significant difference was found in EPSP amplitude between both PINK1−/− and Parkin−/− mice and their WT mice (WT: 22±7.8 mV, n=28; PINK1−/− 21.3±9.1 mV, n=34; Parkin−/−: 22.1±6.6, n=33; p>0.05). Moreover, EPSPs were equally sensitive to a combination of the AMPA and NMDA ionotropic glutamate receptor antagonists, CNQX (10 μM) and MK-801 (30 μM), respectively (not shown, p>0.05). The GABAB receptor agonist baclofen has been shown to presynaptically inhibit corticostriatal glutamate-mediated synaptic potentials (Calabresi et al., 1991). Indeed, perfusion with baclofen (10 μM, 2 min) largely reduced the EPSP amplitude both in WT and in transgenic mice without affecting the RMP of the recorded neurons (Fig. 1B; n=4 for WT and 5 for each −/−; p<0.05). The extent of the baclofen-induced decrease in amplitude was similar in both genotypes (Fig. 1B p>0.05). The inhibitory effect of baclofen was associated to an increase in PPR in WT as well as in PINK1−/− and Parkin−/− mice, consistent with a presynaptic site of action of baclofen (Schulz et al., 1994) (Fig 1B, p<0.05). No significant difference was observed in the baclofen-induced increase in PPR between the two genotypes. The activity of striatal MSNs is primarily driven by cortical glutamatergic inputs. Thus, as a further indicator of glutamatergic activity, we recorded sEPSCs from MSNs of WT and transgenic mice (Fig. 1C). MSNs were clamped at −80 mV and sEPSCs were recorded in the presence of the GABAA receptor antagonist bicuculline (10 μM). A combination of NMDA and AMPA glutamate receptor antagonists, MK-801 (30 μM) and CNQX (10 μM) fully blocked spontaneous synaptic events (not shown). sEPSCs recorded from both PINK1−/−, Parkin−/− mice and their respective WT littermates, showed comparable frequencies and amplitudes (Fig. 1C; WT PINK1: 5±0.4 Hz, 10.1±0.4 pA, n=19; PINK1−/−: 5.2±0.4 Hz, 10.6±0.5 pA, n=22; WT Parkin: 5.4±0.4 Hz, 10.3±0.4 pA, n=23; Parkin−/−: 5.7±0.3 Hz, 10.1±0.3 pA, n=30; p>0.05). Likewise, miniature currents that represent a measure of spontaneous neurotransmitter release probability (Bekkers et al., 1990) did not differ (Fig. 1C; WT PINK1: 4.6±0.3 Hz, 10±0.4 pA, n=10; PINK1−/−: 4.6±0.7 Hz, 9.9±0.5 pA, n=11; WT Parkin: 4.1±0.4 Hz, 10.5±0.3 pA, n=10; Parkin−/−: 4.4±0.4, 10.1±0.4 pA, n=12, p>0.05). Kinetic properties, such as rise time, decay time constant and half width, were similar (not shown; p>0.05).
Fig. 1.
Normal intrinsic and synaptic properties in PINK1−/− and Parkin−/− mice. (A) Current-clamp recordings obtained from a WT MSN showing a tonic firing activity induced by a depolarizing current step (800 pA, 700 ms, upper trace). No significant difference is observed when the same protocol is applied to MSN from PINK1−/− (middle trace) or Parkin−/− (lower trace) mice. Similar voltage responses are observed when current steps in the hyperpolarizing direction (−800 pA, 700 ms) were applied in the three strains (downward deflections). (B) Sharp intracellular recordings showing synaptic responses to paired stimulation (50 ms interstimulus interval) in WT (upper trace), PINK1−/− (middle trace) and Parkin−/− mice (lower trace) in control condition and in the presence of baclofen (10 μM) in the perfusing solution. Note the large decrease in EPSP amplitude as well as the increase in paired-pulse ratio (PPR, EPSP2/EPSP1) induced by baclofen (grey trace). No difference is observed in the extent of inhibition in WT and PINK1−/− or Parkin−/− mice. The graph shows that the inhibitory effect of baclofen was associated to an increase in PPR in the different genotypes, as expected for a presynaptic site of action. Each data point represents the mean±SEM of at least four individual experiments. (C) Sample traces of sEPSCs (downward deflections) recorded from MSNs in the presence of bicuculline (10 μM) in PINK1−/− and Parkin−/− and their respective WT littermates. Cells were clamped at −80 mV. The graphs show no significant changes in mean frequency of both sEPSCs and mEPSCs (recorded in the presence of TTX), between WT and knockouts.
