Abstract
Since AMP-activated protein kinase (AMPK) plays important roles in modulating metabolism in response to diet and exercise, both of which influence bone mass, we examined the influence of AMPK on bone mass in mice. AMPK is an αβγ heterotrimer where the β subunit anchors the α catalytic and γ regulatory subunits. Germline deletion of either AMPK β1 or β2 subunit isoforms resulted in reduced trabecular bone density and mass, but without effects on osteoclast (OC) or osteoblast (OB) numbers, as compared to wild-type littermate controls. We tested whether activating AMPK in vivo would enhance bone density but found AICA-riboside treatment caused a profound loss of trabecular bone volume (49.5%) and density and associated increased OC numbers. Consistent with this, AICA-riboside strongly stimulated OC differentiation in vitro, in an adenosine kinase-dependent manner. OCs and macrophages (unlike OBs) lacked AMPK β2 subunit expression, and when generated from AMPK β1−/− mice displayed no detectable AMPK activity. Nevertheless, AICA-riboside was equally effective at stimulating OC differentiation from wild-type or β1−/− progenitors, indicating that AMPK is not essential for OC differentiation or the stimulatory action of AICA-riboside. These results show that AMPK is required to maintain normal bone density, but not through bone cell differentiation, and does not mediate powerful osteolytic effects of AICA-riboside.—Quinn, J. M. W., Tam, S., Sims, N. A., Saleh, H., McGregor, N. E., Poulton, I. J., Scott, J. W., Gillespie, M. T., Kemp, B. E., van Denderen, B. J. W. Germline deletion of AMP-activated protein kinase β subunits reduces bone mass without altering osteoclast differentiation or function.
Keywords: osteolysis, AMPK, AICA-riboside, osteoblast, knockout mouse
Metabolic energy homeostasis has long been known to critically influence bone mass. For example, obesity is generally associated with high bone mass and severe caloric restriction with reduced bone mass (1, 2). AMP-activated protein kinase (AMPK) is a metabolic stress sensing enzyme that plays key roles in regulating cellular and whole body energy homeostasis (reviewed in ref. 3). AMPK signaling is thought to be impaired in obesity and enhanced with caloric restriction, which suggests that AMPK may be a negative regulator of bone density.
A number of recent studies using clinically important drugs have shown a link between AMPK activity and bone cell function. The cholesterol-lowering statins strongly activate AMPK in vitro and in vivo, and in a separate study were shown to be proanabolic in bone and accelerated fracture repair (4, 5). Similarly, the antidiabetic drug Metformin stimulated mineralization by MC3T3-E1 cells [which derive from mouse osteoblasts (OBs) or bone-forming cells], and this occurred in an AMPK-dependent manner (6). However, significant bone loss and increased fracture risk in humans is associated with thiazolidinedione therapies (7,8,9). These drugs both stimulate PPARγ and indirectly activate AMPK (10, 11). Inflammatory cytokines such as TNF-α and IL-6, which profoundly affect bone mass, can suppress or enhance AMPK signaling (12,13,14). Furthermore, growing evidence indicates that energy homeostatic mechanisms and bone metabolism influence each other, with shared hormonal and neuronal controls (15), e.g., the central actions of the circulating adiposity factor leptin to repress both appetite and osteogenesis (16,17,18), and the actions of OB-derived osteocalcin to stimulate insulin production (15). These considerations are consistent with a possible role of AMPK in bone homeostasis. To date, however, no genetic evidence has been available to support the concept that AMPK is important for maintaining bone density.
Skeletal bone mass and quality are determined principally by osteoclasts (OCs), which degrade bone, and OBs, which form new bone. OBs are highly metabolically active mesenchymally derived cells whose function is regulated by numerous local factors, including bone morphogenetic proteins and Wnt family members (19, 20). OCs form rapidly in vivo in response to OB-derived osteoclastogenic molecules M-CSF and RANKL, the latter a TNF-super family member whose expression is induced by osteolytic hormones and cytokines, including parathyroid hormone and 1,25-dihydroxyvitamin D3 (1,25(OH)2-D3) (21, 22). OCs and OBs work together to remodel or reshape bone structures to optimize their resistance to mechanical forces as well as to repair the bone and enable bone growth. This occurs through bone resorption by OCs and is followed by bone formation by OBs, actions that are closely coupled and coordinated to maintain bone architecture such that OC stimulation may result in elevated OB numbers and vice versa.
AMPK occurs in all cells as an αβγ heterotrimer with multiple subunit isoforms (3). The β subunit acts as a scaffold that supports binding of the catalytic α and regulatory γ subunits and is essential for AMPK function (23). AMPK is activated by phosphorylation of Thr-172 within the activation loop of the α-subunit kinase by upstream kinases that include LKB1 and Ca2+/calmodulin-dependant protein kinase (CAMKKβ) (24,25,26). It is also allosterically activated by binding of AMP to the γ subunit (27, 28). AMPK can be activated similarly by the AMP analog ZMP, which is generated from AICA-riboside by adenosine kinase. Thus, AICA-riboside may mimic a depleted cellular energy state and may be an exercise mimetic (29).
The AMPK β subunit exists in two isoforms, β1 and β2, encoded by separate genes. We examined bones from our germline AMPK β1- and β2-knockout mice, which have reduced AMPK levels in most tissues we have examined. Compared to wild-type littermate controls, both knockout lines displayed a significant reduction in trabecular bone volume. Since no change was found in OC or OB numbers in β1−/− or β2−/− mice, this indicates that the reduced bone density does not result from effects on bone cell differentiation. We then tested whether activation of AMPK using AICA-riboside may lead to improved bone density but found, on the contrary, short-term administration of AICA-riboside led to a rapid high-turnover osteopenia with elevated OC numbers. AICA-riboside also greatly stimulated OC formation from bone marrow progenitors in vitro. However, we found strong evidence that these effects of AICA-riboside were AMPK independent, since OC formation was also stimulated to a similar extent in OC β1−/− progenitors, which lacked detectable AMPK activity.
MATERIALS AND METHODS
Generation of germline AMPKβ2-knockout mice
A targeting construct was assembled from two PCR-amplified fragments of the Prkab2 gene (Gene ID 108097): a 3144-bp NarI-AgeI 5′ homology arm, and a 6020-bp AgeI-AflII fragment (see Supplemental Fig. 1). Complementary oligonucleotide primers encompassing a loxP recombination site and EcoRI site were annealed and cloned into the AgeI site in intron 2. A loxP-flanked PGK-neomycin resistance gene was cloned into an SpeI site in intron 4. We deleted exons 2, 3, and 4 of the β2 gene because predictive splicing of the transcript of the deleted locus would result in an exon 1 to exon 5 splicing event, resulting in a frameshift mutation and premature stop codon in exon 6. Homologous recombination was performed by Ozgene Pty. Ltd. (Perth, Australia). Briefly, the targeting construct was electroporated into C57BL/6 embryonic stem (ES) cells and neomycin resistant colonies screened for successful homologous recombination by Southern blot using 3′, 5′, and neo specific probes (not shown). Successfully targeted ES cells were transferred to blastocysts and implanted into Balb/c foster mothers. Mixed-coat-color chimeras were bred with wild-type C57BL/6 mice, and progeny with germline transmission of the targeted locus were crossed with a transgenic Cre-deletor strain [Tg(CMV-cre)1Cgn/J] (30). These progeny were screened by Southern blot for deletion of exons 2, 3, and 4 and the neomycin cassette. Mice with a heterozygous deletion were intercrossed to generate homozygous knockout mice.
