Abstract
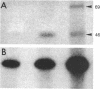
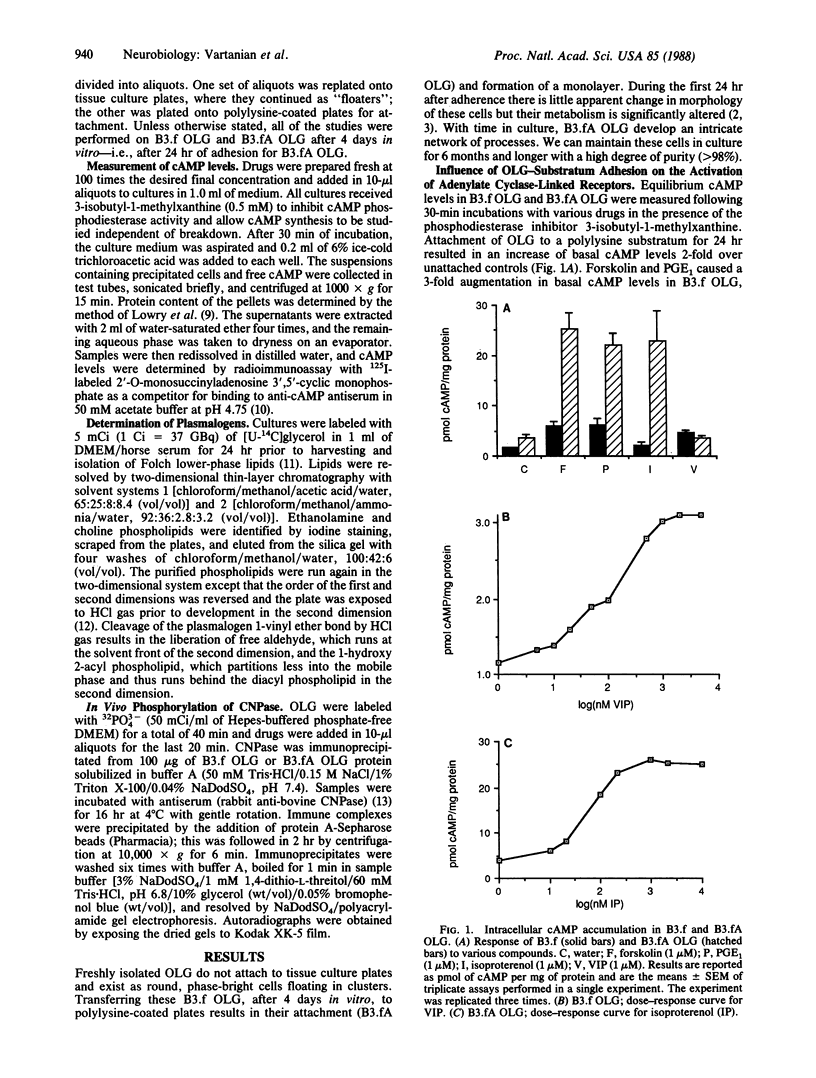
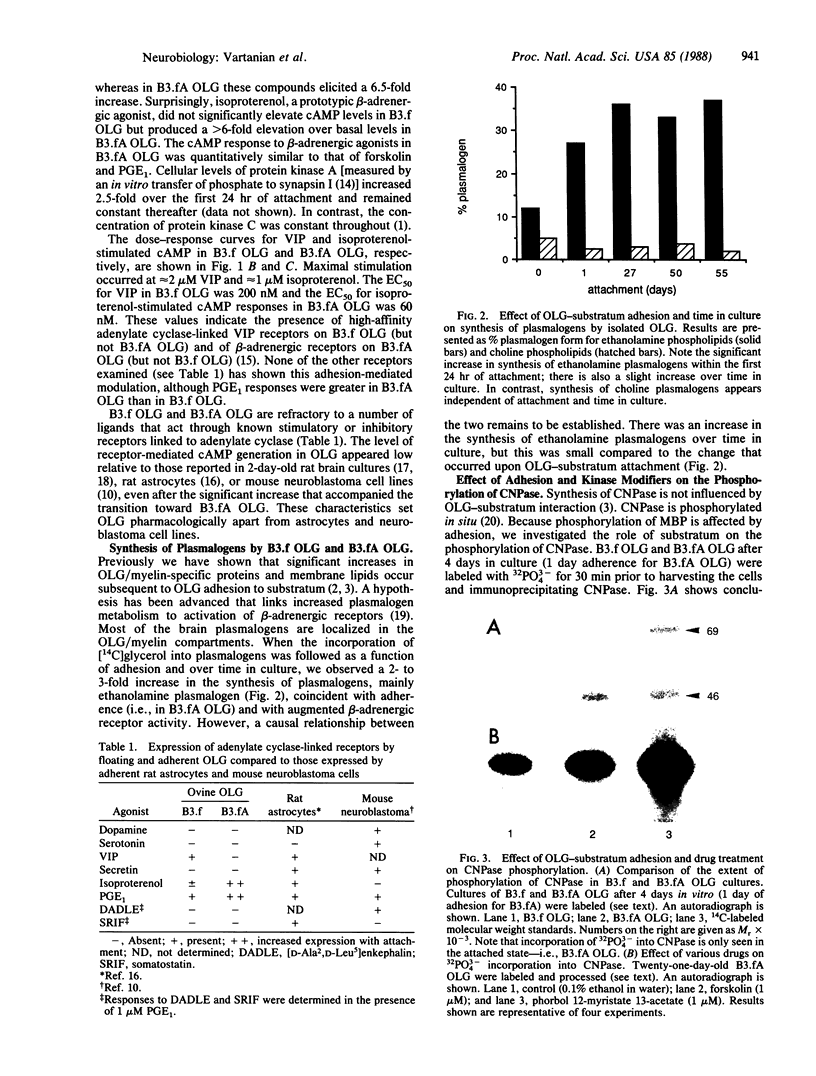
The molecular mechanisms of myelin formation/reformation in the central nervous system are unknown. In previous work we have demonstrated that mature oligodendrocytes (OLG) respond to a signal(s), elicited by their adhesion to a substratum, by turning on a myelinogenic metabolism. Events occurring within 24 hr of adhesion include generation of diacylglycerol, activation of protein kinase C, phosphorylation of myelin basic protein, and enhanced synthesis of myelin lipids and proteins. To elucidate the mechanism(s) of signal transduction, we have investigated whether OLG-substratum interaction influences the level of basal cAMP and the expression of receptors coupled to adenylate cyclase. By using ovine brain OLG we have found that adhesion to a polylysine-coated surface for 24 hr increased the basal level of cAMP 2-fold and altered the expression (assessed by cAMP production) of receptors coupled to adenylate cyclase. Isoproterenol (beta-adrenergic agonist) augmented cAMP from 4 to 26 pmol/mg of protein in adhering OLG but had no such effect in nonattached OLG. Adhesion of OLG was accompanied by rapid synthesis of ethanolamine plasmalogen, a class of lipids believed to be associated with beta-adrenergic receptors. Nonattached OLG responded to prostaglandin E1 with only a 3-fold stimulation in their cAMP content; in attached OLG, 6-fold stimulation was observed. In contrast, vasoactive intestinal polypeptide elicited a 3-fold increase in cAMP in nonattached OLG but, following 24 hr of attachment, OLG did not respond to vasoactive intestinal polypeptide. The increase of cellular cAMP levels was accompanied by a 2.5-fold gain in protein kinase A. OLG-substratum adhesion resulted also in phosphorylation of the OLG/myelin protein, 2',3'-cyclic nucleotide 2'-phosphodiesterase, which proved to be a substrate for cAMP and phospholipid-, Ca2+-dependent protein kinases. These findings, in conjunction with our earlier work, implicate cAMP and diacylglycerol in signaling myelinogenesis; they suggest that phosphorylation/dephosphorylation of myelin basic protein and 2',3'-cyclic nucleotide 2'-phosphodiesterase may be key processes in the cascade of events that are initiated by adhesion of OLG to a polylysine surface (possibly acting as a surrogate for axons) and culminate in the reformation of myelin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amur S. G., Shanker G., Pieringer R. A. Beta-adrenergic stimulation of protein (arginine) methyltransferase activity in cultured cerebral cells from embryonic mice. J Neurosci Res. 1986;16(2):377–386. doi: 10.1002/jnr.490160205. [DOI] [PubMed] [Google Scholar]
