Abstract
Platelets are activated by adhesion to vascular collagen via the immunoglobulin receptor, glycoprotein VI (GPVI). This causes potent signaling toward activation of phospholipase Cγ2, which bears similarity to the signaling pathway evoked by T- and B-cell receptors. Phosphoinositide 3-kinase (PI3K) plays an important role in collagen-induced platelet activation, because this activity modulates the autocrine effects of secreted ADP. Here, we identified the PI3K isoforms directly downstream of GPVI in human and mouse platelets and determined their role in GPVI-dependent thrombus formation. The targeting of platelet PI3Kα or -β strongly and selectively suppressed GPVI-induced Ca2+ mobilization and inositol 1,4,5-triphosphate production, thus demonstrating enhancement of phospholipase Cγ2 by PI3Kα/β. That PI3Kα and -β have a non-redundant function in GPVI-induced platelet activation and thrombus formation was concluded from measurements of: (i) serine phosphorylation of Akt, (ii) dense granule secretion, (iii) intracellular Ca2+ increases and surface expression of phosphatidylserine under flow, and (iv) thrombus formation, under conditions where PI3Kα/β was blocked or p85α was deficient. In contrast, GPVI-induced platelet activation was insensitive to inhibition or deficiency of PI3Kδ or -γ. Furthermore, PI3Kα/β, but not PI3Kγ, contributed to GPVI-induced Rap1b activation and, surprisingly, also to Rap1b-independent platelet activation via GPVI. Together, these findings demonstrate that both PI3Kα and -β isoforms are required for full GPVI-dependent platelet Ca2+ signaling and thrombus formation, partly independently of Rap1b. This provides a new mechanistic explanation for the anti-thrombotic effect of PI3K inhibition and makes PI3Kα an interesting new target for anti-platelet therapy.
Introduction
Exposed collagen in a damaged vessel wall activates platelets via their immunoglobulin family receptor, glycoprotein VI (GPVI),3 by using a complex signal transduction pathway, which is reminiscent to the pathway employed by immune receptors in T and B cells (1, 2). In platelets, tyrosine phosphorylation of the Fc receptor γ-chain, linked to GPVI via Src family kinases, leads to a cascade of protein phosphorylation events, cumulating in the activation of phospholipase Cγ2 (PLCγ2). This key effector enzyme triggers many downstream events, including production of inositol 1,4,5-trisphosphate (InsP3), mobilization of cytosolic Ca2+, activation of integrin αIIbβ3, secretion of platelet granules loaded with autocrine-stimulating agents (ADP and ATP), and exposure of negatively charged phosphatidylserine (PS) at the platelet surface to ensure coagulation (1, 3, 4). All these responses are potently triggered by GPVI ligands, which, besides collagen, include collagen-related peptides and the snake venom convulxin (5–7).
One of the GPVI-induced signaling events contributing to PLCγ2 activation is activation of the protein/lipid kinase, phosphoinositide 3-kinase (PI3K) in both human and mouse platelets (8–11). Evidence for this role came from the finding that, in platelets stimulated with GPVI agonists, the p85α regulatory subunit of PI3K coprecipitates with the Fc receptor γ-chain and the LAT adaptor protein (8). The p85α subunit pulls p110 catalytic subunits to the membrane, where they catalyze the formation of 3-phosphorylated inositol phospholipids, primarily the phosphoinositide 3,4,5-trisphosphate (PI(3,4,5)P3) (10).
Currently, there is evidence that individual class I PI3K isoforms, which are distinguished according to their catalytic subunits, have specific cellular and physiological functions. For instance, the p110α isoform (PI3Kα) has been implicated in oncogenesis, and isoform-selective PI3Kα inhibitors can reduce tumor formation (12). The p110γ isoform (PI3Kγ) is involved in innate immunity and various inflammatory diseases (13), whereas p110δ has a more important role in adaptive immunity, e.g. in T and B cells (14). Human and mouse platelets contain four different PI3K isoforms, among which are the class IA catalytic subunits, p110α, -β, and -δ (PI3Kα, -β, and -δ), and the class IB catalytic subunit, p110γ (PI3Kγ) (15–17). For class IA, the corresponding regulatory subunits are p85α/β, p55α/γ, and p50α, whereas for class IB the regulatory subunit is p101γ. Structural studies in other cells have indicated that the regulatory class IA subunits, particularly p85α, can interact with tyrosine kinase-linked receptors via the SH2 domains (18). In contrast, class IB isoforms may rather interact with G-protein-coupled receptors (16). This concept was recently challenged by the observation that, in platelets, both PI3Kβ and -γ are activated via the P2Y12 receptor for ADP, which is coupled to Gi, and that both isoforms contribute to integrin αIIbβ3 activation and platelet aggregation (17, 19–21). Hence, it is clear that PI3K isoforms can be activated by other platelet receptors than only GPVI.
To date, it is debated which of the PI3K isoforms become directly activated by GPVI signaling, and which are activated indirectly, e.g. following ADP receptor stimulation. Also unclear is which are the downstream events mediated by the various isoforms. Reasons for this lack of clarity include: (i) the large contribution to GPVI-induced responses of secondary, autocrine stimulators, particularly ADP and thromboxane (1, 17, 22); (ii) the proposed stimulation by PI3K and its product PI(3,4,5)P3 to Ca2+ entry rather than to PLC activity (23, 24); (iii) the limited knowledge on the effector targets of PI3K and PI(3,4,5)P3 in platelets, of which only phosphoinositide-dependent kinase and Akt are well studied (21, 25); (iv) the observation that p110δ is not a major isoform implicated in GPVI-induced platelet activation (26); and (v) the limited availability of mice deficient in PI3K subunits.
One possible, poorly explored target of PI3K in platelets is the small GTPase Rap1b, which is highly expressed in these cells and is considered to play a key role in the activation process particularly toward αIIbβ3 activation. Platelet agonists such as ADP and thrombin produce the active GTP-bound form of Rap1b, partly in a PI3K-dependent manner (27, 28). Recent findings suggest that PI3Kβ is the main isoform responsible for the ADP-induced activation of Rap1b and Akt, whereas PI3Kγ contributes to αIIbβ3 activation by a separate pathway (21). However, whether and how Rap1b is activated by GPVI is unresolved, as is the role of different PI3K isoforms herein.
In the present report, we studied the identity of the PI3K isoforms downstream of GPVI and determined their role in platelet activation and thrombus formation. To discriminate between direct and indirect GPVI-induced effects, the cells were stimulated in the presence of autocrine stimulation inhibitors, blocking the signaling contributions of both ADP and thromboxane. For the studies, we combined a pharmacological approach using a panel of isoform-specific PI3K inhibitors with experiments using mice deficient in distinct PI3K catalytic or regulatory subunits. We found that PI3Kα and -β are key mediators of GPVI-induced thrombus formation and show that these isoforms affect the activity of both PLCγ2 and Rap1b.