Mice, cell culture, and reagents
C57BL/6J mice were purchased from the Animal Resource Centre (Perth, Australia). The generation and characterization of AMPKβ1-knockout mice has been described previously (ref. 31 and unpublished results). For all experiments involving AMPK β-subunit-knockout mice, wild-type littermates served as controls. All procedures on animals were approved by the St. Vincent’s Health Animal Ethics Committee (Melbourne, Australia). Cells were cultured in α minimal essential medium (Gibco BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum (CSL Biosciences, Parkville, Australia) (MEM/FBS). Recombinant GST-RANKL 158-316 (RANKL) was produced in-house using a bacterial construct kindly provided by Prof. F. Patrick Ross (Washington University School of Medicine, Dept of Pathology and Immunology, St. Louis, MO, USA). 1,25(OH)2-D3 was purchased from the Wako Pure Chemicals Co. (Osaka, Japan). Adenosine uptake inhibitors [dipyridamole, S-(4-nitrobenzyl)-6-thioinosine], adenosine receptor antagonist (CGS-15943), adenosine kinase inhibitor (A134974; 5-iodo-tubericidin), adenosine, AICA-riboside, mevalonolactone, and other biochemical reagents were analytical grade and purchased from Sigma Chemical Co (Castle Hill, Australia). Fasudil hydrochloride was purchased from Tocris Bioscience (Ellisville, MO, USA). KUSA O cells are a bipotential bone marrow stroma-derived cell line previously characterized by Allan et al. (32), and were used between passages 5 and 20. RAW264.7 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Primary murine calvarial cell cultures were prepared from neonatal calvaria by sequential digestion with 0.1% bacterial collagenase (Worthing Biochemical Co., Freefold, Australia) and 0.2% dispase (Godo Shusei, Tokyo, Japan), as described previously (21). Bone marrow cells were obtained by flushing the marrow cavities of the long bones with sterile saline. Bone marrow macrophages (BMMs) were prepared as described previously by incubating bone marrow cells in the presence of L-cell conditioned medium (30%) for 3 d and removing the nonadherent cell fraction containing enriched macrophage and macrophage progenitors (33).
Western blot analysis of protein expression
Cultured cells were washed with ice-cold PBS, scraped off using a rubber policeman, and collected and lysed in lysis buffer A (50 mM Tris, pH 7.5; 1 mM EDTA; 1 mM EGTA; 50 mM NaF; 5 mM NaPO7; 10% glycerol (v/v); 1% TritonX-100 (v/v); 10 μg/ml trypsin inhibitor; 2 μg/ml aprotinin; 1 mM benzamidine; 1 mM phenylmethylsulfonyl fluoride; and 1 mM dithiothreitol). Samples were snap-frozen in liquid nitrogen and stored at −80°C. Lysate total protein concentrations were determined using a colorimetric BCA™ Protein Assay Kit (Pierce, Rockford, IL, USA), and the absorbance at 562 nm was measured using a Polarstar Optima microplate reader (BMG Labtech, Australia). Samples were diluted to equal protein concentration using lysis buffer A. AMPK heterotrimers were immunoprecipitated from lysates as described previously (23). Briefly, Protein A-Sepharose beads coupled to anti-AMPKα1 and α2 antibodies were added to lysates for 2 h at 4°C on an orbital shaker, followed by successive washing with ice-cold 1× PBS, 2% Triton X-100/PBS, and again with PBS. Immunocomplexed beads were resuspended in either 50 mM Tris, pH 7.5, for AMPK activity assay (see below), or 3× sample loading buffer for Western blot analysis. Acetyl CoA carboxylase-1 (ACC1) was affinity-purified from cell lysates in a similar manner using streptavidin-sepaharose beads (Amersham). Purified complexes were heated (95°C, 5 min), separated on 10% SDS-PAGE gels and transferred to PVDF membranes (Millipore, Bedford, MA, USA). Membranes were preincubated with blocking buffer (PBS, 0.1% v/v Tween20, and 5% w/v skim milk powder), rinsed in PBS, and incubated with primary antibodies at ∼1:1000 for 1 h at room temperature. In the case of detection of total ACC, streptavidin-horseradish peroxidase conjugate (GE Healthcare, UK) was used. Rabbit polyclonal anti-α and -β AMPK subunit specific antibodies were produced in our laboratory and have been previously described (34, 35). Anti-γ1, -γ2, and -γ3 polyclonal antibodies were raised in rabbits against the following peptide sequences; rat γ1 (319-331) CQALVLT GGEKKP, human γ2 (555-569) CLTPAGAKQKETETE, and human γ3 (59-75) AESTGLEATFPKTTPLC. Peptide antibodies were produced and purified by peptide affinity chromatography as previously outlined (35). Membranes were washed in PBS containing 0.1% Tween (PBS-T), incubated with horseradish peroxidase-conjugated protein G secondary antibody (1:2000; Bio-Rad, Hercules, CA, USA), and washed, and the immunoreactive proteins were detected with enhanced chemiluminescence (ECL) reagent.
AMPK assays
AMPK assays were performed as described previously (36) using a SAMS peptide substrate (HMRSAMSGLHVKRR, which corresponds to the Ser-79 phosphorylation site in ACC1, an AMPK target. For each sample, 20 μl of a bead slurry of immunoprecipitated AMPK (see above) was incubated for < 10 min at 30°C with 20 μl of reaction solution to give a final concentration of 100 μM SAMS peptide substrate, 50 mM Hepes (pH 7.5), 1 mM DTT, 0.05% TritonX-100, 200 μM AMP, 250 μM ATP (500–1000 cpm [γ-32P]/pmol unlabeled ATP), 10 mM MgCl2, and 5% glycerol. Each reaction (25 μl) was spotted onto P81 phosphocellulose paper (Whatman, Maidstone, UK), washed twice in 1% phosphoric acid (v/v) for 30 min, and dried. Samples were placed in individual scintillation vials containing 10 ml of scintillation fluid (Opti-Fluor O; PerkinElmer, Wellesley, MA, USA). Baseline values were calculated by performing the assay in the absence of AMPK and subtracting these counts from the experimental values. AMPK-specific activities were calculated as picomoles of phosphate transferred to the SAMS peptide per minute per milligram of protein used in the initial AMPK immunoprecipitation.