- Bradbury J. M., Campbell R. S., Thompson R. J. Endogenous cyclic AMP-stimulated phosphorylation of a Wolfgram protein component in rabbit central-nervous-system myelin. Biochem J. 1984 Jul 15;221(2):351–359. doi: 10.1042/bj2210351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., McCarthy K. D., Harden T. K. Regulation of cyclic AMP accumulation by peptide hormone receptors in immunocytochemically defined astroglial cells. J Neurochem. 1984 Jul;43(1):131–138. doi: 10.1111/j.1471-4159.1984.tb06688.x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gunawan J., Vierbuchen M., Debuch H. Studies on the hydrolysis of 1-alk-1'-enyl-sn-glycero-3-phosphoethanolamine by microsomes from myelinating rat brain. Hoppe Seylers Z Physiol Chem. 1979 Jul;360(7):971–978. doi: 10.1515/bchm2.1979.360.2.971. [DOI] [PubMed] [Google Scholar]
- Horrocks L. A., Yeo Y. K., Harder H. W., Mozzi R., Goracci G. Choline plasmalogens, glycerophospholipid methylation, and receptor-mediated activation of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:263–292. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Massa P. T., Szuchet S., Mugnaini E. Cell-cell interactions of isolated and cultured oligodendrocytes: formation of linear occluding junctions and expression of peculiar intramembrane particles. J Neurosci. 1984 Dec;4(12):3128–3139. doi: 10.1523/JNEUROSCI.04-12-03128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris F. A. Cyclic AMP induction of the myelin enzyme 2',3'-cyclic nucleotide 3'-phosphohydrolase in rat oligodendrocytes. J Neurochem. 1983 Aug;41(2):506–515. doi: 10.1111/j.1471-4159.1983.tb04768.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Pleasure D., Parris J., Stern J., Grinspan J., Kim S. U. Incorporation of tritiated galactose into galactocerebroside by cultured rat oligodendrocytes: effects of cyclic adenosine 3',5'-monophosphate analogues. J Neurochem. 1986 Jan;46(1):300–302. doi: 10.1111/j.1471-4159.1986.tb12963.x. [DOI] [PubMed] [Google Scholar]
- Quach T. T., Rose C., Schwartz J. C. [3H]Glycogen hydrolysis in brain slices: responses to neurotransmitters and modulation of noradrenaline receptors. J Neurochem. 1978 Jun;30(6):1335–1341. doi: 10.1111/j.1471-4159.1978.tb10464.x. [DOI] [PubMed] [Google Scholar]