EXPERIMENTAL PROCEDURES
Mouse Strains
Mice deficient in the p85α regulatory PI3K subunit had a C57BL/6 genetic background (11, 29). Mice deficient in p110γ or p110δ (17) or deficient in Rap1b (30) were from sources previously described. All knock-out mice had normal platelet counts. Wild-type mice were used of the same background and same breeding program. Animal experiments were approved by the local animal experimental committees.
Materials
Fura-2 and Fluo-3 acetoxymethyl esters, Pluronic F-127 and Oregon Green 488-labeled fibrinogen (OG-fibrinogen) were from Molecular Probes; OG-annexin A5 was from Nexins Research; fluorescein isothiocyanate-labeled anti-human CD62 monoclonal antibody was from Sanquin. Antibodies against Akt were from Cell Signaling Technology; anti-Rap1 antibody was from BD Biosciences. LY294002 and H-Phe-Pro-Arg chloromethyl ketone (PPACK) were from Calbiochem. Wortmannin, apyrase (grade V), α-thrombin, and MRS-2179, a P2Y1 antagonist, were from Sigma. Inhibitors of PI3K isoforms (supplemental Table I) and control substance PIK-112 were kind gifts from the Baker Heart Research Institute and synthesized as described (17, 31). Cangrelor (AR-C69931MX), a P2Y12 antagonist, was kindly provided by The Medicines Co. Other reagents were from sources indicated before (32, 33).
Blood Collection and Platelet Isolation
For platelet isolation, blood was collected into one-sixth volume of acid-citrate-dextrose anticoagulant (85 mm sodium citrate, 78 mm citric acid, and 11 mm d-glucose). Donors gave full informed consent according to the Helsinki declaration and had not taken medications for 2 weeks. Platelet-rich plasma (PRP) and washed platelets were obtained by centrifugation, as described for human (7) and mouse (32, 33) platelets. Washed human platelets were resuspended in Hepes buffer, pH 7.45 (137 mm NaCl, 10 mm Hepes, 2.7 mm KCl, 2 mm MgSO4, 0.42 mm d-glucose, 0.2 unit/ml apyrase, and 0.1% bovine serum albumin, pH 7.45). Mouse platelets were resuspended in a modified Hepes buffer (33). Platelets were counted with a Coulter counter. For whole blood flow experiments, human blood was collected into 40 μm PPACK (34), whereas mouse blood was collected into 40 μm PPACK plus 5 units/ml heparin (32).
Intracellular Ca2+ Measurement in Platelet Suspensions
Human PRP (2 × 108 platelets/ml) was loaded with 2.5 μm Fura-2 acetoxymethyl ester in the presence of aspirin (100 μm) and apyrase (0.2 unit of ADPase/ml) at 37 °C for 45 min (35). The loaded platelets were resuspended in Hepes buffer, pH 7.45 (1 × 108/ml), and were used within 90 min. Before addition of agonist, the cells were preincubated with apyrase (0.1 unit/ml) and ADP receptor blockers (40 μm MRS-2179 for P2Y1 and 10 μm ARC-69931MX for P2Y12) to inhibit autocrine stimulation. In some experiments, apyrase was added at a high concentration (0.6 unit/ml). Platelet preincubation with indicated PI3K inhibitors or Me2SO vehicle was for 10 min (37 °C). Calcium responses were recorded under stirring with an SLM-Aminco or a Cairn Research spectrofluorometer, at alternate excitation wavelengths of 340 and 380 nm (37 °C). The 340/380 nm ratio values were converted into nanomolar concentrations of [Ca2+]i, as described (35). Separate calibrations were done for incubations containing colored PI3K inhibitors. Rises in [Ca2+]i were expressed as 5-min time integrals, to quantify prolonged Ca2+-signaling effects (36).
Platelet Aggregation and Flow Cytometry
Human PRP was pretreated with aspirin, and platelets were washed. The washed platelets were preincubated for 10 min with autocrine stimulation inhibitors (see above) and PI3K blockers or Me2SO vehicle. After 10 min of activation, samples were analyzed by flow cytometry for P-selectin expression using fluorescein isothiocyanate-anti-CD62 monoclonal antibody (1:100) or for αIIbβ3 activation using fluorescein isothiocyanate-labeled PAC1 monoclonal antibody (37).
Washed mouse platelets were prepared from PRP (21) and preincubated with a mixture of autocrine stimulation inhibitors (100 μm MRS-2179, 10 μm AR-C69931MX, and 10 μm indomethacin). Platelet aggregation was measured, as described (21). OG488-fibrinogen binding to the platelets was assessed by flow cytometry (33).
InsP Measurement
Accumulation of InsP1 due to InsP3 production was measured with an IP-One ELISA kit from CisBio, determining InsP1 levels. Aspirin-treated platelets (1 × 108/ml) were preincubated with ADP receptor blockers, LiCl (1 mm), and Me2SO vehicle or PI3K blocker. After stimulation for 5–10 min with convulxin or thrombin, the platelets were lysed. Cell lysates were incubated with InsP1-horseradish peroxidase conjugate and anti-InsP1 monoclonal antibody, according to the manufacturer's instructions.
Activation of Akt and Rap1 by Western Blotting
Akt activation was measured by Western blot analysis of platelet lysates. A polyclonal anti-phosphoserine-473 Akt antibody was used to detect active Akt and a polyclonal anti-Akt antibody to determine total Akt (19). Activation of Rap1 was measured in platelet lysates by selective precipitation of GTP-bound Rap1 using glutathione S-transferase-RalGDS bound to glutathione-Sepharose (28). Western blotting was performed with anti-Rap1 antibody (250 ng/ml) and a sheep anti-mouse horseradish peroxidase-coupled secondary antibody (1:5000).
Thrombus Formation and Procoagulant Activity under Flow
Perfusion of PPACK-anti-coagulated human blood (34) and of PPACK/heparin-anti-coagulated mouse blood (32) was done in the absence of coagulation as described. Blood samples were preincubated with PI3K inhibitor or Me2SO vehicle for 10 min. Blood was flowed through a transparent, parallel-plate flow chamber, containing a collagen-coated coverslip, at a defined shear rate for 4 min. The thrombi in flow chambers were stained by rinse with Hepes buffer, pH 7.45, supplemented with 1 unit/ml heparin, 2 mm CaCl2, and OG488-annexin A5 (1 μg/ml). Microscopic images were recorded in real-time using a Visitech imaging system, equipped with two intensified charge-coupled device cameras (34). Bright-field contrast and fluorescence digital images were taken from >10 randomly chosen fields. ImagePro software (Media Cybernetics) was used to quantify platelet deposition (38).
Intracellular Ca2+ Measurement under Flow
Human PRP (2 × 108/ml) was incubated with 7 μm Fluo-3 acetoxymethyl ester for 45 min at 20 °C under gentle rotation. Washed mouse platelets (2 × 108/ml) were incubated with 5 μm Fluo-3 acetoxymethyl ester plus 0.2 mg/ml Pluronic F-127. The dye-loaded cells were added to autologous blood (10% labeled platelets) and used for flow experiments within 1 h. Fluorescence images were recorded during high speed (5 Hz) perfusion of blood over a collagen surface (39). Off-line, regions of interest representing one adhered cell were analyzed for fluorescence changes. Raw fluorescence data were converted into F/Fo values by pseudo-ratio analysis, and then into nanomolar concentrations of [Ca2+]i (40). For quantification, traces from individual cells were superimposed, so that frame numbers of initial [Ca2+]i increases coincided.
