Abstract
Evidence suggests that the orexigenic peptide, galanin (GAL), in the hypothalamic paraventricular nucleus (PVN) has a role in stimulating the consumption of ethanol, in addition to a high-fat diet. This possibility was further examined in mutant mice that overexpress the GAL gene. Two sets of GAL-overexpressors (GALOE) compared to wild-type (WT) controls, maintained on lab chow and water, were trained to voluntarily drink increasing concentrations of ethanol, from 3% to 15%. In the GALOE vs WT mice, the results revealed: 1) a 35–40% increase in ethanol intake and ethanol preference, which was evident only at the highest (15%) ethanol concentration, in male but not female mice, and was seen with comparisons to littermate as well as non-littermate WT controls; 2) a significantly larger, 60–75% increase in ethanol intake and ethanol preference after a day of food deprivation, again only in male GALOE mice; 3) no change in consumption of sucrose or quinine solutions in preference tests; and 4) a 55% increase in consumption of a fat-rich diet during a 2-h test period, in both male and female GALOE mice. These results obtained with overexpression of the GAL gene provide strong support for a physiological role of this peptide in stimulating the consumption of ethanol, as well as a fat-rich diet. They reveal gender differences in the behavioral phenotype, which may reflect GAL’s functional relationship to reproductive hormones in the stimulation of consummatory behavior.
Keywords: Ethanol intake, galanin, paraventricular nucleus, dietary fat
Introduction
Studies demonstrate a stimulatory effect of galanin (GAL) on food intake (Kyrkouli et al., 1986). This effect is strongest when GAL is administered directly into the hypothalamic paraventricular nucleus (PVN), where injection of a GAL receptor antagonist suppresses food intake (Leibowitz and Kim, 1992). A variety of studies have linked PVN GAL to dietary fat (Leibowitz, 2007). When injected into this nucleus, GAL induces a preference for a high-fat diet over a low-fat diet and, with repeated injections, stimulates daily food consumption, only on diets with at least 35% fat (Akabayashi et al., 1994; Leibowitz and Kim, 1992; Nagase et al., 2002; Yun et al., 2005). Further, fat intake and preference for a fat-rich diet is suppressed by PVN injection of a GAL antagonist or antisense oligonucleotide to GAL mRNA that reduces GAL peptide levels (Akabayashi et al., 1994; Leibowitz and Kim, 1992). Measurements of endogenous peptide show GAL mRNA and peptide levels in the PVN to be increased in fat-preferring rats and stimulated by acute or chronic consumption of a fat-rich diet or injection of a fat emulsion, in association with a rise in circulating triglycerides (Akabayashi et al., 1994; Chang et al., 2004; Gaysinskaya et al., 2007; Leibowitz et al., 2004). Together, these findings demonstrate that GAL-induced feeding has a specific relationship to dietary fat that is bidirectional in nature. In a positive feedback loop, GAL increases the ingestion of a high-fat diet that, in turn, further stimulates the GAL system to produce overeating of the diet.
Similar to food intake, GAL is found to stimulate the consumption of ethanol (Lewis et al., 2004; Rada et al., 2004). This effect, occurring with GAL injection in the ventricles as well as PVN, is evident even in the presence of food, with GAL stimulating ethanol but not food intake in rats trained to voluntarily drink ethanol. Also, the opposite effect, a marked decrease in ethanol intake, can be observed with PVN injection of the GAL antagonist, M40 (Rada et al., 2004). These findings suggest that this orexigenic peptide has an additional function in promoting the consumption of ethanol, similar to a high-fat diet. Clinical evidence for the involvement of endogenous GAL in ethanol intake is supported by studies showing an association of GAL haplotypes with alcoholism in distinct populations (Belfer et al., 2007) and also with circulating triglycerides (Plaisier et al., 2009), which rise with consumption of ethanol as well as fat-rich diet (Contaldo et al., 1989; Schrezenmeir, 1996).
This positive relationship of GAL with ethanol intake, similar to fat intake, is found to be bidirectional, with ethanol stimulating the expression and production of endogenous GAL. In rats trained to voluntarily drink 9% ethanol or given an injection of ethanol (0.8 g/kg), GAL mRNA or GAL peptide is increased specifically in the PVN (Chang et al., 2007; Leibowitz et al., 2003). Further, levels of PVN GAL mRNA are positively correlated with the amount of ethanol consumed, as well as levels of blood ethanol and circulating lipids, and naloxone-induced withdrawal from the opioid effects of ethanol ingestion reverses this ethanol effect on GAL, significantly reducing peptide expression below baseline levels. These studies support the existence of a positive feedback loop between this peptide and ethanol, with GAL stimulating the drinking of ethanol that, in turn, enhances the endogenous peptide, causing overconsumption of ethanol. This GAL-ethanol relationship may involve elevated triglyceride levels induced by ethanol intake, similar to that proposed for the GAL-fat feedback loop (Leibowitz, 2007). With behavioral studies revealing a close, positive relationship between ethanol and fat intake (Carrillo et al., 2004), it is possible that GAL and perhaps other peptides known to stimulate both ethanol and fat intake play a mediating role (Leibowitz, 2007).
Investigations of GAL mutants demonstrate that mice with deletions of the GAL gene or GAL receptor genes or that overexpress the GAL gene are able to defend their daily food intake and body weight when fed ad libitum on a standard, low-fat diet or after a period of food deprivation (Hohmann et al., 2003). Whereas this suggests that GAL may not have a major role in controlling feeding under these conditions, this lack of response in GAL mutants compared to wild-type (WT) controls may be attributed to other factors, e.g., the activation of compensatory mechanisms that help the animals maintain nutrient homeostasis or an insufficiency of dietary fat that may be required for GAL to exhibit its behavioral effects. This latter possibility is suggested by a chronic injection study, which showed GAL to produce a significant increase in daily food intake and body weight in rats on a high-fat but not low-fat diet (Yun et al., 2005), and also by a study in GAL knockout mice (GALKO), which compared to WT mice exhibited a decrease in food intake and specifically fat intake when tested on a mixed fat-rich diet or on pure macronutrients (Adams et al., 2008). These findings with chronic disturbances in GAL support a specific role for this peptide in controlling feeding behavior on a high-fat diet. The question addressed in the present study is whether GAL may have a similar function in controlling ethanol intake.