- Rougon G., Noble M., Mudge A. W. Neuropeptides modulate the beta-adrenergic response of purified astrocytes in vitro. Nature. 1983 Oct 20;305(5936):715–717. doi: 10.1038/305715a0. [DOI] [PubMed] [Google Scholar]
- Sprinkle T. J., Grimes M. J., Eller A. G. Isolation of 2',3'-cyclic nucleotide 3'-phosphodiesterase from human brain. J Neurochem. 1980 Apr;34(4):880–887. doi: 10.1111/j.1471-4159.1980.tb09661.x. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Arnason B. G., Polak P. E. Separation of ovine oligodendrocytes into two distinct bands on a linear sucrose gradient. J Neurosci Methods. 1980 Oct;3(1):7–19. doi: 10.1016/0165-0270(80)90030-8. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Polak P. E., Yim S. H. Mature oligodendrocytes cultured in the absence of neurons recapitulate the ontogenic development of myelin membranes. Dev Neurosci. 1986;8(4):208–221. doi: 10.1159/000112254. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Yim S. H. Characterization of a subset of oligodendrocytes separated on the basis of selective adherence properties. J Neurosci Res. 1984;11(2):131–144. doi: 10.1002/jnr.490110203. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Yim S. H., Monsma S. Lipid metabolism of isolated oligodendrocytes maintained in long-term culture mimics events associated with myelinogenesis. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7019–7023. doi: 10.1073/pnas.80.22.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer J. B., Edwards A. M., McMorris F. A., Braun P. E. Cyclic AMP decreases the phosphorylation state of myelin basic proteins in rat brain cell cultures. J Biol Chem. 1987 Feb 5;262(4):1748–1755. [PubMed] [Google Scholar]
- Vartanian T., Szuchet S., Dawson G., Campagnoni A. T. Oligodendrocyte adhesion activates protein kinase C-mediated phosphorylation of myelin basic protein. Science. 1986 Dec 12;234(4782):1395–1398. doi: 10.1126/science.2431483. [DOI] [PubMed] [Google Scholar]
- Ventimiglia R., Greene M. I., Geller H. M. Localization of beta-adrenergic receptors on differentiated cells of the central nervous system in culture. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5073–5077. doi: 10.1073/pnas.84.14.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim S. H., Szuchet S., Polak P. E. Cultured oligodendrocytes. A role for cell-substratum interaction in phenotypic expression. J Biol Chem. 1986 Sep 5;261(25):11808–11815. [PubMed] [Google Scholar]