Statistics
Differences between groups were tested with a Mann-Whitney U test or analysis of variance. Effects of inhibitors were tested with a Student's paired t test. The statistical package for social sciences was used (SPSS 15.0).
RESULTS
Prominent Roles of PI3Kα and -β Isoforms in GPVI-induced Ca2+ Responses
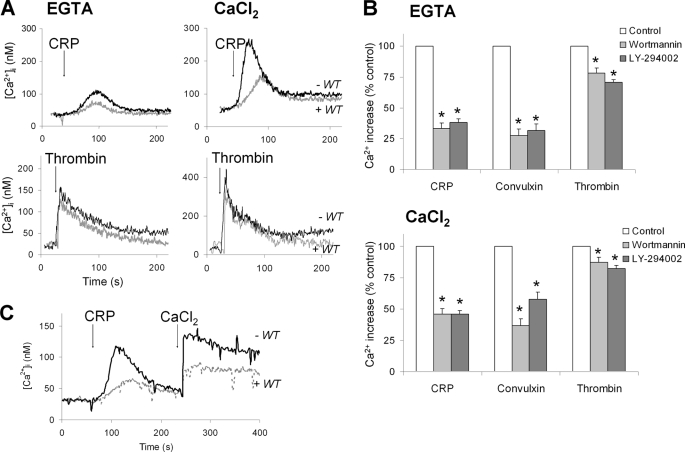
Although PI3K activity has been implicated in GPVI-mediated platelet activation, it is unclear whether its role is direct or indirect, e.g. via autocrine ADP release and P2Y12 signaling. Another proposal is that PI3K may specifically stimulate GPVI-induced Ca2+ entry rather than mobilization of Ca2+ from internal stores (23). To investigate this further, human Fura-2-loaded platelets were activated with GPVI agonist under conditions where indirect effects of autocrine stimulators (thromboxane A2 and ADP) were prevented with aspirin and a high concentration of ADP-degrading apyrase (0.6 unit/ml). When platelets were stimulated with CRP in the presence of extracellular EGTA (where Ca2+ is only mobilized from internal stores), the general PI3K inhibitor wortmannin (0.1 μm) caused ∼60% suppression of the Ca2+ signal (Fig. 1A). In the presence of extracellular CaCl2 (where Ca2+ entry takes place in addition), wortmannin had a similar inhibitory effect on the Ca2+ signal. The treatment of platelets with another general PI3K inhibitor, LY-294002 (25 μm), was as effective as wortmannin. Furthermore, similar effects were obtained with both inhibitors, when platelets were stimulated with the GPVI ligand, convulxin (Fig. 1B). To directly determine the inhibitor effect on Ca2+ entry, platelets were first stimulated with GPVI ligand causing Ca2+ mobilization, after which CaCl2 was added and Ca2+ entry was allowed. Strikingly, wortmannin suppressed both parts of the Ca2+ signal at similar extent (Fig. 1C). A control experiment indicated that blocking anti-GPIb antibodies did not affect the convulxin-induced Ca2+ response (not shown), thus excluding involvement of GPIb-induced Ca2+ signal generation with this agonist.
FIGURE 1.
Major contribution of PI3K to GPVI-induced versus thrombin receptor-induced Ca2+ responses in absence of autocrine mediators. Human platelets, treated with aspirin and loaded with Fura-2, were preincubated for 10 min with Me2SO vehicle (control), wortmannin (0.1 μm), or LY-294002 (10 μm) in the presence of apyrase (0.6 unit/ml). The platelets were stimulated with GPVI ligand, CRP (5 μg/ml), or convulxin (70 ng/ml), or with thrombin (10 nm), in the presence of 1 mm EGTA or 1 mm CaCl2. A, effect of wortmannin (WT) on CRP-induced [Ca2+]i increases. B, quantitative effect of wortmannin and LY-294002 on integrated Ca2+ responses in the presence of EGTA or CaCl2. Per agonist, time-[Ca2+]i integrals of the relevant control condition were set at 100%. C, effect of wortmannin on convulxin-induced Ca2+ mobilization and secondary Ca2+ entry (1 mm CaCl2). Representative traces are shown. Means ± S.E. (n = 4–5); *, p < 0.05 compared with control.
Interestingly, treatment of the platelets with wortmannin or LY-294002 caused only small inhibition of the thrombin-evoked Ca2+ responses in both EGTA and CaCl2 medium (Fig. 1B). Together, these data suggest that PI3K activity primarily enhances PLCγ2-evoked Ca2+ mobilization from stores, whereas it only secondarily affects Ca2+ entry.
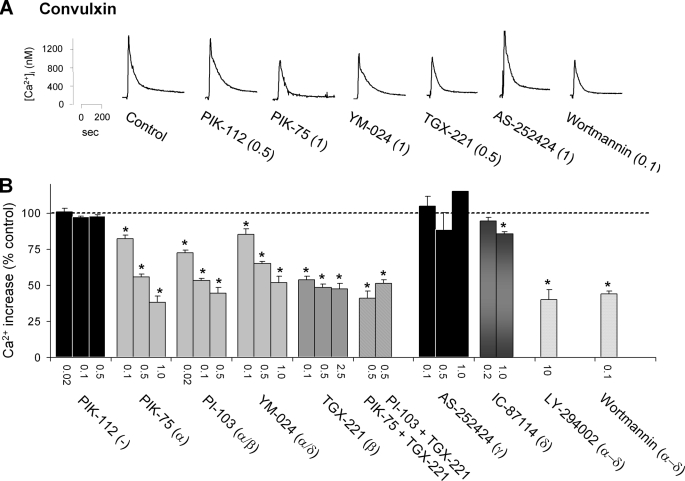
Various new isoform-specific catalytic site inhibitors of class I PI3Ks have been developed and used for functional studies in other cell types (31, 41). The selectivity of these inhibitors for PI3Kα through δ isoforms is mostly determined in cell-free in vitro studies using isolated enzymes (IC50 values are given in supplemental Table I). The compound PIK-75 has a far higher affinity for PI3Kα (IC50 8 nm) than for other isoforms. At nanomolar concentrations, the compound PI-103 inhibits both PI3Kα and -β (PIK-112 is an inactive analog). YM-024 has a relatively high affinity for PI3Kα and -δ, whereas TGX-211 is most active against PI3Kβ (IC50 7 nm). The compounds AS-252424 and IC-87114 show highest activity toward PI3Kγ and PI3Kδ isoforms, respectively. It should be remarked that effective doses of these compounds for complete inhibition of the kinase activity in adipocytes, hepatoma cell lines, and platelets are reported to be in the low micromolar range (17, 21, 41). Hence, for adequate inhibition of PI3K activities in intact platelets, higher doses are needed than apparent from the IC50 values. Reasons for this are the high platelet counts in experiments and the high ATP concentration in cells (at least 10-fold higher than of in vitro kinase assays).