The present study examined transgenic mice that overexpress the GAL gene, to test the possibility that endogenous GAL has a physiological role in stimulating the consumption of ethanol. Two sets of GAL overexpressors (GALOE), known to exhibit a 2–5-fold increase in GAL expression in the brain (Crawley et al., 2002; He et al., 2005; Steiner et al., 2001), were trained to voluntarily drink increasing concentrations of ethanol, and their daily intake was compared to that of their respective WT controls. With evidence showing GAL to act in a close, positive relationship with the ovarian steroids and prolactin in controlling ingestive behavior (Leibowitz et al., 1998; Leibowitz et al., 2007; Leibowitz et al., 2009) and male GALOE mice to exhibit a marked rise in circulating prolactin levels (Perumal and Vrontakis, 2003), we examined both males and females to determine if any behavioral changes induced by GAL overexpression are gender specific. Further, to gain a better understanding of the relationship of GAL to the consumption of both ethanol and fat, we conducted two additional experiments, to determine whether a period of food deprivation that stimulates PVN GAL (Wang et al., 1998) enhances the effect of genotype on ethanol intake and whether GALOE mice show enhanced consumption of a fat-rich diet provided during a brief period at onset of the natural feeding cycle. The results of these tests provide support for the hypothesis that GAL acts physiologically to stimulate the ingestion of ethanol as well as a high-fat diet.
Materials and methods
Animals
Three heterozygous (HET) GALOE females and two HET male mice at 44 days of age (Strain Name: B6.Cg-TG(DBH-Gal)1923Stei/J) were obtained from the Jackson Laboratories (Bar Harbor, ME, USA). These GALOE mice were originally produced at the University of Washington, as previously described (Steiner et al., 2001). Briefly, a transgenic construct containing a 5.8Kb sequence of human dopamine beta-hydroxylase promoter and a 4.6 Kb sequence of mouse GAL gene was injected into fertilized SJL X C57BL/6 mouse egg, and the resulting transgenic mice were backcrossed onto the C57BL/6J background for at least 7 generations. For the present study, the female and male HET mice were bred to generate two groups of WT, HET and homozygous GALOE mice, with genotyping performed by Transnetyx Inc. (see below). Both groups contained male and female mice, with Group 1 comparing GALOE mice to non-littermate WT controls and Group 2 comparing GALOE mice to littermate WT mice. Experimental testing occurred when the mice were between 2–5 months of age, with both females and males tested in each experiment. The mice, which were sex- and age-matched in all experiments, were individually housed in plastic cages, in a fully accredited AAALAC facility (22°C, with a 12:12-h light-dark cycle with lights off at 1 pm), according to institutionally approved protocols as specified in the NIH Guide to the Use and Care of Animals and also with the approval of the Rockefeller University Animal Care Committee. The mice were maintained ad libitum on laboratory chow and water, and their food intake, water intake, and body weights were measured 2–3 times per week.
Genotyping
At 19–20 days of age, the mice from both Group 1 and Group 2 were anesthetized with isoflourene, and a sample from the tip of their tail was collected and sent to Transnetyx (Cordova, TN) for genotype analysis.
Ad libitum ethanol consumption and preference
The female and male WT and GALOE mice in Groups 1 and 2, while maintained on lab chow, were individually housed and habituated in their home cage to drink from two bottles. These bottles were 50 ml conical centrifuge tubes purchased from Nalgene Nunc International (Rochester, NY). For the first week, both bottles contained plain water, and no differences were seen between the genotypes in their daily water intake, as well as chow intake. After this adaptation week, the mice were given access to one bottle of ethanol mixed with water and the other bottle of plain water. The concentration of ethanol (v/v) was increased every 4 days, from 3% to 8%, 10% and then 15% over the course of the experiment. The position of the bottles was changed daily to control for position preference. Intake of ethanol, water and chow was measured daily, and body weight was measured twice weekly. The measure of daily ethanol intake (g/kg body weight) was obtained by averaging ethanol intake over the 4 days of measurements at each concentration. Relative ethanol preference was calculated in terms of the ratio of ethanol intake to total fluid volume consumed.
Ethanol intake in GALOE mice under food deprivation conditions
In the next behavioral test, ethanol intake in Group 1 was measured under conditions of food deprivation. This experiment involved two sets of daily measurements, of 15% ethanol, water and chow intake, on the day before and the day after a 24-h deprivation period. The ethanol, water and chow were available ad libitum for the pre-deprivation and post-deprivation periods, while only ethanol and water were available during the intervening day of food deprivation. Three such tests were conducted over a 3-week period, and the final 24 h intake values were the average of the scores obtained across these 3 tests.
Assessment of high-fat meal consumption
While maintained ad libitum on 15% ethanol, the female and male mice of Group 1 were then tested with a high-fat diet (5.15 Kcals/g), which was given for a brief period at dark onset. This diet, described in detail elsewhere (Chang et al., 2008), consisted of 50% fat (with 80% lard and 20% vegetable oil), 25% carbohydrate (with 30% dextrin, 30% cornstarch and 40% sucrose), and 25% protein (with casein from Bioserv, Frenchtown, NJ, and 0.3% L-cystine and DL-methionine from MP Biomedicals, Solon, OH) and was supplemented with 4% minerals (Briggs N Salt Mixture, MP Biomedicals) and 3% vitamins (Vitamin Diet Fortification Mixture, MP Biomedicals). To adapt them to the diet before the test, the mice were given at dark onset a daily sample (5 Kcal) over a 5-day period. For the actual experiment, the chow and ethanol were removed 2 h before the test, and the mice were then given the high-fat diet at dark onset, which they were allowed to consume ad libitum over the next 2-h period. This test was repeated 4 times over 4 consecutive days, and the final intake measure (Kcal/2 h) was the average of these scores obtained over the 4 days. One week after completing these behavioral tests, blood was collected by retro-orbital eye bleeds for measurement of blood ethanol levels. On the day of the collection, the GALOE and WT mice were allowed to drink for 3 h after dark onset, during which time they consumed similar amounts of ethanol (Females: 6.5 ± 0.66 vs 6.4 ± 1.77 g/kg; Males: 5.2 ± 0.67 vs 4.4 ± 0.50 g/kg). One week after completing these behavioral tests, blood was collected by retro-orbital eye bleeds for measurement of blood ethanol levels. Ocular blood was then collected, and ethanol levels were measured using the Analox GM7 Fast Enzymatic Metabolizer (A nalox, Lunenburg, MA).
Ad libitum sucrose/quinine access procedure
The mice of Group 2, after being taken off ethanol for 2 weeks, were examined in terms of their preference for sucrose and quinine. Over an 8-day period, the mice were given continuous access to plain water in one bottle and sucrose or quinine in the other, with the position of the bottles switched daily. They had access to these solutions over a 48-h period, with the sucrose solutions (1.70% and 4.25%) tested first, followed by the quinine solutions (0.03 mM and 0.10 M). Preference for these solutions was determined by dividing the volume of the taste solution by the total volume of taste solution and water.
