Abstract
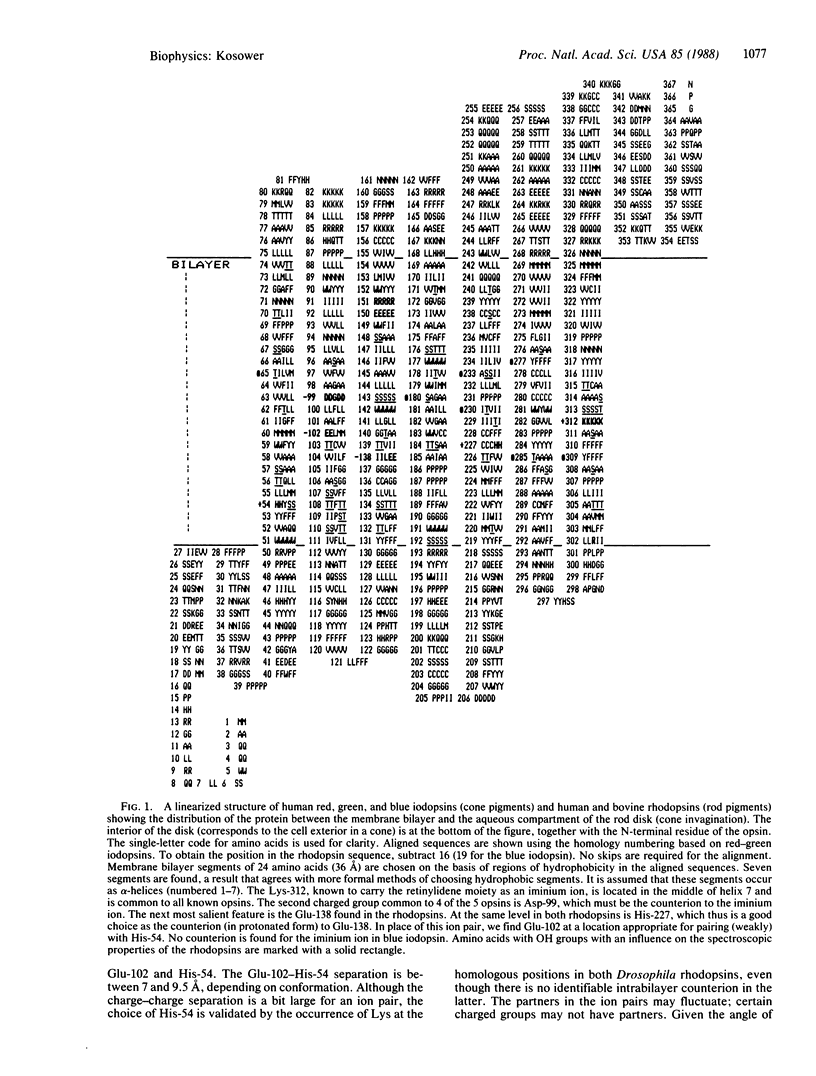
A modified structural model of rhodopsin is presented. Seven (alpha-helical) segments of 24 largely hydrophobic amino acid residues are assembled with exobilayer connecting strands into an aligned set, using the sequences of human red, green, and blue iodopsins (cone pigments) and human and bovine rod rhodopsins. (Aligned set numbering is used in this article). The inner region of the heptahelical hydrophobic domain includes one His-Glu (Asp) ion pair (red, green rod) near the retinylidene moiety in addition to an iminium ion Asp-99 pair. The negative charges posited in the "point-charge model" to cause the shift of the retinylidene iminium ion light absorption to longer wavelengths in the protein ("opsin shift") are Asp-99 (red, green rod), Glu-102 (red, green), and Glu-138 (rod). Blue iodopsin lacks both an ion pair and a counter charge to the iminium ion in the inner region, a fact that explains its absorption relative to rod rhodopsin. The spectroscopic difference between rod rhodopsin and the red/green iodopsins is due to the influence of Glu-102 in the latter. The red-green difference is due to the net effect of seven OH groups around the chromophore, all such groups being found within one helix turn of the retinylidene location. The tryptophan, which rotates as the retinylidene group isomerizes, may be Trp-142 or Trp-177. The geometric change (the rhodopsin "photoswitch") resulting from cis-trans isomerization in the first excited electronic state (S1), ultimately leads to RX (photoactivated rhodopsin, metarhodopsin II) and changes the activity of exobilayer groups, possibly causing dissociation of Lys-83 and Arg-85 from the carboxylate groups at positions 263 and 265.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. J., Tanaka M., Shichi H. Concanavalin A binding to rod outer segment membranes: usefulness for preparation of intact disks. Exp Eye Res. 1978 Nov;27(5):595–605. doi: 10.1016/0014-4835(78)90144-6. [DOI] [PubMed] [Google Scholar]
- Applebury M. L., Hargrave P. A. Molecular biology of the visual pigments. Vision Res. 1986;26(12):1881–1895. doi: 10.1016/0042-6989(86)90115-x. [DOI] [PubMed] [Google Scholar]
- Bennett N., Michel-Villaz M., Kühn H. Light-induced interaction between rhodopsin and the GTP-binding protein. Metarhodopsin II is the major photoproduct involved. Eur J Biochem. 1982 Sep;127(1):97–103. doi: 10.1111/j.1432-1033.1982.tb06842.x. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. GTP-binding proteins. One molecular machine can transduce diverse signals. 1986 Jun 26-Jul 2Nature. 321(6073):814–816. doi: 10.1038/321814a0. [DOI] [PubMed] [Google Scholar]
- Chabre M., Breton J. Orientation of aromatic residues in rhodopsin. Rotation of one tryptophan upon the meta I to meta II transition afer illumination. Photochem Photobiol. 1979 Aug;30(2):295–299. doi: 10.1111/j.1751-1097.1979.tb07150.x. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Molday R. S. Orientation of membrane glycoproteins in sealed rod outer segment disks. Biochemistry. 1979 Dec 25;18(26):5868–5873. doi: 10.1021/bi00593a017. [DOI] [PubMed] [Google Scholar]
- Cobbs W. H., Barkdoll A. E., 3rd, Pugh E. N., Jr Cyclic GMP increases photocurrent and light sensitivity of retinal cones. Nature. 1985 Sep 5;317(6032):64–66. doi: 10.1038/317064a0. [DOI] [PubMed] [Google Scholar]
- Conrad M. P., Strauss H. L. The vibrational spectrum of water in liquid alkanes. Biophys J. 1985 Jul;48(1):117–124. doi: 10.1016/S0006-3495(85)83765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A. F., Zuker C. S., Rubin G. M. An opsin gene expressed in only one photoreceptor cell type of the Drosophila eye. Cell. 1986 Mar 14;44(5):705–710. doi: 10.1016/0092-8674(86)90836-6. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Barclay P. L., Brett M., Davison M., Pappin D. J., Thompson P. The structure of mammalian rod opsins. Vision Res. 1984;24(11):1501–1508. doi: 10.1016/0042-6989(84)90312-2. [DOI] [PubMed] [Google Scholar]