Intrinsic properties of cholinergic interneurons and effects of Group I mGlu receptor activation
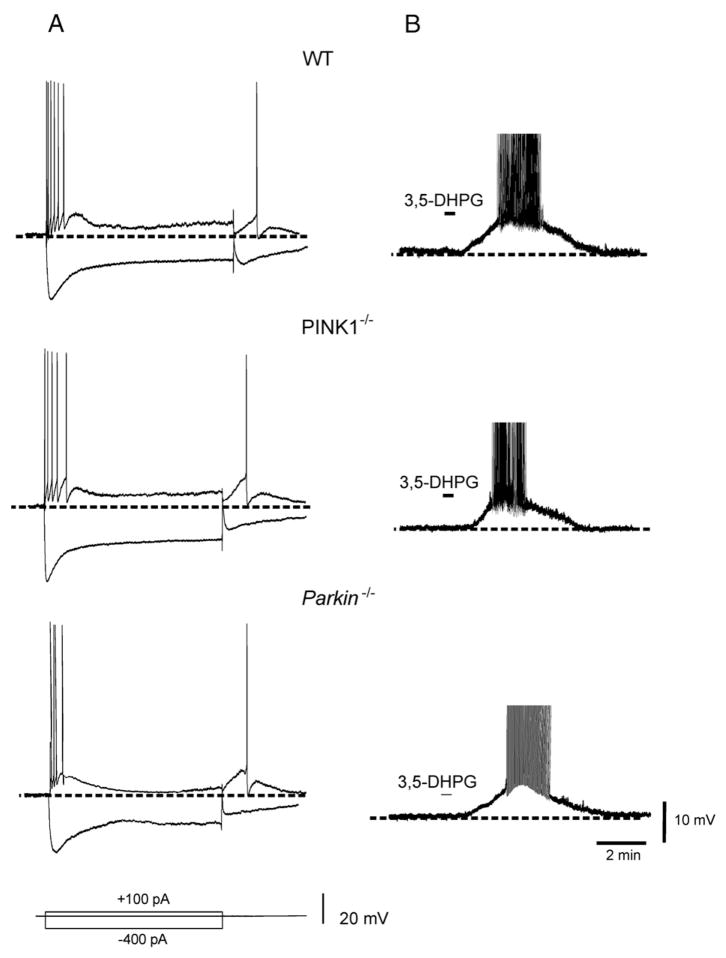
Peculiar electrophysiological characteristics distinguish cholinergic interneurons from other striatal neuronal subtypes (Kawaguchi, 1993; Pisani et al., 2007). Nearly half of the recorded neurons showed a spontaneous, tonic, irregular firing activity, ranging between 1.6±0.5 Hz. Resting membrane potential was close to firing threshold and did not differ significantly among the three genotypes (−58±4.1 mV for WT, n=13; −60±2.5 mV for PINK1−/−, n=10; −62±4.3 for Parkin−/− n=10). Injection of depolarizing current pulses elicited few action potentials (action potential amplitude: WT=75±9 mV; 69±7 mV for PINK1−/−; 67±8 mV for Parkin−/−) followed by a pronounced afterhyperpolarization. Hyperpolarizing current pulses evoked a prominent sag that has been attributed to a cation current (Ih) (Fig. 2) (Jiang and North, 1991). No significant difference in these properties was observed in either PINK1−/− or Parkin−/− mice (Fig. 2; p>0.05).
Fig. 2.
Normal properties and responses to group I mGlu receptor activation in cholinergic interneurons. (A) Sample traces showing the voltage responses to current steps in a cholinergic interneuron from WT mice. Depolarizing steps (100 pA, 1.5 s) evoke firing discharge with a rapid spike accommodation. Note also the pronounced afterhyperpolarization at the end of the depolarizing pulse. Hyperpolarizing current pulse (−400 pA, 1.5 s) evokes a prominent sag conductance, indicative of an Ih current. No significant difference was observed in PINK1−/− (middle trace) or Parkin−/− (lower trace). (B) In WT mice, bath-application of the group I mGlu agonist 3,5-DHPG (50 μM, 30 s) induced a transient membrane depolarization coupled to action potential discharge (upper trace). Similarly in cholinergic interneurons from both PINK1−/− (middle trace) or Parkin−/− (lower trace), 3,5-DHPG (50 μM, 30 s), caused a depolarizing response of similar amplitude. On drug washout, membrane potential rapidly recovered to control levels. Note that action potentials were truncated.
Within the striatum, activation of group I mGlu receptors exerts distinct role depending on the neuronal subtype involved (Bonsi et al., 2008). Cholinergic interneurons respond to the group I mGlu receptor agonist 3,5-DHPG with a robust membrane depolarization and increase in firing frequency (Pisani et al., 2001a; Bonsi et al., 2005). The response to 3,5-DHPG requires the co-activation both of mGlu1 and mGlu5 receptors (Bonsi et al., 2005). We therefore utilized such response as a tool to test the sensitivity to group I mGlu receptor activation. In WT mice, 3,5-DHPG (50 μM, 30 s) caused a transient membrane depolarization coupled to action potential discharge (Fig. 2; 15.3±3.1 mV, n=10). On drug washout, the membrane returned to resting levels. In both PINK1−/− and Parkin−/− mice, 3,5-DHPG caused a membrane depolarization comparable in amplitude to the one observed in WT mice (Fig. 2; 15.8±4.8 mV, n=8 and 15±2.9 mV, n=9, for PINK1−/− and Parkin−/− mice, respectively; p>0.05). Furthermore, the excitatory response to 3,5-DHPG persisted in the presence of TTX (1 μM), supporting the postsynaptic origin of the response to group I mGlu receptor activation (not shown, p>0.05).