OC formation assays and bulk OC preparation
As described previously (33), 10-mm-diameter culture wells (Greiner Bio-One, Frickenhausen, Germany) were seeded with 200 μl of MEM/FBS containing cell bone marrow cells or BMMs (both 105 cells/well) or RAW264.7 cells (104 cells/well) stimulated with soluble recombinant RANKL (100 ng/ml) and M-CSF (30 ng/ml). Medium and mediators were changed after 3 d incubation at 37°C. Cells were fixed after 7 d incubation, then fixed in formaldehyde and histochemically stained to identify tartrate-resistant acid phosphatase positive (TRAP+) multinucleated cells (MNCs), which have been validated previously as OCs (37). Alternatively, spleen cells or BMMs were cocultured with primary OBs (2×104 per well) for 7 d in the presence of 10−8 M 1,25(OH)2-D3 and 10−7 M prostaglandin E2 (PGE2). For the bulk preparation of OC, BMMs were incubated in 10-cm-diameter Petri dishes and stimulated by 100 ng/ml RANKL, 30 ng/ml M-CSF, and 5 ng/ml TGFβ with a change of medium and mediators at d 3. The cultures are utilized at d 5 when OC numbers are maximal; the cultures contain significant numbers of mononuclear macrophages (33).
Investigation of in vivo AICA-riboside action
Male wild-type (10-wk-old) C57Bl/6J mice (Animal Resources Centre, Canning Vale, Australia) were treated for 4 wk with a daily intraperitoneal injection of AICA-riboside dissolved in PBS (500 mg/kg) or PBS alone. Mice were housed in microisolator cages and given ad libitum access to standard rodent chow (Barastoc, Sydney, Australia) and acidified water.
Peripheral quantitative computer tomography (pQCT) analysis of murine femora
Femoral cortical (Ct) and trabecular (Tb) bone mineral density (BMD), femoral circumference, and femoral cortical thickness were measured by pQCT (Stratec X-CT Research SA+, version 5.5; Stratec, Pforzheim, Germany) using methods adapted from Sims et al. (38). Metaphyseal scans of the distal femur were taken at a resolution of 70 μm; trabecular and cortical measurements (including circumference) were taken at a distance proximal to the distal growth plate of 5% and 25% of the length of the femur, respectively; Tb.BMD was determined as the inner 45% of the total area (peel mode 20).
Bone histomorphometric analysis of murine tibia
Tibiae were collected, processed, and analyzed as previously reported (38). Briefly, tibiae were collected at 4, 12, or 26 wk of age; fixed in 4% paraformaldehyde/PBS; and embedded in methylmethacrylate. Sections (5 μm) of the proximal tibia were stained with toluidine blue and measured according to standard procedures using the Osteomeasure system (OsteoMetrics Inc, Decatur, GA, USA). Tibial cortical thickness, periosteal mineral appositional rates, growth plate width, and thickness of the hypertrophic and proliferative zones were measured as described previously (38). Femoral length and width were determined from contact X-rays that were scanned and measured using NIH Image 1.62 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are reported as means ± se. Cell culture data are pooled from ≥4 independent experiments. Bone histomorphometric analyses presented used 8 mice/category and were analyzed by 1-way ANOVA and Fisher’s post hoc test.
RESULTS
AMPK subunit expression and activation in bone cells
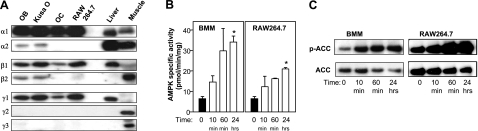
We examined the AMPK subunits present in bone cells by Western blotting. Both osteoblastic lineage cells (cultured primary calvarial OBs and the Kusa O osteoblastic cell line) and macrophage/OC lineage cells were examined. The latter consisted of in vitro-generated OC populations (containing both OC and macrophages but not stromal cells) and the bipotential macrophage/OC cell line, RAW264.7. OCs contained α1-, β1-, and γ1-subunit isoforms to yield exclusively α1β1γ1 heterotrimers; whereas OBs expressed α1, α2, β1, β2, and γ1 and would be predicted to express multiple heterotrimer combinations (Fig. 1A). The γ2 or γ3 subunits were not detected in any of the above cell types.
Figure 1.
AMPK subunit expression and activation in bone cells. A) Western blot analysis of subunit expression. AMPK heterotrimers were immunoabsorbed from cell lysates using α1/α2 specific antibodies. Blots were probed with AMPK subunit isoform-specific antibodies listed at left. Cell lysates included primary calvarial OBs, Kusa O cells, in vitro generated OC-containing cultures, and RAW 264.7 cells. Mouse liver and skeletal muscle (or cardiac for γ2) lysates were included as controls. B) AICAR activation of AMPK in BMMs and RAW264.7 cells. Cells were stimulated with 2 mM AICAR for 10 and 60 min, and 0.2 mM for 24 h time points. AMPK heterotrimers were immunoprecipitated from cell lysates, and AMPK activity was measured by SAMS peptide phosphorylation assay. C) Phosphorylation of AMPK target ACC resulting from AICAR treatment. ACC was precipitated by biotin affinity from cell lysates, and ACC total and phosphorylated ACC (p-ACC) was detected by Western blotting methods. *P < 0.05 vs. positive controls (black columns).
We next examined whether the widely used activator AICA-riboside could activate AMPK in bone cells. In cultured BMMs and macrophage-like RAW264.7 cells, AICA-riboside caused a large and sustained increase in AMPK activity as measured by an in vitro peptide phosphorylation assay (Fig. 1B). Similarly, phosphorylation of ACC, a downstream substrate of AMPK, was elevated by AICA-riboside treatment of these cells (Fig. 1C). Similar effects of AICA-riboside treatment were seen in primary cultured OBs (data not shown).
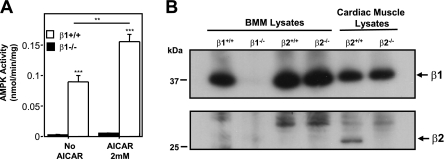
Based on the AMPK subunit composition of OC (Fig. 1A), we predicted that OC derived from our recently described AMPKβ1-knockout mice would be functionally AMPK null (31). As shown in Fig. 2A, AICA-riboside treatment caused robust AMPK activation in BMMs from wild-type but not from AMPKβ1−/− mice, and the latter had extremely low or undetectable endogenous AMPK activity. Western blot analysis confirmed that AMPKβ1−/− BMMs lacked the AMPK β1 subunit (Fig. 2B), with no compensatory up-regulation of the β2 isoform in these cells. Analysis by quantitative real-time PCR revealed an absence of β2 transcripts; threshold levels for β2 in the β1−/− and β1+/+ BMMs were exceeded only after 39 cycles (data not shown).
Figure 2.
BMMs derived from AMPK β1−/− mice are AMPK null. A) Lack of AMPK activity in BMMs derived from AMPK β1−/− mice. BMMs were treated with or without AICAR (2 mM, 30 min) to activate AMPK. AMPK activity was measured as outlined in Materials and Methods. **P < 0.01, ***P < 0.001 vs. respective negative control. B) AMPKβ1−/− BMMs do not express a β2 subunit. BMMs and cardiac muscle tissue lysates prepared from AMPK β1 and β2 wild-type (+/+) and knockout mice (−/−) were immunoabsorbed using α1/α2 antibodies. Western blots were probed with a monoclonal antibody recognizing both β1 and β2 subunits (Epitomics). β1 is expressed in all BMM lysates (except β1−/− BMMs) as well as cardiac muscle. β2 is expressed in cardiac lysates from β2+/+ mice but not β2−/− mice and is absent from all BMM lysates. See Supplemental Material for details describing the generation and preliminary characterization of AMPKβ2−/− null mice.