Data Analysis
All values are expressed as mean ± SEM. With a standard statistical package (SPSS), statistical analyses comparing the different behavioral measures or peptide measures for the subgroups were performed using 2-way ANOVA, with repeated measures where appropriate, or an unpaired, two-tailed Student’s t-test. Effect size was determined by Cohen’s d statistic. The criterion for use of the term “significant” in the text is that the probability value for a given test is p<0.05.
Results
Experiment 1: Ethanol drinking in GALOE mice under ad libitum feeding conditions
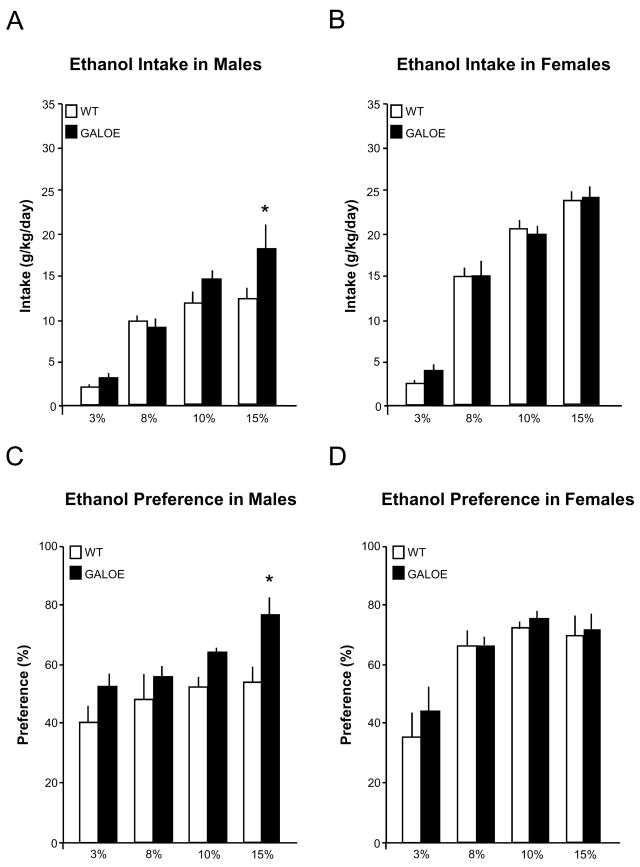
In this experiment, female and male mice overexpressing the GAL gene were given ad libitum access to increasing concentrations of ethanol, from 3% to 15%, and they were compared to their respective female and male WT control mice. In Group 1 with GALOE and non-littermate WT, 2-way ANOVAs relating genotype to ethanol intake across the four concentrations revealed a main genotype effect, increased intake of ethanol, in the male GALOE vs WT mice [F(3,44) = 3.70, p<0.01] but not in the females [F(3,47) = 0.19, p>0.10]. Direct comparisons between the GALOE and WT mice at each of the ethanol concentrations showed that this effect in the males was observed only on the 15% ethanol concentration, when intake was increased by 41% (p<0.001) (Fig. 1A), while the females showed no change at any concentration (Fig. 1B). This effect in the males was observed on their first day of exposure to 15% ethanol (p<0.05), indicating that it was this higher concentration that was needed for the effect to be revealed. Preference for ethanol relative to water was also significantly enhanced in males across concentrations [F(3,43) = 3.99, p<0.01], with the 15% ethanol revealing a 38% increase (Fig. 1C), but it was unaltered in females [F(3,47) = 0.58, p>0.10) (Fig. 1D). In contrast to their intake of ethanol, both the female and male GALOE mice throughout the experiment were similar to their respective WT controls (Table 1) in the measures of daily chow intake, water intake, total fluid intake, and body weight, except for total fluid intake on 15% ethanol, which was significantly increased (p<0.05) in male GALOE mice due to their increase in ethanol intake. The GALOE and WT mice were also similar in their blood ethanol levels when consuming 15% ethanol, as indicated by the measures in both males (62 ± 4.6 vs 59 ± 3.1 mg/dl) and females (67 ± 3.7 vs 61 ± 3.3 mg/dl). Gender comparisons using a 2-way ANOVA across all concentrations showed that female mice, both WT and GALOE, drank significantly more ethanol than the males [F(3,90) = 3.14, p<0.04]. The effect of genotype on 15% ethanol intake was not litter dependent, as it was similarly observed in Group 2 with GALOE and littermate WT controls. Consistent with results in Group 1, increased intake of 15% ethanol but not lower concentrations was seen in the male GALOE compared to littermate WT controls (18 ± 2.3 vs 12 ± 2.1 g/kg/day, p<0.04) but not in the female GALOE vs WT mice (28 ± 2.4 vs 24 ± 1.8 g/kg/day, p>0.10). Ethanol preference was also significantly increased in male GALOE mice (65 ± 3.5% vs 54 ± 2.6%, p<0.04), while unaffected in females (72 ± 6.1% vs 63 ± 7.0%, p>0.10). Together, these results demonstrate that the overexpression of endogenous GAL stimulates ad libitum consumption of ethanol, in males but not females and that this behavioral effect can be seen when comparing GALOE mice to littermate as well as non-littermate WT controls and is not associated with any clear differences in blood ethanol levels.
Figure 1.
Ethanol intake and preference in male and female galanin overexpressing (GALOE) and wild-type (WT) mice voluntarily drinking increasing concentrations of ethanol (3%–15%): (A) Ethanol intake (g/kg/day) in male mice, plotted as a function of ethanol concentration (4-day average) in a two-bottle, continuous-access paradigm; (B) Ethanol intake (g/kg/day) in female mice; (C) Ethanol preference (%) in male mice, calculated as volume of ethanol consumed divided by total volume of solution consumed (x 100); and (D) Ethanol preference (%) in female mice. The male GALOE mice compared to WT, but not female mice, showed a significant increase in ethanol intake and preference on 15% ethanol (*, p<0.01).
Table 1.