Synaptic responses to Group II mGlu receptor activation
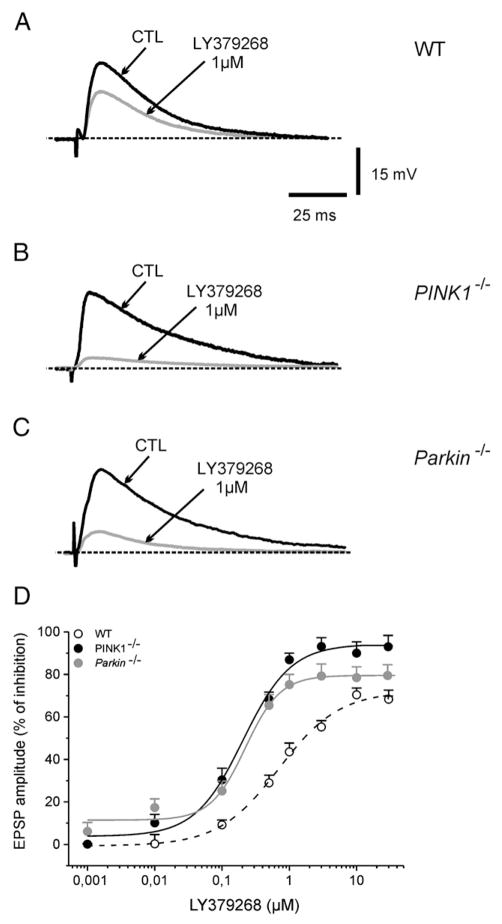
Activation of both mGlu2/3 and mGlu4/7/8 receptors has been shown to cause a presynaptic inhibition at corticostriatal synapses (Lovinger and McCool, 1995; Pisani et al., 1997). In WT mice, bath-application of the selective mGlu2/3 agonist LY379268 (0.001–30 μM, 3 min) depressed in a dose-dependent manner corticostriatal EPSPs (Fig. 3, n=22; p<0.05), without causing any significant change of intrinsic membrane properties of MSNs. The maximal effect was observed at 10 μM, with an estimated IC50 of 0.7±0.1 μM. Interestingly, both in PINK1−/− and Parkin−/− mice, we observed a net increase in the sensitivity to LY379268. As shown (Fig. 3), the maximal inhibition caused by LY379268 was obtained at 1 μM, and the calculated IC50 was 0.2±0.04 μM for PINK1−/− (n=14, p<0.05) and 0.2±0.05 μM for Parkin−/− (n=12, p<0.05), with a leftward shift in the dose–response curve. Hill’s coefficient was 0.42 in WT, 0.76 for PINK1−/− and 0.84 for Parkin−/− mice. On drug washout, the EPSP amplitude recovered to control levels. Additionally, these responses were fully sensitive to the group II antagonist LY341495 (2 μM; n=6 for each strain, not shown).
Fig. 3.
Enhanced sensitivity to group II mGlu receptor activation in PINK1−/− and Parkin−/− ice. (A) Representative traces of glutamate-mediated corticostriatal excitatory postsynaptic potentials (EPSPs) recorded from MSN of WT mice. The superimposed EPSPs show the inhibitory effect of the group II mGlu receptor agonist LY379268 (1 μM, 3 min) as compared to the control EPSP (CTL). (B, C) In PINK1−/− as well as in Parkin−/− mice, the same concentration of LY379268 causes a significantly stronger inhibition of EPSP. (D) The dose–response curve for the inhibitory effects of LY379268 on the EPSP amplitude in the three genotypes expressed as percent of control. Note the leftward shift in the response to LY379268 in both Parkin−/− and PINK1−/− mice. The IC50 was significantly lower in the latter strains as compared to their WT littermates (see Results for details). Each point represents the mean of at least 8 independent observations.
As a further validation of these results, we analyzed the responses to group II mGlu receptor activation on the glutamate-mediated EPSP recorded from cholinergic interneurons. These interneurons have been shown to express mGlu2 receptors, but not mGlu3 (Pisani et al., 2002). Moreover, mGlu2 receptor activation reversibly depresses glutamatergic EPSP amplitude in these interneurons (Pisani et al., 2002). Thus, under this experimental condition, we could virtually isolate the responses to mGlu2 receptor. In WT mice, bath-applied LY379268 (1 μM, 3 min) reversibly reduced EPSP amplitude (Fig. S1; 68.15±4.2%; n=5, p<0.05), without any effect on intrinsic membrane properties. Interestingly, both in PINK1−/− or Parkin−/− mice, LY379268 showed a significantly higher efficacy (Fig. S1; 91.1±9.7% and 83.6±8.1; n=5 for both strains; p<0.05) as compared to WT.