Analysis of the bone phenotype of AMPKβ1−/− and AMPKβ2−/− mice
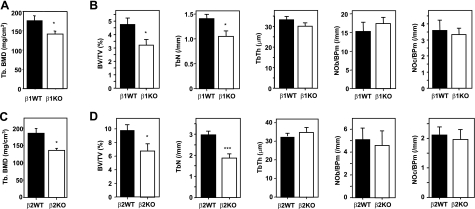
We examined the femora of 12-wk-old female AMPKβ1−/− mice by pQCT. Compared to wild-type littermates, β1−/− mice had significantly reduced (∼20%) trabecular bone density (Fig. 3A) but normal cortical bone density, circumference, and thickness (data not shown). Histomorphometric analysis revealed tibial trabecular bone volume was also significantly lower (∼30%), and trabecular number but not thickness was lower than wild-type controls (Fig. 3B). The reason for the lower trabecular bone mass is not clear, as OC and OB numbers did not differ between the groups (Fig. 3B), indicating that neither OB nor OC formation was abnormal. In 18–20 wk old male AMPKβ1−/− mice, no significant changes in trabecular structure were observed (BV/TV: wild-type, 9.45±1.94, n=4; β1−/−, 7.32±1.17, n=5; P=0.16; TbN: wild-type, 2.28±0.26, n=4; β1−/−, 2.23±0.30, n=5; P=0.45; TbTh: wild-type, 40.2±4.8, n=4; β1−/−, 32.3±1.9, n=5; P=0.06). Again, in males OC numbers were normal (NOc/BPm: wild-type, 1.66±0.23, n=4; β1−/−, 1.20±0.40, n=5; P=0.19), OB numbers and osteoid surface (OS/BS) were not significantly reduced (NOb/BPm: wild-type, 5.70±3.08, n=4; β1−/−, 1.48±0.62, n=5; P=0.19; OS/BS: wild-type, 6.00±2.76, n=4; β1−/−, 1.57±0.63, n=5; P=0.06), and osteoid volume (OV/BV) was significantly lower in AMPKβ1−/− males, suggesting a lower level of bone formation (OV/BV: wild-type, 0.489±0.223, n=4; β1−/−, 0.103±0.047, n=5; P=0.04).
Figure 3.
Bone phenotypes of germline AMPK β1- and β2-subunit-knockout mice. A) pQCT analysis of trabecular bone of femora of female WT and AMPKβ1−/− mice. B) Histomorphometric analysis of female AMPKβ1−/− mouse bones. C) pQCT analysis of trabecular bone of femora of male WT and AMPKβ2−/− mice. D) Histomorphometric analysis of male WT and AMPKβ2−/− mouse bones. *P < 0.05, ***P < 0.001 vs. WT (black columns).
We also generated germline AMPKβ2−/− mice (see Supplemental Material) and examined the femora and tibia of 12-wk-old male animals by pQCT and histomorphometry, respectively. β2−/− femora also demonstrated reduced trabecular bone density (Fig. 3C) and normal cortical bone density, circumference, and thickness (data not shown). Tibial trabecular bone volume and trabecular number were significantly lower than matched wild-types, but again OC and OB numbers were not significantly different between groups (Fig. 3D).
In summary, both β1−/− and β2−/− lines had lower bone mass but normal OC and OB numbers. While differences in cell numbers too small to measure by these methods may have biologically significant effects, our results provide unequivocal genetic evidence that AMPK is not essential for OC differentiation in vivo and that either β1 or β2 alone is sufficient to support OB differentiation. Although the underlying mechanism for the bone abnormality is unclear, we propose that AMPK may influence bone cell functions other than OC and OB differentiation; the change in osteoid volume in the male mice only suggests a possible role in the production of osteoid (the nonmineral component of bone).
In vivo influence of AICA-riboside on bone mass
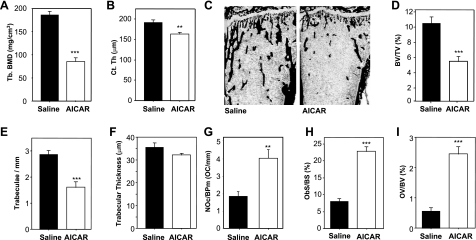
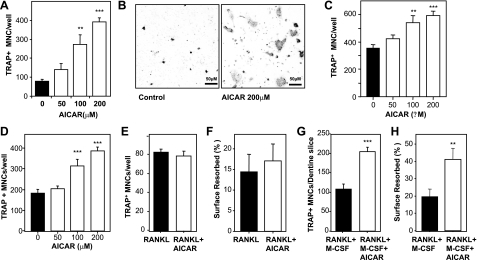
Since the loss of AMPK β-subunit expression in our β1−/− and β2−/− mice led to a low-bone-mass phenotype, we hypothesized that activation of AMPK might lead to increased bone mass. We treated normal wild-type mice with AICA-riboside (also called acadesine or AICAR), as this compound has been used widely as a cell-permeable activator of AMPK. AICA-riboside is a prodrug that is converted by adenosine kinase to the nucleotide ZMP in metabolically active cells (39, 40). ZMP can act as an AMP mimetic and activates AMPK (27). To investigate the effects of chronic administration of AICA-riboside on bone homeostasis, we adapted a protocol previously used to examine the effects of AMPK activation in skeletal muscle of mice (41). Male wild-type (10-wk-old) C57Bl/6 mice were injected with either PBS or AICA-riboside at a dose of 500 mg/kg daily for 4 wk. After sacrifice, pQCT analysis revealed that femoral trabecular bone density of AICA-riboside treated mice was dramatically lower (54%) than in control mice (Fig. 4A). In addition, this treatment caused an ∼15% reduction in cortical thickness (Fig. 4B). Since the rate of turnover of murine cortical bone is much lower than trabecular bone, such a significant drop in cortical bone density suggests that the influence of AICA-riboside on bone is very powerful. Histomorphometric measurement of the volume of mineralized bone showed a similar effect of AICA-riboside treatment; i.e., 49% less trabecular bone volume relative to PBS-treated control mice (Fig. 4C, D). This finding was characterized by a reduction in trabecular number but not trabecular thickness (Fig. 4E, F), consistent with an elevated rate of bone resorption. Indeed, OC numbers (Fig. 4G) and OC surface (data not shown) were significantly elevated in treated mice compared to controls. We also observed greater osteoid volume and OB numbers in treated animals (Fig. 4H, I), which suggests increased bone formation coupled to the elevated bone resorption, which was clearly insufficient to compensate for the proosteolytic actions, a similar effect to that observed after ovariectomy (42). Together with the elevated OC numbers, this finding indicates that AICA-riboside causes a high turnover osteopenia phenotype in male mice. We have not examined whether this same response occurs in female mice.
Figure 4.
AICAR causes bone loss and elevated bone turnover. Male C57Bl/6 mice (10 wk old) were treated with AICAR (500 mg/kg/d) or saline for 4 wk. A, B) pQCT analysis of femoral trabecular density (A) and femoral cortical thickness (B). C) Low-power views of undecalcified sections of tibia from saline and AICAR-treated mice histochemically stained by von Kossa method (black indicates mineralized bone) and counterstained with ponceau-orange G. D) Tibial trabecular volume, BV/TV. E) Tibial trabecular numbers/mm (Tb.N). F) Tibial trabecular thickness (Tb.Th). G) Tibial OC numbers on trabecular bone (Oc.N). H) Surface of tibial bone occupied by OBs, Ob.S/BS. I) Tibial osteoid volume (OV/BV). **P < 0.01, ***P < 0.001 vs. saline control (black columns); n = 6 mice/treatment group.