Behavioral Measures in Male and Female Mice Drinking Ethanol Ad Libitum at Different Concentrations
| Male | Female | |||
|---|---|---|---|---|
| WT | GALOE | WT | GALOE | |
| Chow Intake (kcal/day) | ||||
| 3% | 18 ± 0.81 | 16 ± 0.98 | 14 ± 0.68 | 14 ± 2.10 |
| 8% | 15 ± 0.30 | 15 ± 0.91 | 17 ± 1.60 | 15 ± 0.56 |
| 10% | 12 ± 0.49 | 13 ± 2.40 | 15 ± 0.82 | 15 ± 1.55 |
| 15% | 17 ± 0.78 | 16 ± 3.30 | 15 ± 1.50 | 14 ± 0.60 |
| Water Intake (g/kg/day) | ||||
| 3% | 101 ± 9.1 | 84 ± 8.2 | 172 ± 15 | 183 ± 10 |
| 8% | 97 ± 2.3 | 82 ± 5.2 | 97 ± 6.3 | 97 ± 2.1 |
| 10% | 73 ± 8.0 | 89 ± 7.1 | 82 ± 7.0 | 67 ± 1.4 |
| 15% | 71 ± 4.9 | 64 ± 8.6 | 76 ± 3.3 | 68 ± 8.7 |
| Total Fluid Intake (g/kg/day) | ||||
| 3% | 168 ± 7.2 | 191 ± 6.7 | 269 ± 15 | 280 ± 10 |
| 8% | 210 ± 7.4 | 196 ± 8.6 | 285 ± 9.9 | 285 ± 14 |
| 10% | 193 ± 8.4 | 220 ± 12 | 292 ± 3.9 | 267 ± 7.1 |
| 15% | 154 ± 5.4 | 184 ± 5.6* | 263 ± 14 | 220 ± 15 |
| Body Weight (g) | ||||
| 3% | 26 ± 1.50 | 25 ± 0.42 | 19 ± 0.28 | 21 ± 0.28 |
| 8% | 28 ± 1.30 | 26 ± 1.10 | 19 ± 0.11 | 21 ± 1.10 |
| 10% | 28 ± 1.40 | 27 ± 1.70 | 20 ± 0.29 | 21 ± 0.26 |
| 15% | 30 ± 1.60 | 28 ± 1.70 | 21 ± 0.33 | 22 ± 0.33 |
p < 0.05, comparing female GALOE to WT mice
Experiment 2: Ethanol intake in GALOE mice under food deprivation conditions
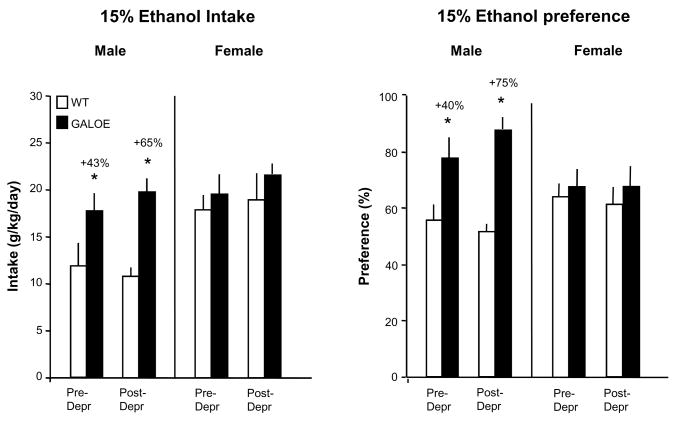
With evidence showing food deprivation or restriction to increase ethanol intake (Hansen et al., 1995; Middaugh et al., 1999) and stimulate endogenous GAL in the PVN (Wang et al., 1998), we examined the possibility that food deprivation might help to reveal a behavioral effect of GAL overexpression in females, which was absent under ad libitum feeding conditions, and possibly potentiate the stimulation of ethanol intake in males. The GALOE and WT mice of Group 1 while on 15% ethanol were deprived of food for a 24-h period, and measurements of daily ethanol intake were taken on the day before (pre-deprivation) and the day after (post-deprivation) the 24-h food deprivation. A 2-way ANOVA relating genotype to condition revealed a main genotype effect in the male mice [F(1,23) = 28.28, p<0.001], but not in the females [F(1,24) = 0.67, p>0.10] (Fig. 2). Consistent with the results in Experiment 1 with ad libitum feeding, the pre-deprivation measure in male GALOE mice compared to WT revealed a significant, 40% increase in both ethanol intake and preference. While the main effect for condition (pre- vs post-deprivation) in the males did not quite reach statistical significance [F(1,23) = 3.00, p<0.10), a significant interaction was revealed [F(1,23) = 4.77, p<0.04), indicating that the effect of genotype in the males was significantly enhanced by the 24-h period without food. As revealed by the post-deprivation measures, ethanol intake and preference in male GALOE were increased by 60–75% compared to WT mice (Fig. 2), with no genotype difference seen in the measures of water or chow intake during these test days (data not shown). Despite enhancing this effect in the male mice, the food deprivation failed to reveal any genotype difference in females, as indicated by their pre-deprivation and post-deprivation measures of ethanol intake and preference (Fig. 2). These results, while confirming the genotype difference revealed in male GALOE compared to WT mice, show this effect to be enhanced by an acute period of food deprivation.
Figure 2.
Ethanol intake and preference in male and female GALOE compared to WT mice drinking 15% ethanol and tested on the day before food deprivation (pre-depr) and the day after deprivation (post-depr). Compared to WT, the male GALOE mice, but not females, exhibited a significant genotype effect (*, p<0.01) before deprivation, an increase in ethanol intake (+43%, p<0.01, d=3.19) and preference (+45, p<0.01, d=1.99), which became significantly greater after deprivation for intake (+75%, p<0.01, d=5.28) and preference (+65%, p<0.01, d=4.40).
Experiment 3: Taste preference tests with sucrose and quinine solutions
This experiment tested whether the increase in ethanol intake observed in male GALOE mice at the 15% concentration is attributed to a change in their preference for flavored solutions. The mice in Group 2 were given preference tests with solutions of sucrose and quinine, two weeks after the ethanol was withdrawn. These tests failed to reveal anydifference between genotypes in their intake of these solutions relative to water (Table 2). The GALOE and WT mice were similar in their preference for the sucrose, as shown in the male [F(1,20) = 0.105] and female [F(1,22) = 0.012] mice, and also for quinine in the male [F[1,20] = 0.115] and female [F(1,22) = 1.171]. These results indicate that the increase in ethanol intake observed in male GAL OE mice on the 15% ethanol solution is not attributed to any obvious change in taste preference.
Table 2.