Normal responses of group III mGlu receptors
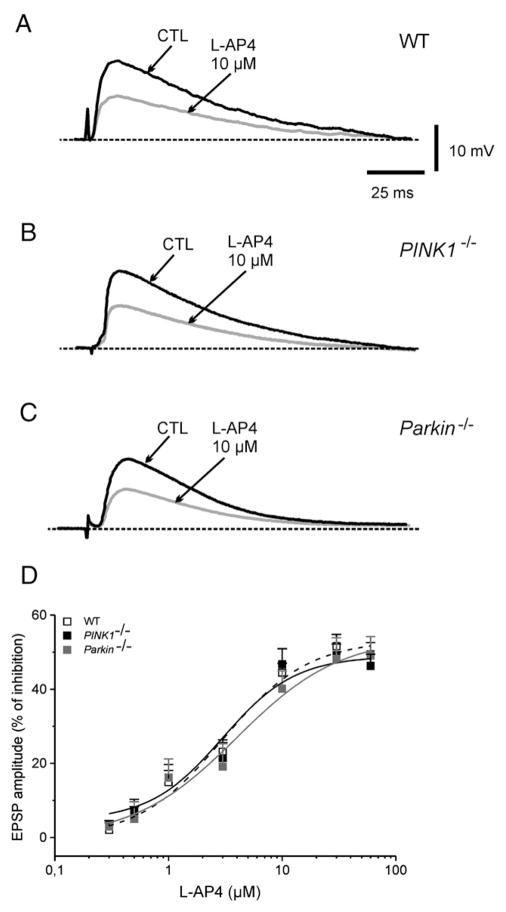
Another set of experiments was performed to test the efficacy of the group III mGlu receptor agonist, L-AP4. Bath-applied L-AP4 dose-dependently (0.3–100 μM, 3 min) reduced the EPSP amplitude, without affecting membrane properties of MSNs recorded from WT mice (Fig 4; n=10; p<0.05). The reduction of EPSP amplitude observed in WT MSNs was comparable to that measured in MSNs from both PINK1−/− or Parkin−/− mice (Fig. 4; n=13 for PINK1−/− and 10 for Parkin−/−, respectively; p>0.05). The peak-inhibition was obtained at 30 μM L-AP4, with an IC50 of 3.7±0.9 μM in WT mice, 2.98±1.3 μM for PINK1−/−, and 3.9±1.8 μM for Parkin−/−. The calculated Hill’s coefficient was 0.065 for WT, 0.083 for PINK1−/−, and 0.071 for Parkin−/− mice. The inhibitory effects caused by L-AP4 were fully prevented by preincubation with the group III antagonist (RS)-alpha-cyclopropyl-4-phosphonophenylglycine (CPPG, 100 μM, n=5) (data not shown).
Fig. 4.
Group III mGlu receptor activation in PINK1−/− and Parkin−/− mice. (A) Sample EPSPs recorded from WT mice in controls (CTL) and after bath-application of the group III mGlu receptor agonist L-AP4 (10 μM, 3 min). No significant difference in the efficacy of L-AP4 was observed either in PINK1−/− and in Parkin−/− mice (B, C). (D) The dose-dependent inhibitory action of L-AP4 did not differ among the two mutant mice, compared to WT mice. Each data point is the average of at least 6 individual experiments.
Changes in paired-pulse facilitation
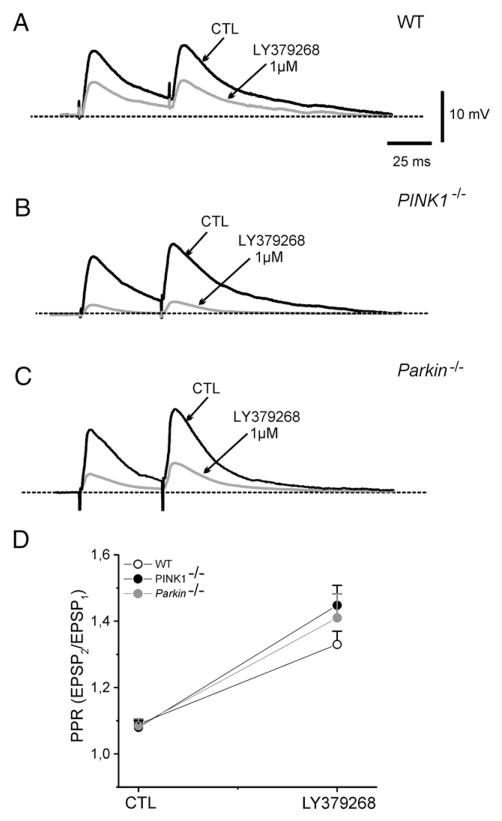
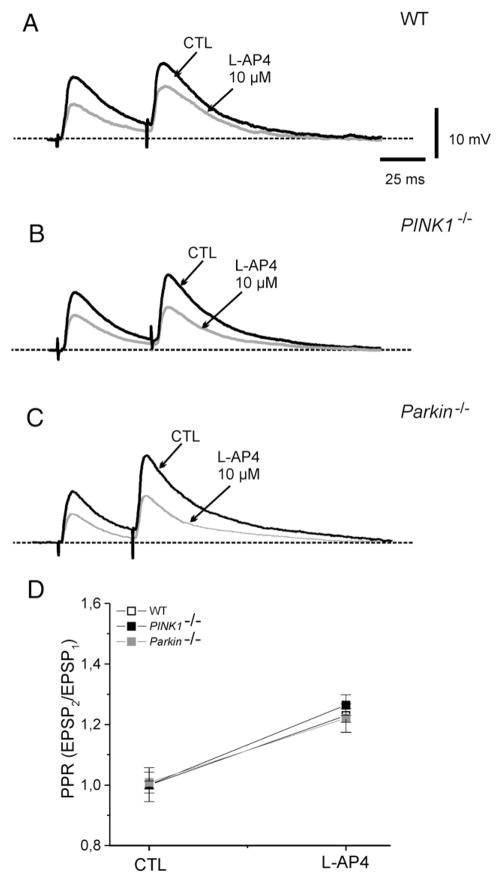
Additionally, to verify whether LY379268 and L-AP4 were acting presynaptically, we measured the effects of both agonists on the paired-pulse ratio (PPR) of corticostriatal EPSP. Changes in PPR are considered as reliable indicators of modifications in transmitter release probability (Schulz et al., 1994). Pairs of synaptic stimuli, 50 ms apart, were delivered at a stimulation rate of 0.1 Hz., in the presence of bicuculline in the bathing medium (10 μM). In MSNs recorded from WT mice, the PPR increased from a mean of 1.09±0.014 to 1.33±0.04 in the presence of 1 μM LY379268 (Fig. 5; n=9; p<0.05). In slices from PINK1−/− mice, PPR increased from 1.08±0.02 to 1.44±0.06 in LY379268 (Fig. 5; n=8; p<0.05). Similarly, in Parkin−/− mice, the PPR increased from 1.08±0.02 to 1.41±0.07 (Fig. 5; n=8; p<0.05). No significant difference was found among the three genotypes (p>0.05). Similarly, bath-applied L-AP4 (10 μM, 3 min) caused an increase in PPR in WT mice (Fig. 6, n=7; p<0.05). Such an effect was not significantly different both in PINK1−/− and Parkin−/− mice (Fig. 6; n=8 and 7, respectively; p>0.05).