Effects of AICA-riboside on OC formation, survival, and function in vitro
Since we observed greatly elevated OC numbers in AICA-riboside treated mice, we investigated AICA-riboside action on OC formation and function in vitro. We first used OB/bone marrow cocultures, as this system recapitulates key aspects of OC formation in vivo. Bone marrow cells cocultured with OBs were stimulated with high concentrations (10 nM) of 1,25(OH)2-D3 as described previously (33). Numerous OCs were formed after 7 d of culture and AICA-riboside treatment dose-dependently increased this number (Fig. 5A). Consistent with accelerated OC formation, these cultures typically contained larger OCs than control cultures (Fig. 5B). AICA-riboside effects were maximal at 200 μM; higher concentrations of AICA-riboside resulted in cell toxicity, which was evident on microscopic examination of cells.
Figure 5.
AICAR causes supramaximal OC formation in vitro without effects on OC survival or activity. A) Dose-response effect of AICAR on OC formation in cocultures of bone marrow cells with calvarial OBs, stimulated by 10–8 M 1,25(OH)2-D3 for 7 d. B) Photomicrographs of cocultures after staining for TRAP (dark gray in image), showing control and 200 μM AICAR-treated cells. C) Dose-response effect of AICAR on OC formation in bone marrow cells maximally stimulated by 100 ng/ml RANKL and 30 ng/ml M-CSF. D) Dose response of AICAR on OC formation in 100 ng/ml RANKL-stimulated RAW264.7 cells. E) AICAR (200μM) effects on 24 h survival of RANKL-treated dispersed OCs. F) AICAR (200 μM) effects on dentine resorption area by 100 ng/ml RANKL-stimulated dispersed OCs incubated for 3 d. G) Numbers of OCs formed on dentine in cultures of bone marrow cells stimulated for 10 d by RANKL (100 ng/ml) and M-CSF (30 ng/ml) in the presence and absence of AICAR. H) AICAR effects on resorption by OCs formed on dentine, as in G. **P < 0.01, ***P < 0.001 vs. positive control (black columns).
In this coculture experimental system, and in vivo, both OBs and bone marrow progenitors are present. Since 1,25(OH)2-D3-stimulated OBs support OC formation by delivery of RANKL and M-CSF, to clarify the actions of AICA-riboside, we used recombinant RANKL- and M-CSF-stimulated bone marrow cultures. AICA-riboside also dose-dependently stimulated OC formation in these assays, elevating OC numbers above the maximum obtained by the RANKL stimulus (Fig. 5C). This finding suggests that its actions are independent of the presence of OBs or 1,25(OH)2-D3. To confirm that the target of the proosteoclastogenic action of AICA-riboside is the hematopoietic progenitors (and not some other bone marrow cell population), we examined RANKL-stimulated BMMs (data not shown) and the RAW264.7 cell line (Fig. 5D). In both cases, OC formation was elevated by AICA-riboside treatment.
Since AICA-riboside influenced OC differentiation so strongly, we investigated its actions on OC survival and activity. We generated OCs in bulk (33), then dispersed and seeded them in culture wells for overnight incubation in the presence of RANKL (100 ng/ml) as a survival factor, or with no survival factor. After 24 h without survival factors, all OCs were dead; 200 μM AICA-riboside did not affect this (Fig. 5E), nor did it affect OC survival in cultures treated with lower concentrations of RANKL (20 ng/ml), or influence M-CSF- or TNF-treated OC survival (data not shown). AICA-riboside did not affect the ability of mature RANKL-stimulated OCs to make resorption pits in dentine (Fig. 5F). However, in cultures of bone marrow cells on a dentine surface stimulated for 11 d with RANKL/M-CSF to stimulate OC formation, AICA-riboside at 200 μM caused an approximate doubling of dentine resorption. However, approximately double the number of OCs were also observed on the dentine surface when AICA-riboside was included (Fig. 5G, H), which suggests that the increase in resorption is accounted for by increases in OC numbers; i.e., AICA-riboside does not affect OC activity. These data also confirm that the additional OCs formed because of AICA-riboside treatment are functional.
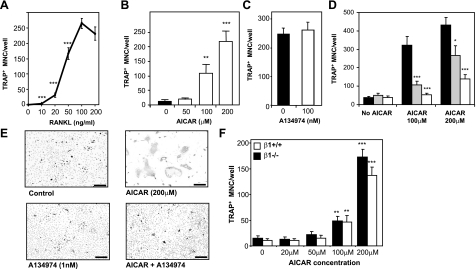
Because we observed increased numbers of OCs with AICA-riboside with the maximal concentration of RANKL (100 ng/ml), we examined the effects of AICA-riboside at lower RANKL concentrations. Bone marrow cultures stimulated by low RANKL (20 ng/ml) resulted in typically 20–30 OCs formed per well (Fig. 6A). In these cultures 200 μM AICA-riboside caused a roughly 25-fold increase in OC formation relative to controls (Fig. 6B).
Figure 6.
AICAR-increased OC formation is ZMP dependent but AMPK independent. A) Dose response of RANKL on OC formation. ***P < 0.01 vs. 100 ng/ml RANKL. B) Dose response of AICAR on OC formation in bone marrow cells stimulated with submaximal RANKL (20 ng/ml) and 30 ng/ml M-CSF. C) Effects of A134974 on bone marrow cell cultures maximally stimulated by RANKL (100 ng/ml) and M-CSF (30 ng/ml). D) Ablation of AICAR action on OC formation by A134974; bone marrow cell cultures submaximally stimulated with RANKL (20 ng/ml) and 30 ng/ml M-CSF in presence and absence of AICAR. Gray columns, 100 pM A134974; white columns, 1 nM A134974. E) Photomicrographs of TRAP-positive cells (dark gray) in bone marrow cell cultures stimulated with submaximal (20 ng/ml) RANKL, as in B; control, A134974 treated, AICAR-treated, and AICAR plus A134974-treated cultures. Scale bars = 50 μm. F) Effects of AICAR treatment on OC formation from 20 ng/ml RANKL-stimulated BMMs from AMPKβ1−/− and AMPKβ1+/+ mice. *P < 0.05, **P < 0.01, ***P < 0.001 vs. respective controls.
Because AICA-riboside is structurally related to adenosine and is an intermediate in the purine biosynthesis pathway, we examined whether AICA-riboside action on OC formation may be mediated by adenosine receptors. We tested the effects of adenosine, adenosine uptake inhibitors dipyridamole and 4-nitrobenyl-6-theoinosine, and the non-specific adenosine receptor antagonist CGS15943 on RANKL/M-CSF-stimulated bone marrow cultures. We found no effects of these agents either on OC formation or on AICA-riboside actions (data not shown).