- Findlay J. B., Brett M., Pappin D. J. Primary structure of C-terminal functional sites in ovine rhodopsin. Nature. 1981 Sep 24;293(5830):314–317. doi: 10.1038/293314a0. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Papermaster D. S., Hargrave P. A. Rhodopsin carbohydrate. Structure of small oligosaccharides attached at two sites near the NH2 terminus. J Biol Chem. 1979 Sep 10;254(17):8201–8207. [PubMed] [Google Scholar]
- Gilson M. K., Rashin A., Fine R., Honig B. On the calculation of electrostatic interactions in proteins. J Mol Biol. 1985 Aug 5;184(3):503–516. doi: 10.1016/0022-2836(85)90297-9. [DOI] [PubMed] [Google Scholar]
- Gray T. M., Matthews B. W. Intrahelical hydrogen bonding of serine, threonine and cysteine residues within alpha-helices and its relevance to membrane-bound proteins. J Mol Biol. 1984 May 5;175(1):75–81. doi: 10.1016/0022-2836(84)90446-7. [DOI] [PubMed] [Google Scholar]
- Grunwald G. B., Gierschik P., Nirenberg M., Spiegel A. Detection of alpha-transducin in retinal rods but not cones. Science. 1986 Feb 21;231(4740):856–859. doi: 10.1126/science.3080807. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Courtin J. M., Lugtenburg J., Herzfeld J., Mathies R. A., Griffin R. G. Solid-state 13C NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry. 1985 Nov 19;24(24):6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Feldmann R. J., Atkinson P. H., Rao J. K., Argos P. Rhodopsin's protein and carbohydrate structure: selected aspects. Vision Res. 1984;24(11):1487–1499. doi: 10.1016/0042-6989(84)90311-0. [DOI] [PubMed] [Google Scholar]
- Harris W. A., Stark W. S., Walker J. A. Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol. 1976 Apr;256(2):415–439. doi: 10.1113/jphysiol.1976.sp011331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L., Yau K. W. Cyclic GMP-sensitive conductance in outer segment membrane of catfish cones. Nature. 1985 Sep 5;317(6032):61–64. doi: 10.1038/317061a0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Ho Y. K., Fung B. K. Characterization of transducin from bovine retinal rod outer segments. The role of sulfhydryl groups. J Biol Chem. 1984 May 25;259(10):6694–6699. [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Kakitani H., Kakitani T., Rodman H., Honig B. On the mechanism of wavelength regulation in visual pigments. Photochem Photobiol. 1985 Apr;41(4):471–479. doi: 10.1111/j.1751-1097.1985.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Dixon R. A., Frielle T., Dohlman H. G., Bolanowski M. A., Sigal I. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower E. M. A structural and dynamic model for the nicotinic acetylcholine receptor. Eur J Biochem. 1987 Oct 15;168(2):431–449. doi: 10.1111/j.1432-1033.1987.tb13437.x. [DOI] [PubMed] [Google Scholar]
- Kosower E. M. Partial tertiary structure assignment for the acetylcholine receptor on the basis of the hydrophobicity of amino acid sequences and channel location using single group rotation theory. Biochem Biophys Res Commun. 1983 Mar 29;111(3):1022–1026. doi: 10.1016/0006-291x(83)91402-x. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Lancet D. Vertebrate olfactory reception. Annu Rev Neurosci. 1986;9:329–355. doi: 10.1146/annurev.ne.09.030186.001553. [DOI] [PubMed] [Google Scholar]
- Liang C. J., Yamashita K., Muellenberg C. G., Shichi H., Kobata A. Structure of the carbohydrate moieties of bovine rhodopsin. J Biol Chem. 1979 Jul 25;254(14):6414–6418. [PubMed] [Google Scholar]