Fig. 5.
LY379268 increases the Paired-Pulse Ratio in PINK1−/− and Parkin−/− mice. (A) Representative traces showing pairs of synaptic stimuli, separated by 50 ms, applied with a repetition rate of 0.1 Hz. LY379268 (1 μM, 3 min) increased the Paired-Pulse Ratio (PPR) in WT mice, as well as in PINK1−/− and Parkin−/− mice (B, C). (D) The plot summarizes the PPR experiments in the three strains, showing the net increase in PPR, measured as the ratio between EPSP2/EPSP1 in control conditions (CTL) or in the presence of the drug.
Fig. 6.
L-AP4 increases the Paired-Pulse Ratio in PINK1−/− and Parkin−/− mice. (A) Superimposed traces showing the efficacy of L-AP4 (10 μM, 3 min) in reducing the EPSP amplitude and increasing the PPR in WT, PINK1−/− and Parkin−/− mice (B, C). (D) Summary of the PPR experiments in the three strains, showing no significant change among the three strains.
Effects of dopamine replacement
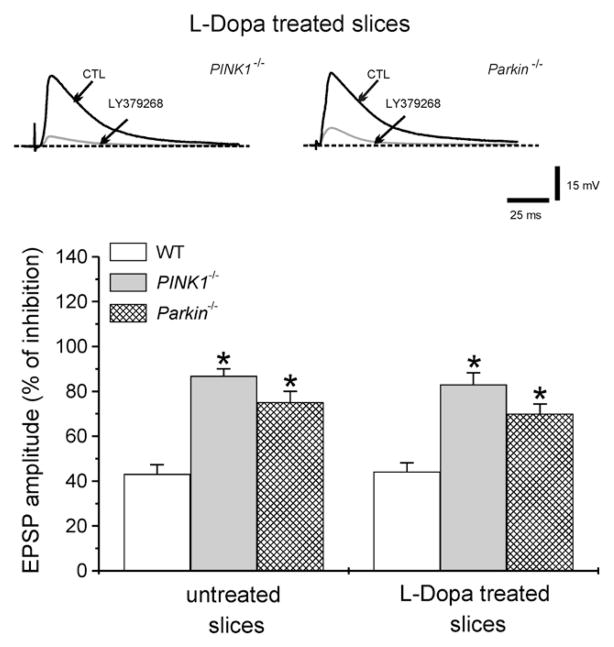
In a final set of experiments, we tested the possibility that dopamine replacement could restore a normal sensitivity to group II mGlu receptor activation. In slices from either PINK1−/− or Parkin−/− mice, incubated with the dopamine precursor L-Dopa (100 μM, 20–30 min), LY379268 (1 μM) exhibited similar potency in depressing EPSP amplitude as compared to non-treated slices (Fig. 7.; 44.1±3.9% for WT, n=4; 83.1±5.6% for PINK1−/− n=4; 70±5% for Parkin−/− n=4, p<0.05) suggesting that the adaptive changes occurring in PINK1−/− and Parkin−/− mice could not be reverted by an acute pharmacological treatment.
Fig. 7.
Effects of dopamine replacement. Sample recordings from either PINK1−/− or Parkin−/− mice obtained from slices pretreated (20–30 min) with 100 μM L-Dopa. Superimposed traces showing the net reduction of EPSP amplitude by LY379268 (1 μM). The plot summarizes the effects of LY379268 in WT, PINK1−/− and Parkin−/− mice in untreated or L-Dopa-treated slices.