Preference for sucrose and quinine solutions at different concentrations
| Male | Female | |||
|---|---|---|---|---|
| WT | GALOE | WT | GALOE | |
| Sucrose Preference (%) | ||||
| 1.70% | 82 ± 7.2 | 89 ± 6.4 | 92 ± 3.0 | 91 ± 3.0 |
| 4.30% | 81 ± 7.8 | 87 ± 9.1 | 96 ± 9.5 | 87 ± 4.0 |
| Quinine Preference (%) | ||||
| 0.03 mM | 18 ± 2.3 | 17 ± 1.6 | 15 ± 2.1 | 15 ± 3.2 |
| 0.10 mM | 19 ± 2.6 | 15 ± 1.0 | 13 ± 1.0 | 15 ± 1.3 |
Experiment 4: Consumption of a high-fat diet in GALOE mice
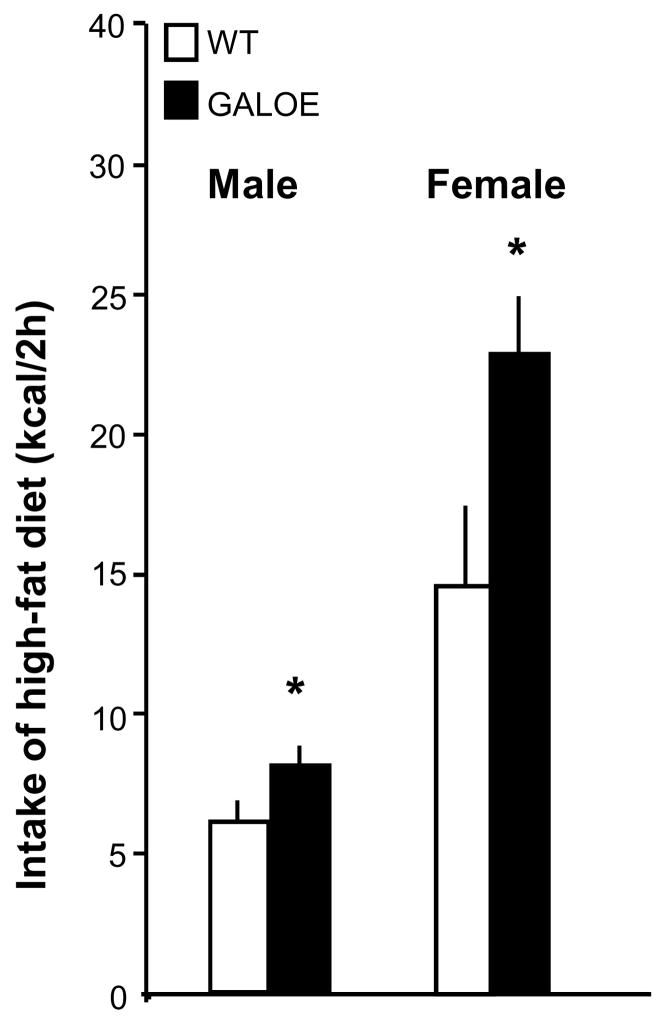
In a recent study that examined GALKO mice of mixed sex on a chronic fat-rich diet (Adams et al., 2008), daily consumption of dietary fat, in experiments using a mixed high-fat diet or jars of pure macronutrients, was found to be reduced by 40–50%, consistent with the proposed role for GAL in stimulating preference for fat (Leibowitz, 2007). To further examine the function of endogenous GAL in eating behavior and preference for a fat-rich diet, the WT and GALOE male and female mice of Group 1, while being maintained on lab chow and drinking 15% ethanol, were tested for their ingestion of a high-fat diet during a brief period at dark onset. Measurements of caloric intake during the 2-h test revealed a marked change in the GALOE compared to WT mice (Fig. 3). While showing normal daily intake of lab chow (Table 1), both the female and male GALOE mice demonstrated a significant increase in the amount of high-fat diet consumed during the acute test. The female mice, while consuming significantly more of the high-fat diet than the males (p<0.01), exhibited a larger genotype effect, a 56% increase in 2-h intake in the GALOE (vs WT) mice compared to only 29% increase in the males. These results demonstrate for the first time that GAL overexpression stimulates intake of a fat-rich diet and that this effect on food intake, while occurring in both sexes, is considerably stronger in females, which also exhibit a stronger preference for the fat-rich diet.
Figure 3.
High-fat diet intake (kcal/2h) in male and female GALOE and WT mice during a 2-h test period at dark onset (average of 4 tests), with chow and ethanol removed. Compared to WT, the GALOE males and females exhibited a significant increase in high-fat diet intake (*, p<0.01), and this genotype effect was significantly stronger in females (+56%, p<0.001, d=2.18) compared to males (+29%, p < 0.01, d=1.3).
Discussion
There is considerable evidence, from studies with injections and measurements of GAL, suggesting a positive relationship between this peptide and ethanol intake that is bidirectional in nature and impacts on voluntary ethanol intake in Sprague-Dawley rats. The present results in mice that overexpress the GAL gene provide further support for this relationship.
Effect of GAL overexpression on ethanol intake in relation to gender
In accordance with the known stimulatory effect of GAL injection on the consumption of ethanol, the male GALOE mice compared to WT controls exhibited a significant increase in their daily intake of ethanol and a parallel increase in preference for ethanol relative to water. This change in male mice became evident only at the 15% concentration of ethanol, when the switch from a 10% to 15% ethanol solution increased ethanol intake (in g/kg) by more than 160% in GALOE mice but only 60% in WT mice. This difference between genotypes suggests that endogenous GAL in stimulating ethanol intake becomes active only at the highest, 15% concentration of ethanol, when the overexpression of endogenous GAL induces the mice to consume more ethanol. This increase in ethanol intake and preference was not litter dependent, since it was observed when the GALOE mice were compared to littermate as well as non-littermate WT controls. It was also not associated with any changes in preference for sweet (sucrose) and bitter (quinine) solutions, as revealed by the tests in both the male and female GALOE mice after being taken off ethanol. Whereas the possibility of genotype differences prior to ethanol exposure can not be excluded, these results suggest that the increase in ethanol intake observed in male GALOE mice is not attributed to a change in their taste for flavored solutions. Blood levels of ethanol were also not altered in the GALOE mice on the 15% concentration, with the small, statistically insignificant increase in males presumably reflecting their higher ethanol intake. This suggests that the metabolism of ethanol was not markedly affected. A particularly interesting finding of this study, however, was that the increase in ethanol intake and preference induced by GAL overexpression was gender specific, occurring in male GALOE mice but not in females at any concentration. This result suggests possible mechanisms involving reproductive hormones that may contribute to GAL’s role in the control of ethanol intake (see below).