- Liu R. S., Asato A. E. The primary process of vision and the structure of bathorhodopsin: a mechanism for photoisomerization of polyenes. Proc Natl Acad Sci U S A. 1985 Jan;82(2):259–263. doi: 10.1073/pnas.82.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstaff C., Calhoon R. D., Rando R. R. Chemical modification of rhodopsin and its effect on regeneration and G protein activation. Biochemistry. 1986 Oct 7;25(20):6311–6319. doi: 10.1021/bi00368a072. [DOI] [PubMed] [Google Scholar]
- Martin R. L., Wood C., Baehr W., Applebury M. L. Visual pigment homologies revealed by DNA hybridization. Science. 1986 Jun 6;232(4755):1266–1269. doi: 10.1126/science.3010467. [DOI] [PubMed] [Google Scholar]
- Matthew J. B. Electrostatic effects in proteins. Annu Rev Biophys Biophys Chem. 1985;14:387–417. doi: 10.1146/annurev.bb.14.060185.002131. [DOI] [PubMed] [Google Scholar]
- Michel-Villaz M., Roche C., Chabre M. Orientational changes of the absorbing dipole or retinal upon the conversion of rhodopsin to bathorhodopsin, lumirhodopsin, and isorhodopsin. Biophys J. 1982 Mar;37(3):603–616. [PMC free article] [PubMed] [Google Scholar]
- Mollevanger L. C., Kentgens A. P., Pardoen J. A., Courtin J. M., Veeman W. S., Lugtenburg J., de Grip W. J. High-resolution solid-state 13C-NMR study of carbons C-5 and C-12 of the chromophore of bovine rhodopsin. Evidence for a 6-S-cis conformation with negative-charge perturbation near C-12. Eur J Biochem. 1987 Feb 16;163(1):9–14. doi: 10.1111/j.1432-1033.1987.tb10729.x. [DOI] [PubMed] [Google Scholar]
- Mollon J. D. Perception. Questions of sex and colour. Nature. 1986 Oct 16;323(6089):578–579. doi: 10.1038/323578a0. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Neitz J., Jacobs G. H. Polymorphism of the long-wavelength cone in normal human colour vision. Nature. 1986 Oct 16;323(6089):623–625. doi: 10.1038/323623a0. [DOI] [PubMed] [Google Scholar]
- O'Tousa J. E., Baehr W., Martin R. L., Hirsh J., Pak W. L., Applebury M. L. The Drosophila ninaE gene encodes an opsin. Cell. 1985 Apr;40(4):839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Seiff F., Wallat I., Westerhausen J., Heyn M. P. Location of chemically modified lysine 41 in the structure of bacteriorhodopsin by neutron diffraction. Biophys J. 1986 Oct;50(4):629–635. doi: 10.1016/S0006-3495(86)83502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Stryer L. Molecular design of an amplification cascade in vision. Biopolymers. 1985 Jan;24(1):29–47. doi: 10.1002/bip.360240105. [DOI] [PubMed] [Google Scholar]
- Trewhella J., Popot J. L., Zaccaï G., Engelman D. M. Localization of two chymotryptic fragments in the structure of renatured bacteriorhodopsin by neutron diffraction. EMBO J. 1986 Nov;5(11):3045–3049. doi: 10.1002/j.1460-2075.1986.tb04604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Wang J. K., McDowell J. H., Hargrave P. A. Site of attachment of 11-cis-retinal in bovine rhodopsin. Biochemistry. 1980 Oct 28;19(22):5111–5117. doi: 10.1021/bi00563a027. [DOI] [PubMed] [Google Scholar]
- Warshel A. Bicycle-pedal model for the first step in the vision process. Nature. 1976 Apr 22;260(5553):679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]
- Warshel A., Russell S. T. Calculations of electrostatic interactions in biological systems and in solutions. Q Rev Biophys. 1984 Aug;17(3):283–422. doi: 10.1017/s0033583500005333. [DOI] [PubMed] [Google Scholar]
- Warshel A., Russell S. T., Churg A. K. Macroscopic models for studies of electrostatic interactions in proteins: limitations and applicability. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4785–4789. doi: 10.1073/pnas.81.15.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T. Photophysiological functions of visual pigments. Adv Biophys. 1984;17:5–67. doi: 10.1016/0065-227x(84)90024-8. [DOI] [PubMed] [Google Scholar]
- Zuker C. S., Cowman A. F., Rubin G. M. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985 Apr;40(4):851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]