Discussion
In the present study, we demonstrate that in two distinct mouse models of familial PD linked to mutations in PINK1 or Parkin genes, in which an altered dopamine signaling has been documented (Kitada et al., 2007, 2008), the electrophysiological responses to group II mGlu receptor activation are selectively upregulated. MGluR2/3 agonists were indeed able to reduce corticostriatal EPSP with a greater efficacy both in PINK1- or Parkin-deficient mice. Such increase in sensitivity could not be reverted by acute dopamine replacement. Moreover, the lack of any effect on the intrinsic membrane properties of the recorded cell, together with the net increase in paired-pulse ratio suggests a presynaptic effect. Group II mGlu receptor expression has been demonstrated on glutamatergic corticostriatal terminals, on cholinergic interneurons, as well as on MSNs projecting to the globus pallidus in the rat, and also in primates and humans (Phillips et al., 2000; Pisani et al., 2002; Samadi et al., 2008; Smith et al., 2001; Testa et al., 1998; for rev. see Conn et al., 2005). An interesting question is whether the observed changes are linked to mGlu2 or mGlu3 receptors. Although LY379268 cannot differentiate between the two group II mGlu receptor subtypes, our evidence of an enhanced sensitivity to LY379268 also in cholinergic interneurons suggests a primary involvement of mGlu2 receptors, since cholinergic interneurons do not express mGlu3 receptors (Pisani et al., 2002).
On the contrary, pharmacological activation of both group I and group III mGlu receptors did not reveal significant differences either in PINK1- or in Parkin-deficient mice. Yet, the lack of any significant change in sensitivity to a wide range of doses of L-AP4 rules out a major involvement of mGlu4, mGlu7 or mGlu8 receptors.
Functional interplay between dopamine and glutamate transmission in the striatum
Within the striatum, glutamatergic terminals make asymmetrical synapses on the heads of dendritic spines of MSNs, whereas nigrostriatal fibers establish synaptic contacts on spine necks, dendritic shafts and perykaria of MSNs. The proximity of corticostriatal and nigrostriatal dopaminergic terminals forms an anatomical basis for a close functional interplay (Smith and Bolam, 1990). Moreover, recent studies demonstrated a strong interaction between mGlu2/3 receptors and dopamine signaling (David and Abraini, 2003; Morishima et al., 2005). Indeed, a compensatory increase in corticostriatal glutamatergic synaptic activity was observed in 6-hydroxy-dopamine (6-OHDA)-denervated rats (Calabresi et al., 1993; Gubellini et al., 2006). Similarly to our findings from PINK1 and Par-kin-deficient mice, in the striatum of 6-OHDA-treated rats the efficacy of group II mGlu receptor agonists in inhibiting corticostriatal EPSPs was significantly increased (Picconi et al., 2002). However, contrarily to what observed in 6-OHDA-denervated striata, we did not detect any alteration in spontaneous glutamate-mediated synaptic events either in PINK1−/− or in Parkin−/− mice, suggesting that, compared to the acute denervation of dopaminergic fibers occurring in the 6-OHDA model, in these mice the gene mutation produces adaptive changes that lead to a compensation across development. Consistent with the notion of a functional link between group II mGlu receptors and dopamine, in 6-OHDA-denervated rats chronic L-Dopa treatment reverted both the increase in glutamatergic activity as well as the enhanced sensitivity to group II agonists. Conversely, in PINK1−/− or in Parkin−/− mice, acute L-Dopa treatment was not sufficient to restore the normal responsiveness to LY379268. The mechanistic aspects of the relationship between group II and dopamine receptors have not been clarified yet. However, given that both D2 dopamine and group II mGlu receptors are negatively coupled to adenylate cyclase, it could be inferred that if dopaminergic signaling is deficient, upregulation of group II mGlu receptors at post-receptor level, could represent a compensatory change.
Several lines of evidence demonstrate an impaired dopaminergic signaling in PINK1 and Parkin-deficient mice. First, in both genotypes, evoked dopamine overflow was largely reduced. Additionally, quantal cathecolamine release from chromaffin cells was significantly reduced as compared to WT littermates (Kitada et al., 2007, 2008). Finally, impaired corticostriatal long-term depression (LTD) in PINK1 and Parkin-deficient mice was restored by applying agents capable of increasing the availability of striatal dopamine, such as amphetamine (Kitada et al., 2007, 2008).
The presence of an altered nigrostriatal dopaminergic function is also demonstrated by clinical imaging studies in humans carrying the gene mutations. Both Positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies of PD patients carrying PINK1 and Parkin mutations revealed a presynaptic dopaminergic dysfunction in the striatum (Albanese et al., 2005; Binkofski et al., 2007; Varrone et al., 2004).
An effective role of mGlu2/3 receptor agonists has been shown in experimental models of parkinsonism. First, the selective agonist LY354740 administered systemically in rats reversed the parkinsonian rigidity induced by chronic haloperidol treatment (Konieczny et al., 1998). Secondly, mGlu2/3 receptor agonists were shown to possess antiakinetic properties both in the haloperidol-induced catalepsy and in the reserpine-induced akinesia models (Dawson et al., 2000; Murray et al., 2002). In addition, LY379268 has proven effective in protecting nigral neurons against MPTP toxicity (Battaglia et al., 2003).
Our observation that the adaptive changes in group II mGlu receptor sensitivity occur, to a similar extent, in two mouse models lacking distinct genes is not unexpected. In fact, it has been shown that Parkin acts downstream from PINK1 in a common pathway that regulates mitochondrial function. Accordingly, overexpression of Parkin was able to compensate for the loss of PINK1. Moreover, double PINK1–Parkin mutant flies had a phenotype that was indistinguishable from the one seen in single PINK1 or Parkin mutants (Clark et al., 2006; Park et al., 2006).
