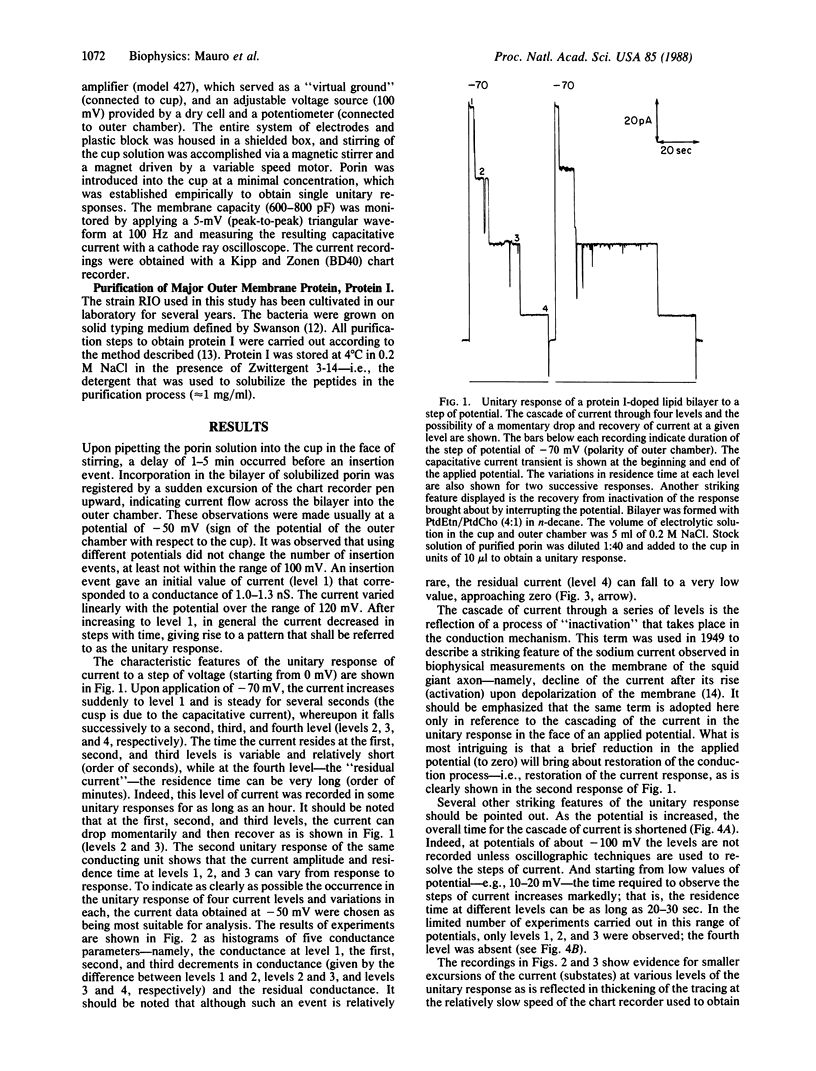
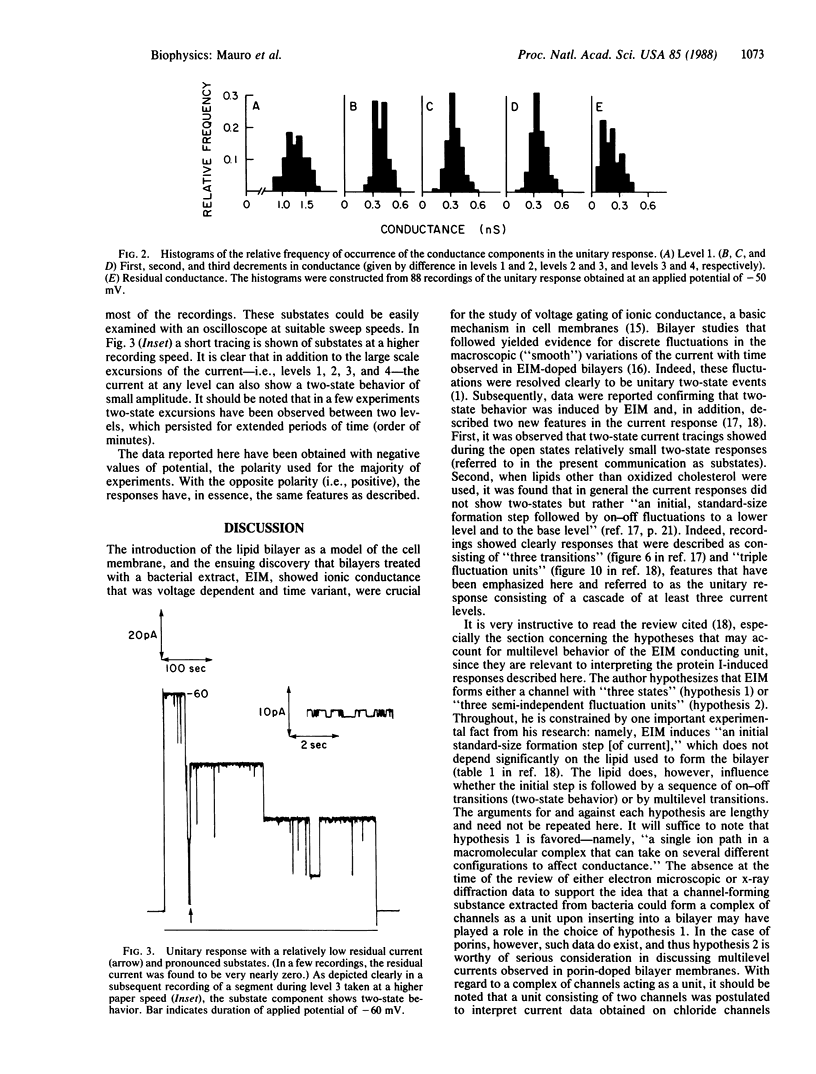
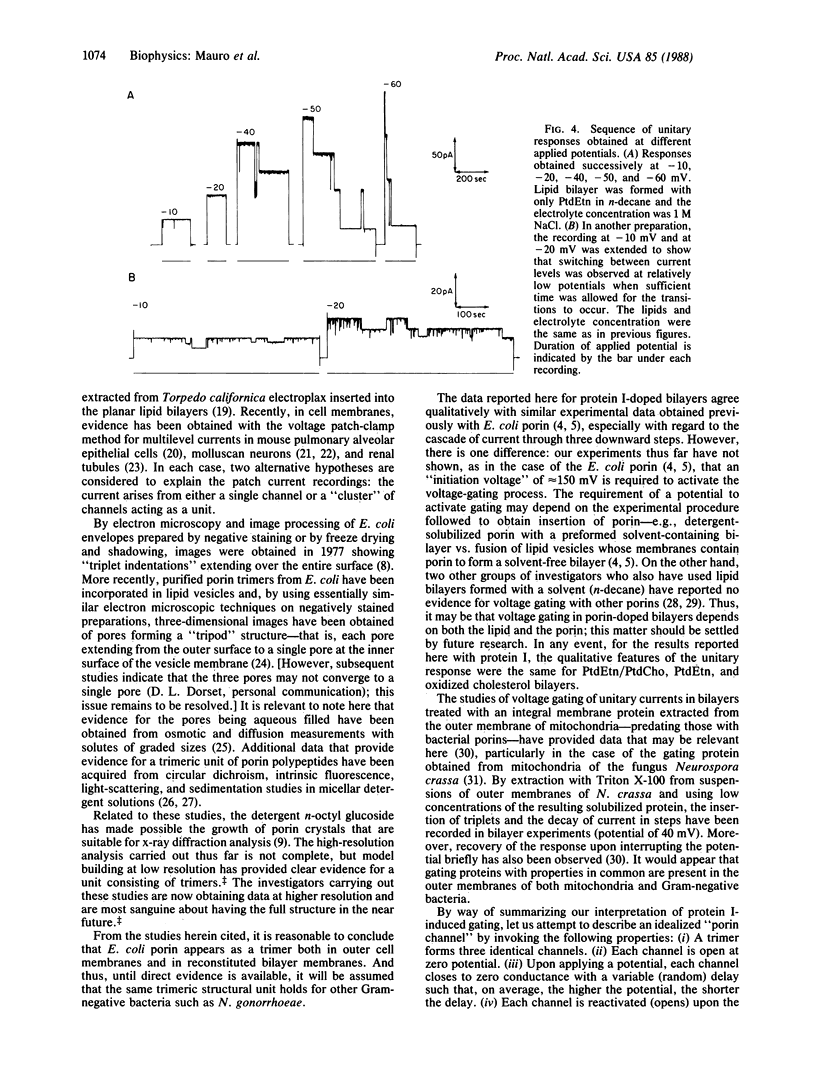
Abstract
Porins, polypeptides of approximately 35 kDa, are present as integral membrane proteins in the outer membranes of a variety of Gram-negative bacteria. As reported previously for a purified porin from Escherichia coli, voltage gating of conductance was found to be induced in a lipid bilayer by the solubilized purified porin, protein I, from Neisseria gonorrhoeae. The unitary response to an applied potential showed a cascade of current from an initial level through at least three levels, more or less equal, to a persisting lower level. The initial level of current corresponded to 1.0-1.3 nS for 0.2 M NaCl on either side of the bilayer. Briefly reducing the potential to zero restored the current to its initial level. Interpretation of the unitary response is suggested by electron microscopic data obtained on negatively stained outer membranes of E. coli indicating the presence of "pores" appearing in triplets. Moreover, low-resolution x-ray and neutron diffraction studies on crystals obtained with an E. coli porin show that three polypeptides associate to form a unit. Combining such structural data with the present electrical data lends support for the hypothesis that the unitary response results from three pores acting as a unit in response to an applied potential. Evidence obtained with the patch-clamp technique is mounting for a similar mechanism of many channels operating as a unit in a variety of cell membranes. The porin channel holds promise as a concrete model for the analysis of voltage gating of ionic conductance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean R. C. Protein-mediated mechanisms of variable ion conductance in thin lipid membranes. Membranes. 1973;2:409–477. [PubMed] [Google Scholar]