For the above-mentioned inhibitors, dose-effect relations were determined for convulxin-induced Ca2+ responses in CaCl2 medium. To prevent autocrine stimulatory effects of thromboxane and ADP, all experiments were carried out with aspirin-treated platelets, and blockers of both ADP receptors were added. Time integrals of increases in [Ca2+]i served as a read-out of the extent of GPVI-induced PLC activation (36). Strikingly, those inhibitors with a high affinity toward PI3Kα (PIK-75, PI-103, and YM-024, 0.5–1 μm) reduced the Ca2+ signal with ∼50%, whereas the control substance PIK-112 was completely inactive (Fig. 2, A and B). In addition, the PI3Kβ selective inhibitor TGX-221 (0.1–2.5 μm) caused a similar reduction in Ca2+ signal. Combined application of PIK-75/TGX-221 or PI-103/TGX-221 did not further suppress the response. In general, the isoform-specific PI3Kα or -β inhibitors were similarly effective as the general PI3K inhibitors, wortmannin and LY-294002. On the other hand, AS-252424 (0.1–1 μm), inhibiting PI3Kγ, was without any effect, whereas the PI3Kδ inhibitor IC-87114 only slightly reduced the Ca2+ response.
FIGURE 2.
Inhibition of platelet PI3Kα and -β isoforms strongly suppresses GPVI-induced Ca2+ responses. Aspirin-treated, Fura-2-loaded platelets were preincubated with ADP receptor blockers (10 μm AR-C69931MX, 40 μm MRS-2179, and 0.1 unit/ml apyrase) and 1 mm CaCl2. Platelets were then treated with one of the following substances (PI3K isoform specificity in parentheses): Me2SO vehicle (control), PIK-112 (inactive analog of PIK-75), PIK-75 (α), PI-103 (α/β), YM-024 (α/δ), TGX-221 (β), AS-252424 (γ), IC-87114 (δ), LY-294002 (α through δ), or wortmannin (α through δ). Numbers give final concentrations in micromolar. A, representative [Ca2+]i traces upon stimulation with convulxin (70 ng/ml). B, effects of inhibitors on Ca2+ responses, expressed as percentages of time-[Ca2+]i integrals relative to the control condition. Note that at a low dose of convulxin (7 ng/ml), PIK-75, TGX-221, or the combination of both (0.5 μm) suppressed the Ca2+ rise to 51 ± 3%, 40 ± 6%, or 31 ± 1% of control, respectively. Data are means ± S.E. (n = 3–5); *, p < 0.05 compared with control.
For comparison, the same panel of inhibitors was tested in measurements of thrombin-induced Ca2+ responses. Here, only compounds with a high affinity toward PI3Kβ (i.e. PI-103 and TGX-221) and the general PI3K inhibitors (wortmannin and LY-294002) reduced the Ca2+ signal by 15–20% (supplemental Fig. 1). In this case, the PI3Kα inhibitor PIK-75 was without effect. Together, these results point to high and non-redundant roles of the PI3Kα and -β isoforms in Ca2+ signaling directly downstream of GPVI, but not of thrombin receptors.
Control experiments were performed to exclude that the high affinity PI3Kα inhibitor, PIK-75, acted by residual inhibition of PI3Kβ. We examined the effect of PIK-75 (0.5–1 μm) on P2Y12-induced platelet responses and integrin αIIbβ3 activation, because these are exclusively mediated by the PI3Kβ and -γ isoforms (20, 21). As shown in supplemental Fig. 2, PIK-75 was completely inactive in both platelet aggregation and integrin activation in response to ADP, in marked contrast to the established PI3Kβ inhibitor, TGX-221. On the other hand, TGX-221 did not affect Akt phosphorylation at Ser473 evoked by insulin-like growth factor-1, which is known to be mediated by only the PI3Kα isoform (S. Kim, data not shown, but see Ref. 42).
Contribution of Both PI3Kα and -β Isoforms to GPVI-induced Signaling
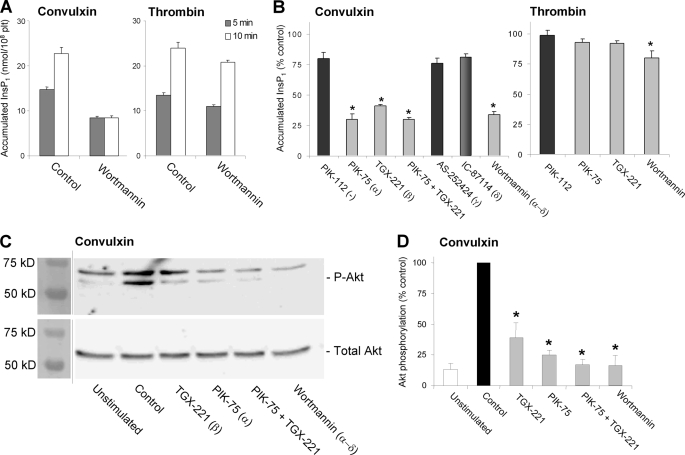
In platelets, the GPVI receptor activates the PH-domain containing PLCγ2, in contrast to thrombin receptors, which activate PLCβ isoforms. For other cells, it was hypothesized that an increase in PI(3,4,5)P3 level caused by PI3K pulls PLCγ2 to the membrane and increases its action (18). To verify this for GPVI-stimulated platelets (again with blocked autocrine responses), we determined overall PLC activity by measuring the accumulation of InsP1 due to InsP3 production. Platelet stimulation with convulxin led to a production of InsP3 for up to 10 min (Fig. 3A). Wortmannin treatment reduced the InsP3 formation and moreover confined it to 5 min. Such a shortening of InsP3 production was not seen in thrombin-stimulated platelets. The isoform-specific inhibitors, PIK-75 or TGX-221 (targeting at PI3Kα and -β, respectively) suppressed the InsP3 production to a similar extent as wortmannin, whereas the combination of the two did not have an additional effect (Fig. 3B). In contrast, inhibition of PI3Kγ (AS-252424) or PI3Kδ (IC-87114) was not effective on InsP3 amounts. Hence, these results point to a GPVI-induced prolongation of PLC activity by PI3Kα/β.
FIGURE 3.
Contribution of PI3Kα and -β isoforms to GPVI-induced InsP3 production and Akt phosphorylation. Aspirin-treated human platelets (1 × 108/ml) were preincubated with ADP receptor blockers (Fig. 2) and Me2SO vehicle (control) or 1 μm PI3K inhibitor (25 μm LY-294002). A and B, effect of PI3K blockers on accumulation of InsP1 due to InsP3 production. Platelets were stimulated for 10 min with convulxin (70 ng/ml) or thrombin (10 nm) in the presence of 1 mm LiCl. C and D, effect of PI3K blockers on convulxin-induced Ser473 Akt phosphorylation. C, representative Western blots of phospho-Akt and total Akt (60 kDa). D, data are densitometric intensities of phospho-Akt/Akt, expressed as percentage of vehicle. Means ± S.E. (n = 3–5); *, p < 0.05 compared with PIK-112 or control.