To determine whether these effects were dependent on the conversion of AICA-riboside to ZMP by adenosine kinase, we used A134974, a potent and specific pharmacological adenosine kinase inhibitor. Titration of this agent showed that at concentrations below 100 nM, no direct effect on OC formation was found at either 100 or 20 ng/ml RANKL (Fig. 6C, D). At 1 nM, A134974 ablated the stimulatory action of AICA-riboside with no effects on OC formation in the absence of AICA-riboside; 100 pM A134974 had a lesser but still significant influence (Fig. 6D, E). In vitro kinase assays revealed A134974 inhibited AMPK (IC50=48 μM) only at concentrations several orders of magnitude higher than the doses used in these experiments. These data suggest that AICA-riboside actions on OC formation required adenosine kinase and involved ZMP, ZDP, or ZTP.
Since we had found that macrophage/OC-lineage cells lack AMPK β2 and that these cells when cultured from AMPKβ1−/− mice are functionally AMPK null, we investigated OC formation and AICA-riboside action in AMPKβ1−/− cells. Bone marrow cells taken from 12-wk-old β1−/− males yielded similar numbers of OCs as wild-type mice when maximally stimulated with 100 ng/ml RANKL and 30 ng/ml M-CSF (data not shown). Furthermore, in similar cultures stimulated with a lower RANKL concentration (20 ng/ml), no differences were seen between WT and AMPKβ1−/− OC formation, and AICA-riboside elevated β1−/− OC numbers to a similar degree as wild-type OCs (Fig. 6F). This finding shows that the in vitro actions of AICA-riboside, though ZMP-dependent, are independent of AMPK. Together with our observation that OC numbers were not abnormal in AMPKβ1−/− mice despite the absence of AMPK β2 subunit, which suggests that AMPK does not play any significant role in OC lineage cells. It also suggests that while AICA-riboside greatly stimulates OC formation, it acts through an AMPK-independent mechanism.
DISCUSSION
In view of the roles of AMPK in controlling energy metabolism, and the emerging relationship between whole-body energy homeostasis and bone metabolism (15), we examined the bones of germline AMPK β1- and β2-subunit-knockout mice. pQCT analysis revealed low femoral trabecular bone mass compared to wild-type littermate controls, which suggests an abnormality in bone metabolism. Consistent with this, analysis of both lines by histomorphometry also revealed lower trabecular bone thickness and trabecular number, but no significant alteration in OB or OC numbers were detected. While differences in bone mass and density are often reflected in altered OC/OB numbers, this is not always the case. For example, their respective activities may be reduced, defects may occur at the growth plate where trabecular bone is formed (43), or an early developmental defect in young mice may occur resulting in lower bone mass as the mouse gets older (44). Our results suggest that the bone abnormalities observed in our mice may be due to reduced bone cellular functions, rather than affecting OC and OB differentiation, which appears to be intact in vivo. Reduced AMPK levels in accessory cells such as osteocytes and local macrophages or AMPK signaling abnormalities in the central nervous system may also play a role. Given the importance of AMPK at the cellular level, it was particularly surprising that in β1−/− mice, the OC populations were normal and that bone resorption clearly occurred, because our in vitro studies clearly showed a lack of detectable β2-subunit protein and AMPK activity in cells of the macrophage/OC lineage. If a similar scenario holds true in vivo, it would indicate that OC and presumably macrophages do not require AMPK functionality, at least under physiological conditions in mice of this age.
The loss of bone in our knockout models prompted us to explore whether systemically activating AMPK might lead to increased bone mass. We investigated the actions in bone of AICA-riboside, as it is a widely used tool for studying the effects of AMPK activation in isolated cells and in vivo. Despite its relatively poor bioavailability (45), interest in using AICA-riboside for treating a number of clinically important conditions has grown (reviewed in ref. 46). The most promising include a phase III trial to treat ischemia-reperfusion injury with AICA-riboside following coronary artery bypass surgery (47, 48) and a phase I trial for the treatment of B-cell chronic lymphocytic leukemia (49). Furthermore, in rodents AICA-riboside exerts both prophylactic and therapeutic effects in an experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis, most likely by attenuating inflammation-associated neurodegeneration via a shift from Th1-type to Th2 cytokine production (50). We treated normal mice with AICA-riboside following a protocol previously used to study effects on AMPK activation in murine skeletal muscle (41), and similar to that employed by Narkar et al. (29). Surprisingly, we found that AICA-riboside had extremely detrimental effects on bone in mice, with an ∼50% reduction in trabecular bone mass observed. These observations themselves suggest that clinical use of this compound would be detrimental to bone density.
The AICA-riboside treated mice displayed greatly increased numbers of OC and OBs, indicating a high-turnover type of osteopenia. Bone resorption by OC is normally followed by bone replacement by activated OBs, which ensures that bone structure and morphology are preserved, a phenomenon that probably involves the recruitment and activation of OBs by activated OCs (51). Indeed, consistent with this notion, clinical treatments that cause a fall in OC numbers (e.g., bisphosphonates) frequently lead to reduced OB numbers, while increased OC numbers (e.g., caused local inflammatory stimuli) increases OB numbers. The latter is observed with AICA-riboside treatment, and as with other types of high turnover osteopenia, the osteoblastic response may ameliorate the bone loss but is insufficient to compensate for it when osteolysis is abnormally high.
OC formation, activity, and survival are regulated by osteolytic hormones such as PTH, 1,25(OH)2-D3, prostaglandins, and inflammatory cytokines. These factors act on osteoblastic cells to increase the production of RANKL, a factor essential for OC differentiation but which also activates OCs and promotes their survival. Excessive and rapid OC formation, seen in a number of common osteolytic diseases, can result from abnormally high stimulation of RANKL production (which under inflammatory conditions can also be released by activated T cells) or by pathophysiological stimuli that increase responsiveness of OC progenitors to RANKL. Our data strongly indicate that, rather than acting through OBs or some other accessory cell, AICA-riboside affects the responsiveness to RANKL of OC progenitors. Several exogenous stimuli have been identified that increase RANKL-dependent OC formation although their mechanism of action are highly controversial; these currently include TGFβ and reactive oxygen species, while pharmacological reagents that do this include rosiglitazone and geldanomycin-derived HSP90-blocking compounds (52). However, the diversity of these OC-stimulating factors is probably not consistent with a single mechanism of action. In addition, since OC progenitors are not a single type of cell with an agreed definition (although they do form from immature cells that also form macrophages and dendritic cells), it is not possible to determine whether their number or fate is altered by AICA-riboside treatment. To date we have not found any effect of AICA-riboside on NF-κB, c-fos, or NFATc1 signaling. It is notable, however, that other functions of RANKL, which include promotion of OC survival and activity, are not enhanced by AICA-riboside action, which may suggest that its actions do not merely magnify RANK signals but have specific effects affecting actions downstream.
A number of effects of AICA-riboside have been shown to be independent of AMPK by showing that their action does not require formation of ZMP. We therefore cotreated osteoclastogenic cultures with AICA-riboside and an adenosine kinase inhibitor, which confirmed that the conversion of AICA-riboside to ZMP was required. This finding also suggests that adenosine receptor dependent pathways are not involved. Consistent with this, neither adenosine, adenosine receptor agonists, nor antagonists mimicked or blocked AICA-riboside action in these assays. It is also notable that AICA-riboside has antiinflammatory and antiproliferative actions that are reminiscent of TGF-β, a factor that also accelerates OC formation (53).