A definite link between PINK1 or Parkin and mGlu receptors remains to be established, also considering that the mechanistic aspects of PINK1 and Parkin gene product function are still largely unknown (Cookson et al., 2007). The PINK1/Parkin pathway has been shown to regulate mitochondrial morphology and function (Poole et al., 2008). In fact, mutations in both genes lead to cellular energetic defects, such as ATP depletion, enhanced sensitivity to oxidative stress (Gautier et al., 2008). Activation of group II mGlu receptors has been shown to exert neuroprotective effects by promoting the release of neurotrophic factors (Corti et al., 2007). Thus, it might be hypothesized that an upregulation of group II mGlu receptors would reflect a compensatory neuroprotective change, through an increased release of neurotrophic factors.
Alternatively, because PINK1 contains a serine/threonine protein kinase domain and mutations are found commonly in the kinase region (Cookson et al., 2007), another explanation could be that PINK1 and mGlu receptors share common substrates for phosphorylation processes, that might undergo plastic rearrangements in brain areas, such as the striatum, where dopaminergic influence is relevant.
Clarifying the possible link between these gene products and the functional rearrangements involving the corticostriatal pathway is relevant for a better understanding both of inherited and sporadic PD, suggesting alternative targets for pharmacological treatment.
Supplementary Material
Acknowledgments
This work was supported by grants from Ministero Salute (Prog. Ricerca Finalizzata, RF06.55); from the Italian Space Agency, DCMC grant and MIUR (PRIN 2006).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.expneurol.2008.11.001.
References
- Albanese A, Valente EM, Romito LM, Bellacchio E, Elia AE, Dallapiccola B. The PINK1 phenotype can be indistinguishable from idiopathic Parkinson disease. Neurology. 2005;64 (11):1958–1960. doi: 10.1212/01.WNL.0000163999.72864.FD. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Busceti CL, Pontarelli F, Biagioni F, Fornai F, Paparelli A, Bruno V, Ruggieri S, Nicoletti F. Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology. 2003;45 (2):155–166. doi: 10.1016/s0028-3908(03)00146-1. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Richerson GB, Stevens CF. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci U S A. 1990;87:5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Reetz K, Gaser C, Hilker R, Hagenah J, Hedrich K, van Eimeren T, Thiel A, Büchel C, Pramstaller PP, Siebner HR, Klein C. Morphometric fingerprint of asymptomatic Parkin and PINK1 mutation carriers in the basal ganglia. Neurology. 2007;69 (9):842–850. doi: 10.1212/01.wnl.0000267844.72421.6c. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299 (5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, De Persis C, Centonze D, Bernardi G, Calabresi P, Pisani A. Modulatory action of metabotropic glutamate receptor (mGluR) 5 on mGluR1 function in striatal cholinergic interneurons. Neuropharmacology. 2005;49 (Suppl 1):104–113. doi: 10.1016/j.neuropharm.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Sciamanna G, Mitrano DA, Cuomo D, Bernardi G, Platania P, Smith Y, Pisani A. Functional and ultrastructural analysis of group I mGluR in striatal fast-spiking interneurons. Eur J Neurosci. 2007;25 (5):1319–1331. doi: 10.1111/j.1460-9568.2007.05383.x. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Platania P, Martella G, Madeo G, Vita D, Tassone A, Bernardi G, Pisani A. Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology. 2008;55 (4):392–395. doi: 10.1016/j.neuropharm.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, De Murtas M, Bernardi G. Involvement of GABA systems in feedback regulation of glutamate- and GABA-mediated synaptic potentials in rat neostriatum. J Physiol. 1991;440:581–599. doi: 10.1113/jphysiol.1991.sp018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Sancesario G, Bernardi G. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson’s disease. Brain. 1993;116 (Pt 2):433–452. [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev, Neurosci. 2005;6 (10):787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- Cookson MR, Dauer W, Dawson T, Fon EA, Guo M, Shen J. The roles of kinases in familial Parkinson’s disease. J Neurosci. 2007;27 (44):11865–11868. doi: 10.1523/JNEUROSCI.3695-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, Mugnaini M, Nicoletti F, Bruno V. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci. 2007;27 (31):8297–8308. doi: 10.1523/JNEUROSCI.1889-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David HN, Abraini JH. Blockade of the locomotor stimulant effects of amphetamine by group I, group II, and group III metabotropic glutamate receptor ligands in the rat nucleus accumbens: possible interactions with dopamine receptors. Neuropharmacology. 2003;44 (6):717–727. doi: 10.1016/s0028-3908(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Dawson L, Chadha A, Megalou M, Duty S. The group II metabotropic glutamate receptor agonist, DCG-IV, alleviates akinesia following intranigral or intraventricular administration in the reserpine-treated rat. Br J Pharmacol. 2000;129 (3):541–546. doi: 10.1038/sj.bjp.0703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105 (32):11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278 (44):43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT. Glutamatergic influences on the basal ganglia. Clin Neuropharmacol. 2001;24 (2):65–70. doi: 10.1097/00002826-200103000-00001. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Eusebio A, Oueslati A, Melon C, Kerkerian-Le Goff L, Salin P. Chronic high-frequency stimulation of the subthalamic nucleus and L-DOPA treatment in experimental parkinsonism: effects on motor behaviour and striatal glutamate transmission. Eur J Neurosci. 2006;24 (6):1802–1814. doi: 10.1111/j.1460-9568.2006.05047.x. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13 (11):4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392 (6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104 (27):11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Karouani M, Martella G, Platania P, Madeo G, Pothos EN, Shen J. Impaired catecholamine release in the striatum of parkin mutant mice. The 38th Society for Neurosceince Annual Meeting; Washington DC. 2008. [Google Scholar]