We measured agonist-induced Ser473 phosphorylation of Akt as an established downstream effector of lipid PI3K (21). In platelets stimulated with convulxin (Fig. 3C) or CRP (not shown), wortmannin treatment suppressed the phosphorylation of Akt, as expected. Interestingly, also inhibition of PI3Kα (PIK-75) or -β (TGX-221) reduced this phosphorylation, whereas the combination was not more effective (Fig. 3D).
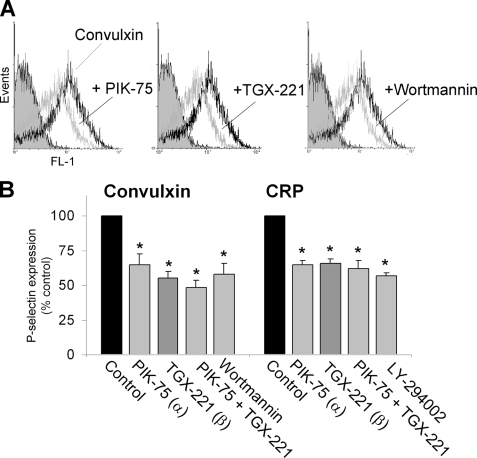
A key downstream effect of [Ca2+]i elevation in platelets is the secretion of α-granules, which is measured as expression of granular P-selectin at the platelet surface. Platelet treatment with PIK-75 or TGX-221, alone or in combination, suppressed convulxin- or CRP-induced P-selectin expression with 40–50%, i.e. to the same extent as did wortmannin or LY-294002 (Fig. 4). This points to a non-additive effect of both isoform-selective compounds on GPVI-induced secretion.
FIGURE 4.
Contribution of PI3Kα and -β isoforms to GPVI-induced α-granule secretion. Human washed aspirin-treated platelets (1 × 108/ml) were preincubated with ADP receptor blockers (Fig. 2) and treated with Me2SO vehicle (control) or specific PI3K inhibitor (in micromolar): PIK-75 (1.0), TGX-221 (0.5), wortmannin (0.1), and LY-294002 (25). The platelets were activated for 10 min with convulxin (70 ng/ml) or CRP (10 μg/ml). A, histogram showing effect of PI3Kα/β blockers on P-selectin expression (FL1 fluorescence). B, quantitative effect of PI3K blockage on P-selectin expression. Data are relative to the control condition. Means ± S.E. (n = 3); *, p < 0.05 compared with control.
Roles of PI3Kα and -β Isoforms in GPVI-induced Platelet Activation and Thrombus Formation on Collagen under Flow
The pharmacological evidence so far points to a common role of the PI3Kα/β isoforms in GPVI-induced Ca2+ signaling and downstream platelet activation events. We then performed studies to determine the importance of these isoforms in flow-dependent thrombus formation at physiological shear rates. Earlier work has shown that, during platelet interaction with collagen under flow, GPVI-induced increases in [Ca2+]i are important for granule secretion, activation of integrins, and exposure of PS (22, 34, 43, 44). In whole blood, supplemented with Fluo-3-loaded platelets, [Ca2+]i increases were measured of individual platelets upon adhesion to collagen under flow. Interestingly, pretreatment with either PIK-75, TGX-221, or wortmannin led to a marked suppression in single cell Ca2+ responses, so that the adhered platelets showed low amplitude spiking changes in [Ca2+]i (Fig. 5). Control experiments indicated that the Ca2+ responses under flow were not influenced by aspirin and blockage of ADP receptors (not shown), thus confirming that autocrine ADP release did not contribute to this initial platelet response. Other controls indicated that these [Ca2+]i increases were completely suppressed by blocking GPVI (22).
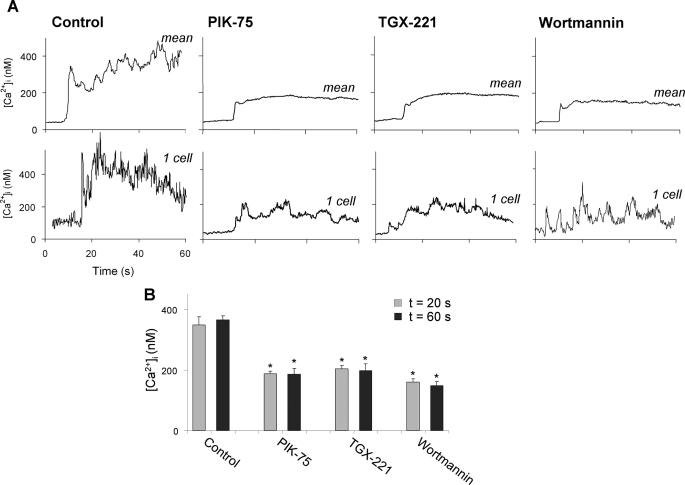
FIGURE 5.
Role of PI3K in collagen-induced Ca2+ responses under flow in the absence of secondary mediators. PPACK-anti-coagulated blood, containing 10% autologous Fluo-3-loaded platelets, was preincubated with Me2SO vehicle (control), PIK-75, TGX-221, or wortmannin (each 1 μm). A, single cell Ca2+ responses during flow of blood over collagen in the presence of indicated inhibitor at a shear rate of 1000 s−1. Upper panels: mean overlays of [Ca2+]i traces from >27 individual platelets; lower panels: traces from representative single platelets. B, quantification of increases in [Ca2+]i at 20 s (gray) or 60 s (dark gray) after initial platelet activation. Means ± S.E. (n = 27–50); *, p < 0.05 compared with control.
The effects of inhibitors on thrombus formation were assessed from measurements of the deposition of platelets on the collagen surface (Fig. 6A). In addition, the exposure of PS of collagen-adhered platelets (detected with OG-annexin A5) was measured, the response for which is shown by a subpopulation of the platelets in direct contact with collagen due to GPVI activation (38). Both parameters were substantially diminished with wortmannin, with the PI3Kα inhibitors PIK-75 and YM-024, or with the PI3Kβ inhibitor TGX-221. In the presence of all inhibitors, platelet deposition on collagen was substantially reduced in comparison to the vehicle control (Fig. 6B). In addition, the number of PS-exposing platelets was reduced to ∼50% by PIK-75, YM-024, or TGX-221. On the other hand, inhibition of PI3Kγ (AS-252424) or -δ (IC-87114) was without effect. Together, these results suggest that inhibition of only PI3Kα or -β isoforms suppresses flow-dependent thrombus formation on collagen, by reducing GPVI-dependent Ca2+ responses and downstream activation processes leading to platelet aggregation and PS exposure.