Effect of GAL overexpression on food intake and body weight
There are numerous studies with injection and measurements of GAL suggesting a role for this peptide in stimulating food intake and body weight in rats and mice (Hohmann et al., 2003; Leibowitz, 2007; Yun et al., 2005). This is not supported by earlier studies, which show GALOE compared to WT mice to be similar in these measures while maintained ad libitum on lab chow or after a period of chow deprivation (Hohmann et al., 2003). The results of the present study also failed to reveal any difference in food intake and body weight between the GALOE and WT mice trained to drink increasing concentrations of ethanol. While this suggests that GAL may not have an important role in controlling feeding and weight gain while feeding on lab chow or chronically drinking ethanol, there are other confounding factors that need to be considered when interpreting such negative results from mutant mice. In addition to the diet which is important for revealing the effects of GAL (see below), recent studies show that PVN injection of GAL in rats trained to voluntarily drink ethanol stimulates only ethanol intake and not food intake as one normally sees in the absence of ethanol (Lewis et al., 2004; Rada et al., 2004). This finding has led to the proposal that chronic availability of ethanol can usurp the role of endogenous GAL in feeding and shift its stimulatory effect on ingestive behavior toward the consumption of ethanol (Leibowitz, 2007).
Possible mechanisms underlying gender-specificity of effects produced by GAL overexpression
The effect of GAL overexpression on the drinking of ethanol, revealed only in male mice, suggests that gender-specific endocrine changes may play an important role in the effects of excess GAL on ethanol intake. Whereas GAL in the PVN of female rats is highly responsive to ovarian steroids (Brann et al., 1993; Rossmanith et al., 1996) and acts in close association with these steroids in stimulating feeding (Leibowitz et al., 1998; Leibowitz et al., 2007; Leibowitz et al., 2009), PVN GAL in male rats is found to be unresponsive to testosterone (Delemarre-van de Waal et al., 1994). Further, whereas GAL injection causes the release of gonadotropins in both males and females (López et al., 1993), pituitary content of these hormones as well as circulating levels of gonadotropins and testosterone are unaltered in GALOE mice (Hohmann et al., 2003). It is noteworthy that administration of GAL in males and females induces a dose-dependent increase in circulating levels of prolactin (Koshiyama et al., 1987; López et al., 1993; Ottlecz et al., 1988), a hormone known to stimulate feeding behavior (Byatt et al., 1993; O’Halloran et al., 1991). Pituitary content of prolactin mRNA and protein is greatly reduced in GALKO mice compared to WT (Wynick et al., 1998), and circulating levels of prolactin are markedly increased, by 9-fold, in male GALOE mice, with females showing only a small, 2-fold change (Perumal and Vrontakis, 2003). This evidence supports a positive association between GAL and prolactin and suggests that the greater responsiveness of males to GAL overexpression may, in part, be attributed to this relationship. Although a causal effect of prolactin on ethanol consumption has yet to be demonstrated, there is one study in a subpopulation of alcoholics that has revealed, in males, a positive association between circulating prolactin levels and ethanol craving during withdrawal (Hillemacher et al., 2006). Together, these findings focus attention on prolactin as one factor that may contribute to the gender differences observed here in the effect of GAL genotype on ethanol intake.
Relationship between GAL and ethanol intake under conditions of food deprivation
Food deprivation, which stimulates the consumption of ethanol (Middaugh et al., 1999), is also found to increase levels of GAL in the PVN (Wang et al., 1998), suggesting that GAL may contribute to deprivation-induced increase in consummatory behavior. There is only one study of food deprivation in GALOE mice, and this report failed to reveal any effect of genotype on feeding in response to deprivation (Hohmann et al., 2003). The present study examined the possibility that a day of food deprivation may enhance the stimulatory effect of GAL overexpression on ethanol consumption observed in males, while possibly revealing some effect in females. Consistent with results obtained under ad libitum feeding conditions, the female GALOE mice after food deprivation still failed to exhibit any difference from WT in their consumption of ethanol. This period of food deprivation, however, had significant impact in the male mice. In the GALOE compared to non-littermate WT mice, it potentiated the magnitude of the genotype effect from a 40% increase in ethanol intake and preference on the day before deprivation to a 60–75% increase on the day after deprivation. Since there was no difference between the intake scores of the WT mice before and after the one day of deprivation, this deprivation effect is most likely driven by the propensity of the male GAL-OE mice to consume more ethanol and to be susceptible to the stimulatory effect of deprivation on intake. Whereas this has yet to be tested in littermate GALOE and WT mice, these results obtained in non-littermates confirm the lack of response in female GALOE mice and demonstrate that male GALOE mice voluntarily drinking ethanol become even more responsive to GAL overexpression under conditions of food deprivation.
Relationship between GAL, dietary fat and ethanol intake
In addition to the steroids and prolactin, GAL is known to have a close, positive relationship to dietary fat, which in turn is related to the consumption of ethanol (Leibowitz, 2007; Yun et al., 2005). This idea is based on studies in rats involving injections and measurements of GAL. Two recent studies in mutant mice provide further support for an association of GAL with dietary fat. When chronically maintained on a mixed, high-fat diet or on macronutrient diets with pure fat available, GAL deficient mice compared to WT (gender unspecified) consume lower amount of the mixed diet and choose less of the pure fat, and this effect is partially reversed by central administration of GAL (Adams et al., 2008). Further, mice lacking the GAL type 1 receptor also show a decrease in chronic consumption of high-fat diet (Zorrilla et al., 2007). The results of the present investigation provide the first evidence that GAL overexpression in mutant mice can produce the opposite effect. In animals maintained on lab chow, they demonstrate that GALOE mice compared to WT consume more calories of the fat-rich diet when provided during a brief period at the onset of the natural feeding cycle. They also show this stimulatory effect to be gender-related, significantly stronger in females compared to males. This may be related to the finding, as shown here in mice and also in rats (Leibowitz et al., 2007; Leibowitz et al., 1991; Leibowitz et al., 2009), that females generally consume more fat calories that males and show a stronger preference for this macronutrient. This gender difference has been attributed to endogenous GAL in the PVN and medial preoptic nucleus, which exhibits greater expression in females compared to males, and also to progesterone, which in estrogen-primed rats stimulates GAL in these nuclei and increases the consumption of fat (Leibowitz et al., 1998; Leibowitz et al., 2007; Leibowitz et al., 2009). These mechanisms may similarly contribute to the stronger effect observed in females of genotype on high-fat diet consumption. In understanding why the females failed to exhibit a genotype effect on ethanol intake, one must consider the evidence that mutation of one gene involved in ingestive behavior can affect the expression of other peptides or neurotransmitters that, in turn, may counteract the expected phenotype (Marsh et al., 1999; Qian et al., 2002). The results obtained in male GALOE mice, revealing increased consumption of both ethanol and fat, are consistent with the positive relationship that exists between these two nutrients and their underlying mechanisms (Leibowitz, 2007). Together with evidence showing a 2–5-fold increase in GAL in the brain of GALOE mice (Crawley et al., 2002; He et al., 2005), this study provides strong support for a causal relationship between these consummatory behaviors and endogenous GAL.