- Bean R. C., Shepherd W. C., Chan H., Eichner J. Discrete conductance fluctuations in lipid bilayer protein membranes. J Gen Physiol. 1969 Jun;53(6):741–757. doi: 10.1085/jgp.53.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the major outer membrane protein of Neisseria gonorrhoeae. Infect Immun. 1982 Apr;36(1):277–283. doi: 10.1128/iai.36.1.277-283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Chen-Schmeisser U., Henning U. Primary structure of major outer-membrane protein I (ompF protein, porin) of Escherichia coli B/r. Biochem J. 1982 Apr 1;203(1):33–43. doi: 10.1042/bj2030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Blumenthal R., Latorre R., Lecar H. Kinetics of the opening and closing of individual excitability-inducing material channels in a lipid bilayer. J Gen Physiol. 1974 Jun;63(6):707–721. doi: 10.1085/jgp.63.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Lecar H., Nossal R. The nature of the negative resistance in bimolecular lipid membranes containing excitability-inducing material. J Gen Physiol. 1970 Jan;55(1):119–133. doi: 10.1085/jgp.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A., Massalski A., Schindler H., Dorset D. L., Rosenbusch J. P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature. 1985 Oct 17;317(6038):643–645. doi: 10.1038/317643a0. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Blake M., Niles W. D., Horwitz M. A. Purification of Legionella pneumophila major outer membrane protein and demonstration that it is a porin. J Bacteriol. 1985 Apr;162(1):85–91. doi: 10.1128/jb.162.1.85-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavito R. M., Jenkins J., Jansonius J. N., Karlsson R., Rosenbusch J. P. X-ray diffraction analysis of matrix porin, an integral membrane protein from Escherichia coli outer membranes. J Mol Biol. 1983 Feb 25;164(2):313–327. doi: 10.1016/0022-2836(83)90079-7. [DOI] [PubMed] [Google Scholar]
- Geletyuk V. I., Kazachenko V. N. Single Cl- channels in molluscan neurones: multiplicity of the conductance states. J Membr Biol. 1985;86(1):9–15. doi: 10.1007/BF01871605. [DOI] [PubMed] [Google Scholar]
- Hunter M., Giebisch G. Multi-barrelled K channels in renal tubules. Nature. 1987 Jun 11;327(6122):522–524. doi: 10.1038/327522a0. [DOI] [PubMed] [Google Scholar]