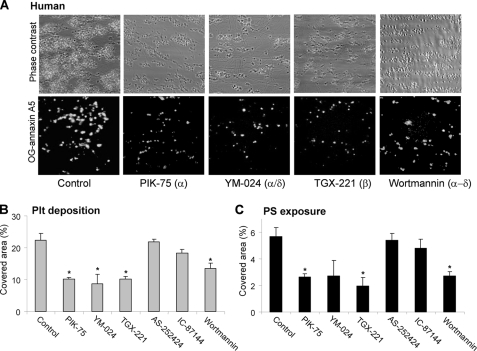
FIGURE 6.
Role of PI3Kα and -β isoforms in collagen-induced thrombus formation and PS exposure under flow. PPACK-anti-coagulated blood was flowed during 4 min over collagen at a shear rate of 1000 s−1. Preincubation was with Me2SO vehicle (control) or one of the PI3K inhibitors: PIK-75, YM-024, TGX-221, AS-252424, IC-87144, or wortmannin (all 1 μm). A, representative images of phase contrast (120 × 120 μm) and OG-annexin A5 fluorescence (150 × 150 μm) after 4-min flow. B and C, surface area coverage of all deposited platelets and PS-exposing platelets. Data are expressed as percentages of control condition. Means ± S.E. (n = 4); *, p < 0.05 compared with control.
Roles of Murine PI3K Isoforms in GPVI-induced Platelet Activation and Thrombus Formation
Platelets from mice lacking the p85α regulatory PI3K subunit are impaired in collagen-induced aggregation, which points to diminished GPVI signaling (11). In the p85α−/− platelets, expression of the p110α subunit (PI3Kα) is almost undetectable, while both p85β and p110β are still present at low levels (11, 29, 45). We used blood from p85α−/− mice to measure collagen-dependent [Ca2+]i increases in adhered platelets, thrombus formation, and PS exposure under flow. In contrast to the potent and prolonged Ca2+ responses of p85α+/+ platelets, p85α-deficient platelets showed a markedly lower Ca2+ signal, which in 36% of the cells showed spiking Ca2+ responses (Fig. 7A). Treatment of p85α+/+ blood with TGX-221 reduced the average Ca2+ signal to the level seen in p85α−/− platelets. Treatment of wild-type blood with YM-024 had a similar suppressive effect on the Ca2+ response (p = 0.012). As a comparison, blood was used from p110γ−/− mice, in which case Ca2+ responses under flow were not different from those of corresponding wild-type platelets.4
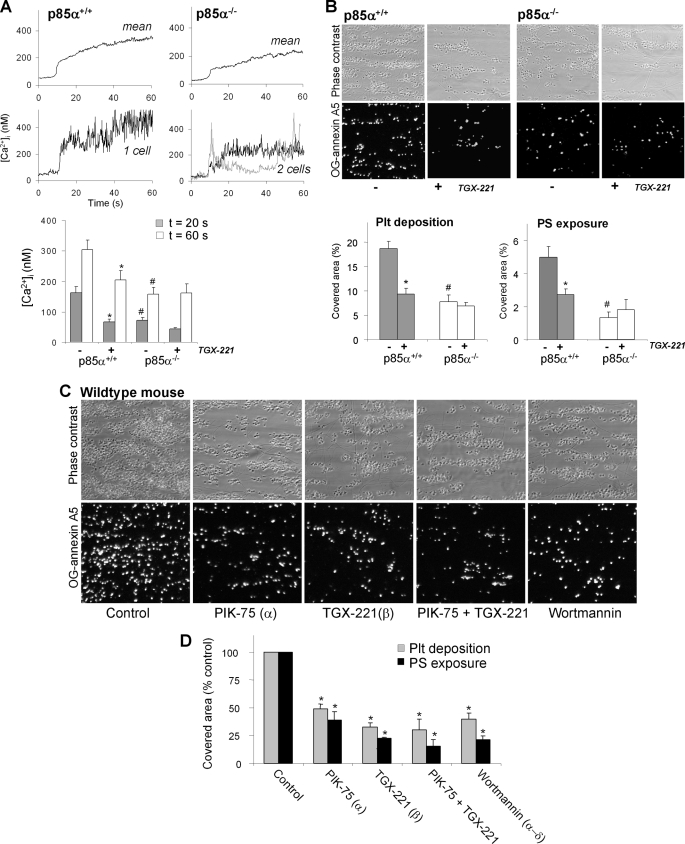
FIGURE 7.
Murine p85α regulates collagen-induced Ca2+ responses and thrombus formation under flow. PPACK/heparin-anti-coagulated blood from p85α+/+ or p85α−/− mice, spiked with 10% Fluo-3-loaded platelets from the same mouse strain, was flowed over collagen at 1000 s−1. The blood was preincubated with Me2SO vehicle or TGX-221 (1 μm). A, contribution of p85α to Ca2+ responses under high shear perfusion. Upper panels: averaged overlays of [Ca2+]i traces from >25 platelets; lower panels: traces from representative single platelets. Bars show quantification of [Ca2+]i increases at 20 s (gray) or 60 s (white) after initial platelet activation. B, contribution of p85α to thrombus formation and PS exposure. Shown are representative images of phase-contrast (120 × 120 μm) and OG-annexin A5 fluorescence (150 × 150 μm) of p85α+/+ and p85α−/− thrombi. Bars represent surface area coverage of all deposited platelets and PS-exposing platelets. C and D, effect of pharmacological PI3K inhibition on thrombus formation and PS exposure. Wild-type blood was preincubated with Me2SO vehicle (control), PIK-75, TGX-221, or wortmannin (each 1 μm). Data are means ± S.E. (n = 3–5); *, p < 0.05 compared with vehicle control; #, p < 0.05 compared with wild type.
Measurements of flow-dependent thrombus formation on collagen showed a reduced deposition of p85α−/− platelets, as well as a reduced PS exposure of the cells in comparison to wild-type blood (Fig. 7B). Similarly, addition of TGX-221 to p85α+/+ blood, but not to p85α−/− blood, significantly decreased thrombus formation and number of PS-exposing platelets. A role for PI3Kα and -β in murine thrombus formation was further supported by inhibitor studies using blood from wild-type mice. Treatment with PIK-75 and/or TGX-221 led to a similar reduction in platelet deposition and PS exposure as treatment with the general PI3K inhibitor, wortmannin (Fig. 7C). Together, these results demonstrated that, in mouse, the regulatory p85α subunit and the catalytic PI3Kα and -β subunits are essential in flow-dependent Ca2+ signaling, PS exposure, and thrombus formation on collagen.
Experiments carried out with washed mouse platelets, in the presence of autocrine stimulation inhibitors, confirmed that GPVI-induced activation relied on PI3Kα/β activity, because LY-294002, PIK-75, and TGX-221 all inhibited CRP-induced aggregation (not shown). In addition, platelets from mice lacking the catalytic p110γ or p110δ subunits were essentially unaltered in aggregation, when compared with wild-type platelets.5 Together, this points to major functions of murine p110α and p110β PI3K isoforms and to minor roles for p110γ and p110δ in the aggregation response.