Thus, AICA-riboside powerfully increases OC differentiation above that possible by RANKL/M-CSF stimulus in vitro. We found that macrophage lineage cells lack the β2 subunit and that macrophages from β1−/− mice do not up-regulate expression of the β2 subunit to compensate for the lack of β1. We also showed that these cells lacked endogenous AMPK activity. Thus, AMPK is not essential for survival and functions of macrophages or OCs reported herein, despite these cells containing large numbers of mitochondria and a presumed high rate of energy demand (54). It also provided compelling evidence that AICA-riboside actions on OC differentiation is independent of AMPK, since RANKL-treated β1−/− bone marrow cells also display enhanced OC formation when treated with AICA-riboside. While ZMP can activate AMPK, other mechanistically important AMP-binding proteins can also be activated by ZMP, including glucokinase (55), glycogen synthase and phosphorylase (56, 57), and fructose 1,6-bisphosphatase (39). In addition, as recently proposed by Guigas and colleagues (58), phosphorylation of AICA-riboside to ZMP would result in depletion of intracellular inorganic phosphate (Pi), resulting in deinhibition of AMP deaminase and a reduction in adenine nucleotides.
CONCLUSIONS
We have shown that genetically modified mice lacking individual AMPK β1 or β2 subunits have a low-bone-mass phenotype. Although the underlying mechanism for this defect remains unclear, this is the first demonstration that AMPK plays a role in maintaining normal bone homeostasis. We have also shown that AICA-riboside has powerful proosteolytic action mediated though increased OC differentiation that, while ZMP-dependent, is independent of AMPK. Indeed, this finding suggests that OCs have no requirement for AMPK to form and function and that the low bone mass seen in the AMPK β1- or β2-subunit-null mice is not mediated by OCs.
Supplementary Material
Acknowledgments
This work was supported by National Health and Medical Research Council (NHMRC) grants; N.A.S. and B.E.K. are NHMRC Fellows.
References
- Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Seibel M J. Nutrition and molecular markers of bone remodelling. Curr Opin Clin Nutr Metab Care. 2002;5:525–531. doi: 10.1097/00075197-200209000-00011. [DOI] [PubMed] [Google Scholar]
- Scott J W, Oakhill J S, van Denderen B J W. AMPK/SNF1 structure: a ménage a trois of energy-sensing. Front Biosci. 2009;14:596–610. doi: 10.2741/3266. [DOI] [PubMed] [Google Scholar]
- Sun W, Lee T S, Zhu M, Gu C, Wang Y, Zhu Y, Shyy J Y. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- Gutierrez G E, Edwards J R, Garrett I R, Nyman J S, McCluskey B, Rossini G, Flores A, Neidre D B, Mundy G R. Transdermal lovastatin enhances fracture repair in rats. J Bone Miner Res. 2008;23:1722–1730. doi: 10.1359/JBMR.080603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3–E1 cells. BMC Cell Biol. 2007;8:51. doi: 10.1186/1471-2121-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glintborg D, Andersen M, Hagen C, Heickendorff L, Hermann A P. Association of pioglitazone treatment with decreased bone mineral density in obese premenopausal patients with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2008;93:1696–1701. doi: 10.1210/jc.2007-2249. [DOI] [PubMed] [Google Scholar]
- Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid I R. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–1310. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- Meier C, Kraenzlin M E, Bodmer M, Jick S S, Jick H, Meier C R. Use of thiazolidinediones and fracture risk. Arch Intern Med. 2008;168:820–825. doi: 10.1001/archinte.168.8.820. [DOI] [PubMed] [Google Scholar]
- Boyle J G, Logan P J, Ewart M A, Reihill J A, Ritchie S A, Connell J M, Cleland S J, Salt I P. Rosiglitazone stimulates nitric oxide synthesis in human aortic endothelial cells via AMP-activated protein kinase. J Biol Chem. 2008;283:11210–11217. doi: 10.1074/jbc.M710048200. [DOI] [PubMed] [Google Scholar]
- Fryer L G, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- Carey A L, Steinberg G R, Macaulay S L, Thomas W G, Holmes A G, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt M J, James D E, Kemp B E, Pedersen B K, Febbraio M A. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- Steinberg G R, Michell B J, van Denderen B J, Watt M J, Carey A L, Fam B C, Andrikopoulos S, Proietto J, Gorgun C Z, Carling D, Hotamisligil G S, Febbriao M A, Kay T W, Kemp B E. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Herman S, Kronke G, Schett G. Molecular mechanisms of inflammatory bone damage: emerging targets for therapy. Trends Mol Med. 2008;14:245–253. doi: 10.1016/j.molmed.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Lee N K, Sowa H, Hinoi E, Ferron M, Ahn J D, Confavreux C, Dacquin R, Mee P J, McKee M D, Jung D Y, Zhang Z, Kim J K, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim C A, Ogawa Y, Liu X, Ware S M, Craigen W J, Robert J J, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Kinzig K P, Aja S, Scott K A, Keung W, Kelly S, Strynadka K, Chohnan S, Smith WW, Tamashiro K L, Ladenheim E E, Ronnett G V, Tu Y, Birnbaum M J, Lopaschuk G D, Moran T H. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci U S A. 2007;104:17358–17363. doi: 10.1073/pnas.0708385104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim Y B, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum M J, Stuck B J, Kahn B B. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol. 2006;76:103–127. doi: 10.1016/S0070-2153(06)76004-5. [DOI] [PubMed] [Google Scholar]
- Leboy P S. Regulating bone growth and development with bone morphogenetic proteins. Ann N Y Acad Sci. 2006;1068:14–18. doi: 10.1196/annals.1346.003. [DOI] [PubMed] [Google Scholar]
- Horwood N J, Elliott J, Martin T J, Gillespie M T. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology. 1998;139:4743–4746. doi: 10.1210/endo.139.11.6433. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseli T J, Walter M, van Denderen B J, Katsis F, Witters L A, Kemp B E, Michell B J, Stapleton D. AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186–270) J Biol Chem. 2005;280:13395–13400. doi: 10.1074/jbc.M412993200. [DOI] [PubMed] [Google Scholar]