- Klein C, Lohmann-Hedrich K, Rogaeva E, Schlossmacher MG, Lang AE. Deciphering the role of heterozygous mutations in genes associated with parkinsonism. Lancet Neurol. 2007;6 (7):652–662. doi: 10.1016/S1474-4422(07)70174-6. [DOI] [PubMed] [Google Scholar]
- Konieczny J, Ossowska K, Wolfarth S, Pilc A. LY354740, a group II metabotropic glutamate receptor agonist with potential antiparkinsonian properties in rats. Naunyn-Schmiedeberg’s Arch Pharmacol. 1998;358 (4):500–502. doi: 10.1007/pl00005284. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, McCool BA. Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol. 1995;73 (3):1076–1083. doi: 10.1152/jn.1995.73.3.1076. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Emre N, Nakamura CM, Allen C, Donohue MK, Buckman JF. Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience. 1999;88 (1):1–16. doi: 10.1016/s0306-4522(98)00189-4. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci U S A. 2005;102 (11):4170–4175. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TK, Messenger MJ, Ward MA, Woodhouse S, Osborne DJ, Duty S, O’Neill MJ. Evaluation of the mGluR2/3 agonist LY379268 in rodent models of Parkinson’s disease. Pharmacol Biochem Behav. 2002;73 (2):455–466. doi: 10.1016/s0091-3057(02)00842-0. [DOI] [PubMed] [Google Scholar]
- Paisán-Ruíz C, Jain S, Evans EW, Gilks WP, Simón J, van der Brug M, López de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Martí-Massó JF, Pérez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44 (4):595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441 (7097):1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Phillips T, Rees S, Augood S, Waldvogel H, Faull R, Svendsen C, Emson P. Localization of metabotropic glutamate receptor type 2 in the human brain. Neuroscience. 2000;95 (4):1139–1156. doi: 10.1016/s0306-4522(99)00353-x. [DOI] [PubMed] [Google Scholar]
- Picconi B, Pisani A, Centonze D, Battaglia G, Storto M, Nicoletti F, Bernardi G, Calabresi P. Striatal metabotropic glutamate receptor function following experimental parkinsonism and chronic levodopa treatment. Brain. 2002;125 (Pt 12):2635–2645. doi: 10.1093/brain/awf269. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Bernardi G. Activation of group III metabotropic glutamate receptors depresses glutamatergic transmission at corticostriatal synapse. Neuropharmacology. 1997;36 (6):845–851. doi: 10.1016/s0028-3908(96)00177-3. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Centonze D, Bernardi G, Calabresi P. Functional coexpression of excitatory mGluR1 and mGluR5 on striatal cholinergic interneurons. Neuropharmacology. 2001a;40 (3):460–463. doi: 10.1016/s0028-3908(00)00184-2. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001b;106 (3):579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Catania MV, Giuffrida R, Morari M, Marti M, Centonze D, Bernardi G, Kingston AE, Calabresi P. Metabotropic glutamate 2 receptors modulate synaptic inputs and calcium signals in striatal cholinergic interneurons. J Neurosci. 2002;22 (14):6176–6185. doi: 10.1523/JNEUROSCI.22-14-06176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30 (10):545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276 (5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105 (5):1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi P, Grégoire L, Morissette M, Calon F, Tahar AH, Bélanger N, Dridi M, Bédard PJ, Di Paolo T. Basal ganglia group II metabotropic glutamate receptors specific binding in non-human primate model of L-Dopa-induced dyskinesias. Neuropharmacology. 2008;54 (2):258–268. doi: 10.1016/j.neuropharm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14 (9):5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, Matsuno-Yagi A, Miller GW, Greenamyre JT. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson’s disease. J Neurochem. 2007;100 (6):1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13 (7):259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Smith Y, Charara A, Paquet M, Kieval JZ, Paré JF, Hanson JE, Hubert GW, Kuwajima M, Levey AI. Ionotropic and metabotropic GABA and glutamate receptors in primate basal ganglia. J Chem Neuroanat. 2001;22 (1–2):13–42. doi: 10.1016/s0891-0618(01)00098-9. [DOI] [PubMed] [Google Scholar]
- Testa CM, Friberg IK, Weiss SW, Standaert DG. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390 (1):5–19. [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304 (5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Varrone A, Pellecchia MT, Amboni M, Sansone V, Salvatore E, Ghezzi D, Garavaglia B, Brice A, Brunetti A, Bonavita V, De Michele G, Salvatore M, Pappatà S, Barone P. Imaging of dopaminergic dysfunction with [123I]FP-CIT SPECT in early-onset parkin disease. Neurology. 2004;63 (11):2097–2103. doi: 10.1212/01.wnl.0000145765.19094.94. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Müller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44 (4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.