Roles of PI3K Isoforms in GPVI-induced Rap1b Activation in Human and Mouse Platelets
In platelets, the low molecular weight GTPase, Rap1b, is a well characterized effector downstream of PI3K (27). However, there is no conclusive evidence how Rap1b functions directly downstream of GPVI. Platelets from mice deficient in Rap1b were used to investigate this. In the presence of autocrine stimulation inhibitors, Rap1b−/− platelets showed a 36% reduction in CRP-induced aggregation in comparison to Rap1b+/+ wild types (Fig. 8, A and B). This suggested a direct role for this GTPase in GPVI-induced platelet aggregation. Inhibition of PI3K with LY-294002 markedly reduced the aggregation of Rap1b+/+ platelets and, surprisingly, of Rap1b−/− platelets, with reductions of 89 and 86%, respectively, in comparison to vehicle-treated controls. The observation that LY-294002 further reduced the aggregation of Rap1b−/− platelets indicated that, even though Rap1b is involved in GPVI-mediated activation, it is not the only effector of PI3K in this response.
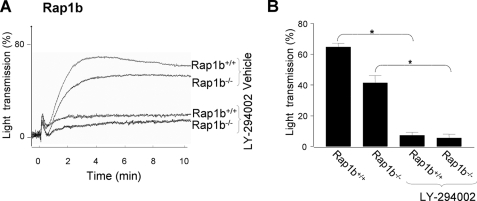
FIGURE 8.
Contribution of Rap1b to GPVI-induced integrin αIIbβ3 activation and platelet aggregation. A, washed platelets (3 × 108/ml) from Rap1b+/+ or Rap1b−/− mice were preincubated with autocrine stimulation inhibitors (AR-C69931MX, MRS-2179, and 10 μm indomethacin), in the presence of Me2SO vehicle or LY-294002 (25 μm). Platelets were stimulated with 10 μg/ml CRP in the presence of purified fibrinogen (0.5 mg/ml), and aggregation was monitored. Representative aggregation traces are shown. B, bars give percentages of light transmission at 3 min after CRP addition. Data are means ± S.E. (n = 3–4); *, p < 0.05 compared with control.
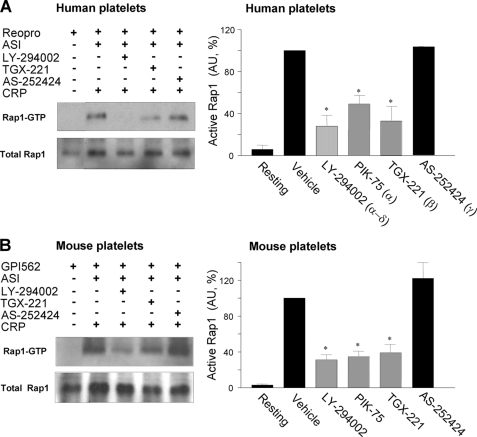
To elucidate the PI3K isoforms implicated in Rap1b activation directly downstream of GPVI, the effect of CRP was evaluated on Rap1b-GTP levels in platelets treated with autocrine stimulation inhibitors. An inhibitor of αIIbβ3 of human (40 μg/ml Reopro) or mouse (100 μm GPI-562) platelets served to suppress platelet aggregation, which process interferes with the assay. In both human and mouse platelets, CRP induced a potent activation of Rap1b (Fig. 9, A and B). Treatment with LY-294002 strongly suppressed Rap1b activation with Rap1-GTP levels being reduced in human and mouse platelets by 72 and 69%, respectively. Markedly, treatment with TGX-221 or PIK-75 resulted in a similar degree of reduction in Rap1b activation, both in mouse or human platelets. In contrast, effects of AS-252424 treatment were negligible. These findings demonstrate a major role for PI3Kα and -β but not -γ in Rap1b activation downstream of GPVI.
FIGURE 9.
Contribution of PI3K isoforms to GPVI-induced activation of Rap1b. Washed human (A) and wild-type mouse (B) platelets (3 × 108/ml) were preincubated with autocrine stimulation inhibitors (ASI) (Fig. 8) and αIIbβ3 inhibitor (human: 40 μg/ml Reopro; mouse: 100 μm GPI-562). Incubations contained Me2SO vehicle or PI3K inhibitor LY-294402 (25 μm), TGX-221 (0.5 μm), or AS-252424 (10 μm). Platelets were stimulated with 20 μg/ml CRP, lysed, and used for immunoprecipitation of GTP-Rap1. A and B, representative Western blots of active Rap1-GTP and total Rap1. Right panels: densitometric analysis of the amount of active Rap1-GTP (arbitrary units). Means ± S.E. (n = 3–5); *, p < 0.05 compared with control.
DISCUSSION
The present report provides the first data showing the contribution of multiple PI3K isoforms in GPVI-induced activation of (human) platelets and the role of these isoforms in GPVI-dependent thrombus formation. Our findings significantly extend the earlier evidence that GPVI agonists increase the formation of PI(3,4,5)P3 and its derivative phosphoinositide 3,4-bisphosphate via PI3K activation (9, 10, 46). A marked finding is that both PI3Kα and -β isoforms are needed for full InsP3 formation and Ca2+ signal generation, thus suggesting that both activities are necessary for optimal activation of the key effector enzyme of GPVI, PLCγ2. Detailed Ca2+ measurements in the presence of extracellular EGTA and CaCl2 pointed to a marked contribution of PI3K to Ca2+ mobilization from intracellular stores, which is a direct read-out of PLC, and not to a (store-independent) effect on Ca2+ entry. Hence, the earlier suggested regulation of Ca2+ entry by PI3K or its product PI(3,4,5)P3 (23, 24) seems to be secondary to the effect of PI3K regulation of Ca2+ store depletion.
Several observations point to a non-redundant contribution of PI3Kα and -β isoforms in GPVI-induced platelet activation. These include the inhibitory effects on human platelets of a panel of compounds with high affinity to PI3Kα (PIK-75, PI-103, and YM-024) or to PI3Kβ (TGX-221 and also PI-103). Other support comes from studies with mouse platelets, which responded similarly to the isoform-specific inhibitors as did human platelets. Furthermore, we found that mouse platelets lacking p85α were strikingly impaired in collagen-induced Ca2+ signaling and thrombus formation. These p85α−/− platelets are fully depleted in p110α, but still express residual p85β and p110β (11). However, treatment of these platelets with TGX-221 did not further suppress Ca2+ signaling and downstream responses such as PS exposure, although this compound did insignificantly reduce p85α−/− platelet deposition under flow. This suggested a function of the residual p110β in ADP-dependent platelet aggregate formation.
In human platelets, inhibition of PI3Kγ (AS-252424) failed to affect the GPVI-induced Ca2+ response, whereas inhibition of PI3Kδ (IC-87114) caused a minor reduction, in contrast to the marked effect of PI3Kα/β inhibition. Similarly, mouse platelets, which were deficient in p110γ or p110δ, showed undiminished activation, when stimulated with GPVI agonist under conditions where autocrine stimulators were blocked. These results are supported by data from others, showing that deficiency in murine p110γ (19) or p110δ (26) has no more than a modest role in GPVI-induced platelet activation.