- Kazachenko V. N., Geletyuk V. I. The potential-dependent K+ channel in molluscan neurones is organized in a cluster of elementary channels. Biochim Biophys Acta. 1984 Jun 13;773(1):132–142. doi: 10.1016/0005-2736(84)90558-3. [DOI] [PubMed] [Google Scholar]
- Krouse M. E., Schneider G. T., Gage P. W. A large anion-selective channel has seven conductance levels. Nature. 1986 Jan 2;319(6048):58–60. doi: 10.1038/319058a0. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Colombini M., Frank J. Structural and functional evidence for multiple channel complexes in the outer membrane of Neurospora crassa mitochondria. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2243–2247. doi: 10.1073/pnas.80.8.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic-Housley Z., Garavito R. M. Effect of temperature and low pH on structure and stability of matrix porin in micellar detergent solutions. Biochim Biophys Acta. 1986 Jan 30;869(2):158–170. doi: 10.1016/0167-4838(86)90290-6. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Induced excitability in reconstituted cell membrane structure. J Theor Biol. 1963 May;4(3):268–280. doi: 10.1016/0022-5193(63)90006-7. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Colombini M., Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976 Dec 28;30(2):99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2302–2306. doi: 10.1073/pnas.78.4.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. Z., Shi B., McGroarty E. J., Tien H. T. Channel-closing activity of porins from Escherichia coli in bilayer lipid membranes. Biochim Biophys Acta. 1986 Nov 6;862(1):57–64. doi: 10.1016/0005-2736(86)90468-2. [DOI] [PubMed] [Google Scholar]
- Young J. D., Blake M., Mauro A., Cohn Z. A. Properties of the major outer membrane protein from Neisseria gonorrhoeae incorporated into model lipid membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3831–3835. doi: 10.1073/pnas.80.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]