- Hawley S A, Boudeau J, Reid J L, Mustard K J, Udd L, Makela T P, Alessi D R, Hardie D G. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S A, Pan D A, Mustard K J, Ross L, Bain J, Edelman A M, Frenguelli B G, Hardie D G. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hurley R L, Anderson K A, Franzone J M, Kemp B E, Means A R, Witters L A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Corton J M, Gillespie J G, Hawley S A, Hardie D G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Scott J W, Hawley S A, Green K A, Anis M, Stewart G, Scullion G A, Norman D G, Hardie D G. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar V A, Downes M, Yu R T, Embler E, Wang Y X, Banayo E, Mihaylova M M, Nelson M C, Zou Y, Juguilon H, Kang H, Shaw R, Evans R M. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J W, van Denderen B J, Jorgensen S B, Honeyman J E, Steinberg G R, Oakhill J S, Iseli T J, Koay A, Gooley P R, Stapleton D, Kemp B E. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol. 2008;15:1220–1230. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Allan E H, Ho P W, Umezawa A, Makashima F, Gillespie M T, Martin T J. Differentiation potential of a mouse bone marrow stromal cell line. J Cell Biochem. 2003;90:158–169. doi: 10.1002/jcb.10614. [DOI] [PubMed] [Google Scholar]
- Quinn J M, Whitty G A, Byrne R J, Gillespie M T, Hamilton J A. The generation of highly enriched osteoclast-lineage cell populations. Bone. 2002;30:164–170. doi: 10.1016/s8756-3282(01)00654-8. [DOI] [PubMed] [Google Scholar]
- Chen Z, Heierhorst J, Mann R J, Mitchelhill K I, Michell B J, Witters L A, Lynch G S, Kemp B E, Stapleton D. Expression of the AMP-activated protein kinase beta1 and beta2 subunits in skeletal muscle. FEBS Lett. 1999;460:343–348. doi: 10.1016/s0014-5793(99)01371-x. [DOI] [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill K I, Gao G, Widmer J, Michell B J, Teh T, House C M, Fernandez C S, Cox T, Witters L A, Kemp B E. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Davies S P, Carling D, Hardie D G. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem. 1989;186:123–128. doi: 10.1111/j.1432-1033.1989.tb15185.x. [DOI] [PubMed] [Google Scholar]
- Quinn J M, Morfis M, Lam M H, Elliott J, Kartsogiannis V, Williams E D, Gillespie M T, Martin T J, Sexton P M. Calcitonin receptor antibodies in the identification of osteoclasts. Bone. 1999;25:1–8. doi: 10.1016/s8756-3282(99)00094-0. [DOI] [PubMed] [Google Scholar]
- Sims N A, Brennan K, Spaliviero J, Handelsman D J, Seibel M J. Perinatal testosterone surge is required for normal adult bone size but not for normal bone remodeling. Am J Physiol Endocrinol Metab. 2006;290:E456–E462. doi: 10.1152/ajpendo.00311.2005. [DOI] [PubMed] [Google Scholar]
- Vincent M F, Marangos P J, Gruber H E, Van den Berghe G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes. 1991;40:1259–1266. doi: 10.2337/diab.40.10.1259. [DOI] [PubMed] [Google Scholar]
- Zimmerman T P, Deeprose R D. Metabolism of 5-amino-1-beta-D-ribofuranosylimidazole-4-carboxamide and related five-membered heterocycles to 5′-triphosphates in human blood and L5178Y cells. Biochem Pharmacol. 1978;27:709–716. doi: 10.1016/0006-2952(78)90508-7. [DOI] [PubMed] [Google Scholar]
- Jorgensen S B, Treebak J T, Viollet B, Schjerling P, Vaulont S, Wojtaszewski J F, Richter E A. Role of AMPKalpha2 in basal training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- Sims N A, Clement-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M, Baron R. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest. 2003;111:1319–1327. doi: 10.1172/JCI17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J M, Sims N A, Saleh H, Mirosa D, Thompson K, Bouralexis S, Walker E C, Martin T J, Gillespie M T. IL-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J Immunol. 2008;181:5720–5729. doi: 10.4049/jimmunol.181.8.5720. [DOI] [PubMed] [Google Scholar]
- Walker E C, McGregor N E, Poulton I J, Pompolo S, Allan E H, Quinn J M, Gillespie M T, Martin T J, Sims N A. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res. 2008;23:2025–2032. doi: 10.1359/jbmr.080706. [DOI] [PubMed] [Google Scholar]
- Dixon R, Gourzis J, McDermott D, Fujitaki J, Dewland P, Gruber H. AICA-riboside: safety, tolerance, and pharmacokinetics of a novel adenosine-regulating agent. J Clin Pharmacol. 1991;31:342–347. doi: 10.1002/j.1552-4604.1991.tb03715.x. [DOI] [PubMed] [Google Scholar]
- Drew B G, Kingwell B A. Acadesine, an adenosine-regulating agent with the potential for widespread indications. Expert Opin Pharmacother. 2008;9:2137–2144. doi: 10.1517/14656566.9.12.2137. [DOI] [PubMed] [Google Scholar]
- Mangano D T. Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. JAMA. 1997;277:325–332. doi: 10.1001/jama.277.4.325. [DOI] [PubMed] [Google Scholar]
- Mangano D T, Miao Y, Tudor I C, Dietzel C. Post-reperfusion myocardial infarction: long-term survival improvement using adenosine regulation with acadesine. J Am Coll Cardiol. 2006;48:206–214. doi: 10.1016/j.jacc.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Campas C, Lopez J M, Santidrian A F, Barragan M, Bellosillo B, Colomer D, Gil J. Acadesine activates AMPK and induces apoptosis in B-cell chronic lymphocytic leukemia cells but not in T lymphocytes. Blood. 2003;101:3674–3680. doi: 10.1182/blood-2002-07-2339. [DOI] [PubMed] [Google Scholar]
- Nath N, Giri S, Prasad R, Salem M L, Singh A K, Singh I. 5-aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:566–574. doi: 10.4049/jimmunol.175.1.566. [DOI] [PubMed] [Google Scholar]
- Martin T J, Sims N A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Price J T, Quinn J M, Sims N A, Vieusseux J, Waldeck K, Docherty S E, Myers D, Nakamura A, Waltham M C, Gillespie M T, Thompson E W. The heat shock protein 90 inhibitor, 17-allylamino-17-demethoxygeldanamycin, enhances osteoclast formation and potentiates bone metastasis of a human breast cancer cell line. Cancer Res. 2005;65:4929–4938. doi: 10.1158/0008-5472.CAN-04-4458. [DOI] [PubMed] [Google Scholar]
- Quinn J M, Itoh K, Udagawa N, Hausler K, Yasuda H, Shima N, Mizuno A, Higashio K, Takahashi N, Suda T, Martin T J, Gillespie M T. Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. J Bone Miner Res. 2001;16:1787–1794. doi: 10.1359/jbmr.2001.16.10.1787. [DOI] [PubMed] [Google Scholar]
- Brown D, Breton S. Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol. 1996;199:2345–2358. doi: 10.1242/jeb.199.11.2345. [DOI] [PubMed] [Google Scholar]
- Vincent M F, Bontemps F, Van den Berghe G. Inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside in isolated rat hepatocytes. Biochem J. 1992;281:267–272. doi: 10.1042/bj2810267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnus S L, Wambolt R B, Parsons H L, Brownsey R W, Allard M F. 5-Aminoimidazole-4-carboxamide 1-beta -D-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol. 2003;284:R936–R944. doi: 10.1152/ajpregu.00319.2002. [DOI] [PubMed] [Google Scholar]
- Shang J, Lehrman M A. Activation of glycogen phosphorylase with 5-aminoimidazole-4-carboxamide riboside (AICAR). Assessment of glycogen as a precursor of mannosyl residues in glycoconjugates. J Biol Chem. 2004;279:12076–12080. doi: 10.1074/jbc.M400431200. [DOI] [PubMed] [Google Scholar]
- Guigas B, Taleux N, Foretz M, Detaille D, Andreelli F, Viollet B, Hue L. AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem J. 2007;404:499–507. doi: 10.1042/BJ20070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.