Acknowledgments
This research was supported by USPHS Grants, AA 12882 and DA 21518. We thank Helen Martirosova and Olga Berman for their technical assistance.
References
- Adams A, Clapham J, Wynick D, Speakman J. Feeding behaviour in galanin knockout mice supports a role of galanin in fat intake and preference. J Neuroendocrinol. 2008;20:199–206. doi: 10.1111/j.1365-2826.2007.01638.x. [DOI] [PubMed] [Google Scholar]
- Akabayashi A, Koenig J, Watanabe Y, Alexander J, Leibowitz S. Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc Natl Acad Sci U S A. 1994;91:10375–10379. doi: 10.1073/pnas.91.22.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfer I, Hipp H, Bollettino A, McKnight C, Evans C, Virkkunen M, Albaugh B, Max M, Goldman D, Enoch M. Alcoholism is associated with GALR3 but not two other galanin receptor genes. Genes Brain Behav. 2007;6:473–481. doi: 10.1111/j.1601-183X.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- Brann D, Chorich L, Mahesh V. Effect of progesterone on galanin mRNA levels in the hypothalamus and the pituitary: correlation with the gonadotropin surge. Neuroendocrinology. 1993;58:531–538. doi: 10.1159/000126587. [DOI] [PubMed] [Google Scholar]
- Byatt J, Staten N, Salsgiver W, Kostelc J, Collier R. Stimulation of food intake and weight gain in mature female rats by bovine prolactin and bovine growth hormone. Am J Physiol. 1993;264:E986–992. doi: 10.1152/ajpendo.1993.264.6.E986. [DOI] [PubMed] [Google Scholar]
- Carrillo CA, Leibowitz SF, Karatayev O, Hoebel BG. A high-fat meal or injection of lipids stimulates ethanol intake. Alcohol. 2004;34:197–202. doi: 10.1016/j.alcohol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Chang G, Gaysinskaya V, Karatayev O, Leibowitz S. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31:249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Contaldo F, D’Arrigo E, Carandente V, Cortese C, Coltorti A, Mancini M, Taskinen M, Nikkilä E. Short-term effects of moderate alcohol consumption on lipid metabolism and energy balance in normal men. Metabolism. 1989;38:166–171. doi: 10.1016/0026-0495(89)90257-6. [DOI] [PubMed] [Google Scholar]
- Crawley J, Mufson E, Hohmann J, Teklemichael D, Steiner R, Holmberg K, Xu Z, Blakeman K, Xu X, Wiesenfeld-Hallin Z, et al. Galanin overexpressing transgenic mice. Neuropeptides. 2002;36:145–156. doi: 10.1054/npep.2002.0891. [DOI] [PubMed] [Google Scholar]
- Delemarre-van de Waal H, Burton K, Kabigting E, Steiner R, Clifton D. Expression and sexual dimorphism of galanin messenger ribonucleic acid in growth hormone-releasing hormone neurons of the rat during development. Endocrinology. 1994;134:665–671. doi: 10.1210/endo.134.2.7507832. [DOI] [PubMed] [Google Scholar]
- Gaysinskaya V, Karatayev O, Chang G, Leibowitz S. Increased caloric intake after a high-fat preload: relation to circulating triglycerides and orexigenic peptides. Physiol Behav. 2007;91:142–153. doi: 10.1016/j.physbeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Hansen S, Fahlke C, Söderpalm A, Hård E. Significance of adrenal corticosteroid secretion for the food restriction-induced enhancement of alcohol drinking in the rat. Psychopharmacology (Berl) 1995;121:213–221. doi: 10.1007/BF02245632. [DOI] [PubMed] [Google Scholar]
- He B, Counts S, Perez S, Hohmann J, Koprich J, Lipton J, Steiner R, Crawley J, Mufson E. Ectopic galanin expression and normal galanin receptor 2 and galanin receptor 3 mRNA levels in the forebrain of galanin transgenic mice. Neuroscience. 2005;133:371–380. doi: 10.1016/j.neuroscience.2005.01.068. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Bayerlein K, Wilhelm J, Frieling H, Sperling W, Kornhuber J, Bleich S. Prolactin serum levels and alcohol craving - an analysis using Lesch’s typology. Neuropsychobiology. 2006;53:133–136. doi: 10.1159/000092543. [DOI] [PubMed] [Google Scholar]
- Hohmann J, Krasnow S, Teklemichael D, Clifton D, Wynick D, Steiner R. Neuroendocrine profiles in galanin-overexpressing and knockout mice. Neuroendocrinology. 2003;77:354–366. doi: 10.1159/000071308. [DOI] [PubMed] [Google Scholar]
- Koshiyama H, Kato Y, Inoue T, Murakami Y, Ishikawa Y, Yanaihara N, Imura H. Central galanin stimulates pituitary prolactin secretion in rats: possible involvement of hypothalamic vasoactive intestinal polypeptide. Neurosci Lett. 1987;75:49–54. doi: 10.1016/0304-3940(87)90073-5. [DOI] [PubMed] [Google Scholar]
- Kyrkouli S, Stanley B, Leibowitz S. Galanin: stimulation of feeding induced by medial hypothalamic injection of this novel peptide. Eur J Pharmacol. 1986;122:159–160. doi: 10.1016/0014-2999(86)90175-5. [DOI] [PubMed] [Google Scholar]
- Leibowitz S. Overconsumption of dietary fat and alcohol: mechanisms involving lipids and hypothalamic peptides. Physiol Behav. 2007;91:513–521. doi: 10.1016/j.physbeh.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz S, Akabayashi A, Alexander J, Wang J. Gonadal steroids and hypothalamic galanin and neuropeptide Y: role in eating behavior and body weight control in female rats. Endocrinology. 1998;139:1771–1780. doi: 10.1210/endo.139.4.5867. [DOI] [PubMed] [Google Scholar]
- Leibowitz S, Akabayashi A, Wang J, Alexander J, Dourmashkin J, Chang G. Increased caloric intake on a fat-rich diet: role of ovarian steroids and galanin in the medial preoptic and paraventricular nuclei and anterior pituitary of female rats. J Neuroendocrinol. 2007;19:753–766. doi: 10.1111/j.1365-2826.2007.01584.x. [DOI] [PubMed] [Google Scholar]
- Leibowitz S, Avena N, Chang G, Karatayev O, Chau D, Hoebel B. Ethanol intake increases galanin mRNA in the hypothalamus and withdrawal decreases it. Physiol Behav. 2003;79:103–111. doi: 10.1016/s0031-9384(03)00110-0. [DOI] [PubMed] [Google Scholar]
- Leibowitz S, Dourmashkin J, Chang G, Hill J, Gayles E, Fried S, Wang J. Acute high-fat diet paradigms link galanin to triglycerides and their transport and metabolism in muscle. Brain Res. 2004;1008:168–178. doi: 10.1016/j.brainres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Leibowitz S, Kim T. Impact of a galanin antagonist on exogenous galanin and natural patterns of fat ingestion. Brain Res. 1992;599:148–152. doi: 10.1016/0006-8993(92)90863-5. [DOI] [PubMed] [Google Scholar]
- Leibowitz S, Lucas D, Leibowitz K, Jhanwar Y. Developmental patterns of macronutrient intake in female and male rats from weaning to maturity. Physiol Behav. 1991;50:1167–1174. doi: 10.1016/0031-9384(91)90578-c. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Akabayashi A, Alexander J, Karatayev O, Chang GQ. Puberty Onset in Female Rats: Relationship With Fat Intake, Ovarian Steroids and the Peptides, Galanin and Enkephalin, in the Paraventricular and Medial Preoptic Nuclei. Journal of Neuroendocrinology. 2009;21:538–549. doi: 10.1111/j.1365-2826.2009.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Johnson D, Waldman D, Leibowitz S, Hoebel B. Galanin microinjection in the third ventricle increases voluntary ethanol intake. Alcohol Clin Exp Res. 2004;28:1822–1828. doi: 10.1097/01.alc.0000148099.12344.c8. [DOI] [PubMed] [Google Scholar]
- López F, Meade EJ, Negro-Vilar A. Endogenous galanin modulates the gonadotropin and prolactin proestrous surges in the rat. Endocrinology. 1993;132:795–800. doi: 10.1210/endo.132.2.7678800. [DOI] [PubMed] [Google Scholar]
- Marsh D, Miura G, Yagaloff K, Schwartz M, Barsh G, Palmiter R. Effects of neuropeptide Y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues. Brain Res. 1999;848:66–77. doi: 10.1016/s0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- Middaugh L, Kelley B, Bandy A, McGroarty K. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Nagase H, Nakajima A, Sekihara H, York D, Bray G. Regulation of feeding behavior, gastric emptying, and sympathetic nerve activity to interscapular brown adipose tissue by galanin and enterostatin: the involvement of vagal-central nervous system interactions. J Gastroenterol. 2002;37(Suppl 14):118–127. doi: 10.1007/BF03326430. [DOI] [PubMed] [Google Scholar]
- O’Halloran D, Jones P, Ghatei M, Bloom S. Rat anterior pituitary neuropeptides following chronic prolactin manipulation: a combined radioimmunoassay and mRNA study. J Endocrinol. 1991;131:411–419. doi: 10.1677/joe.0.1310411. [DOI] [PubMed] [Google Scholar]
- Ottlecz A, Snyder G, McCann S. Regulatory role of galanin in control of hypothalamic-anterior pituitary function. Proc Natl Acad Sci U S A. 1988;85:9861–9865. doi: 10.1073/pnas.85.24.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal P, Vrontakis M. Transgenic mice over-expressing galanin exhibit pituitary adenomas and increased secretion of galanin, prolactin and growth hormone. J Endocrinol. 2003;179:145–154. doi: 10.1677/joe.0.1790145. [DOI] [PubMed] [Google Scholar]
- Plaisier C, Kyttälä M, Weissglas-Volkov D, Sinsheimer J, Huertas-Vazquez A, Riba L, Ramírez-Jiménez S, de Bruin T, Tusié-Luna T, Aouizerat B, et al. Galanin preproprotein is associated with elevated plasma triglycerides. Arterioscler Thromb Vasc Biol. 2009;29:147–152. doi: 10.1161/ATVBAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer M, Novi D, Guan X, Yu H, Shen Z, Feng Y, Frazier E, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena N, Leibowitz S, Hoebel B. Ethanol intake is increased by injection of galanin in the paraventricular nucleus and reduced by a galanin antagonist. Alcohol. 2004;33:91–97. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Rossmanith W, Marks D, Clifton D, Steiner R. Induction of galanin mRNA in GnRH neurons by estradiol and its facilitation by progesterone. J Neuroendocrinol. 1996;8:185–191. doi: 10.1046/j.1365-2826.1996.04473.x. [DOI] [PubMed] [Google Scholar]
- Schrezenmeir J. Hyperinsulinemia, hyperproinsulinemia and insulin resistance in the metabolic syndrome. Experientia. 1996;52:426–432. doi: 10.1007/BF01919311. [DOI] [PubMed] [Google Scholar]
- Steiner R, Hohmann J, Holmes A, Wrenn C, Cadd G, Juréus A, Clifton D, Luo M, Gutshall M, Ma S, et al. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Akabayashi A, Yu H, Dourmashkin J, Alexander J, Silva I, Lighter J, Leibowitz S. Hypothalamic galanin: control by signals of fat metabolism. Brain Res. 1998;804:7–20. doi: 10.1016/s0006-8993(98)00632-5. [DOI] [PubMed] [Google Scholar]
- Wynick D, Small C, Bacon A, Holmes F, Norman M, Ormandy C, Kilic E, Kerr N, Ghatei M, Talamantes F, et al. Galanin regulates prolactin release and lactotroph proliferation. Proc Natl Acad Sci U S A. 1998;95:12671–12676. doi: 10.1073/pnas.95.21.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun R, Dourmashkin J, Hill J, Gayles E, Fried S, Leibowitz S. PVN galanin increases fat storage and promotes obesity by causing muscle to utilize carbohydrate more than fat. Peptides. 2005;26:2265–2273. doi: 10.1016/j.peptides.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Zorrilla E, Brennan M, Sabino V, Lu X, Bartfai T. Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. Physiol Behav. 2007;91:479–485. doi: 10.1016/j.physbeh.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]