The role of PI3Kα and -β isoforms was particularly clear in measurements of GPVI-dependent thrombus formation, where platelets were flowed over collagen at a defined physiological shear rate. Blocking or deficiency in PI3Kα/β resulted in suppression of Ca2+ increases and PS exposure of platelets adhered to collagen, as well as in reduced formation of aggregate formation. For both human and mouse blood, it is known that these responses fully rely on GPVI signaling via Src family kinases to LAT and PLCγ2 (34, 47). Hence, the present findings support a concept that full PLCγ2-evoked activity is required for optimal platelet activation and thrombus formation under flow. Earlier, it was established that at the same flow conditions different populations of platelets assemble into aggregates or expose PS (38). Although this heterogeneity in platelet responses was maintained with PI3Kα/β inhibition or absence, the present results also suggest that the reduced PS exposure in inhibited platelets is a direct consequence of the reduced Ca2+ signal of those platelets that are adhered to collagen.
Earlier, we and others have shown that two PI3K isoforms, namely PI3Kβ and -γ, are implicated in ADP/P2Y12-induced integrin αIIbβ3 activation and subsequent thrombus stabilization (17, 19, 20). Recently, these observations were extended to in vivo thrombosis models, where wortmannin treatment of p110γ-deficient mice was found to cause a dramatic defect in initial thrombus growth following vascular damage (21). The present data shed a new light on these in vivo observations, because it now appears that both collagen- and ADP-dependent platelet activation processes are affected by PI3K inhibition with a likely key role of the PI3Kα/β isoforms.
There is a growing body of evidence that, in many cell systems individual PI3K isoforms, although involved in specific cellular functions, show functional redundancy, e.g. as noted for the additive contribution of PI3Kβ and -γ in complement 5a-stimulated macrophages (48). So far, non-redundancy in PI3K function has been described only in particular cases. For example, in neutrophils producing inflammatory reactive oxygen species, both PI3Kγ and -δ isoforms contribute to PI(3,4,5)P3 formation, but in this case the role of PI3Kγ may be prior to that of PI3Kδ (31, 49). In comparison to platelets, this is reminiscent of the early contribution of PI3Kα/β in GPVI stimulation, and the later involvement of PI3Kβ/γ in responses to autocrine produced ADP.
In the classic scheme of immunoglobulin receptor-linked PLCγ activity, as proposed for lymphocytes, accumulation of PI(3,4,5)P3 in the plasma membrane may function as an anchoring site for the N-terminal pleckstrin homology domain of PLCγ isoforms (18, 50). This scheme can be applicable to GPVI-stimulated platelets as well. Given the high turnover of phosphoinositides in the platelet plasma membrane, we hypothesize that the stimulation and anchoring of PLCγ2 requires a threshold elevation of PI(3,4,5)P3 that can only be achieved by simultaneous activity of PI3Kα and -β. In other words, activation of either PI3Kα or -β alone may be insufficient for PLCγ2 targeting to membrane via its PH domain. It is conceivable that this is a consequence of the need of PLCγ2 to compete with other PH-domain containing signaling proteins such as Akt isoforms. Such a mechanism would provide an attractive, but not the only, explanation for the identified non-redundant contribution to GPVI-induced signaling of both PI3K isoforms. One possible scenario is that one isoform may produce an initial small amount of PI(3,4,5)P3, which in turn promotes activation of the other isoform through PI(3,4,5)P3 regulation of p85 subunits. Future studies examining temporal signaling by individual PI3K isoforms, as previously performed in neutrophils (31), will be needed to address this issue. The enhancing effect of PI3K activity on PLCγ2 is in contrast to the essential contribution of Src family kinases to GPVI-induced activation, because this is apparent from the complete abrogation of GPVI-induced Ca2+ increases by Src family kinase inhibition (39).
The literature contains solid evidence that platelet Rap1b is activated in a PI3K-dependent manner, in particular after occupation of Gi-coupled receptors such as the P2Y12 receptor for ADP (27, 28). By using autocrine stimulation inhibitors, where the P2Y12-dependent component was blocked, we could identify, for both human and mouse platelets, the existence of a direct GPVI-stimulated and PI3K-dependent component of Rap1b activation. Use of the isoform-specific inhibitors revealed involvement of PI3Kα/β in this activation of Rap1b. However, the prominent suppressive effect of PI3K inhibitors on αIIbβ3 activation and aggregation seen in Rap1b−/− platelets indicates that the PI3K isoforms contribute to GPVI-induced integrin activation also through a pathway that is independent of Rap1b. This pathway may involve PI3Kα/β-dependent stimulation of PLCγ2, which can contribute to the Ca2+- and protein kinase C-mediated activation of αIIbβ3.
PI3Kα is the most frequently mutated kinase in human cancer. Hence, this isoform has raised great interest as a target for novel anti-tumor drugs, some of which are currently in early stage clinical trials (51). Given the likely important function of GPVI in (experimental) thrombosis, the present data suggest another important application for PI3Kα and PI3Kβ inhibitors, namely in anti-platelet therapy and arterial thrombosis. In this context, it is relevant to note that arterial thrombosis is a known companion of some cancers. The availability of selective pharmacological agents against specific isoforms may thus offer new potential approaches for prevention of these diseases.
Supplementary Material
Acknowledgments
We thank Dr. A. van Montfoort for performing preliminary experiments. We acknowledge the Baker Heart Research Institute (Melbourne, Australia) for the kind gift of PI3K isoform inhibitors.
Addendum
While this report was in revision, other authors have also reported that PIK3β plays an essential role in GPVI-induced activation of mouse platelets (52).
This work was supported by Grants from the EU (Marie Curie EST 2005-020706), the Netherlands Heart Foundation (2002-B014), and the Netherlands Organization for Scientific Research (NWO 11.400.0076).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2 and Table I.
I. Munnix, unpublished data.
P. Mangin, unpublished data.
- GPVI
- glycoprotein VI
- CRP
- collagen-related peptide
- InsP3
- inositol 1,4,5-trisphosphate
- PI3K
- phosphoinositide 3-kinase
- PI(3,4,5)P3
- phosphoinositide 3,4,5-trisphosphate
- PLC
- phospholipase C
- PPACK
- H-Phe-Pro-Arg chloromethyl ketone
- PRP
- platelet-rich plasma
- PS
- phosphatidylserine
- OG
- Oregon Green
- PH
- pleckstrin homology.
REFERENCES
- 1.Nieswandt B., Watson S. P. (2003) Blood 102, 449–461 [DOI] [PubMed] [Google Scholar]
- 2.Watson S. P., Gibbins J. M. (1998) Immunol. Today 19, 260–264 [DOI] [PubMed] [Google Scholar]
- 3.Heemskerk J. W., Kuijpers M. J., Munnix I. C., Siljander P. R. (2005) Trends Cardiovasc. Med. 15, 86–92 [DOI] [PubMed] [Google Scholar]
- 4.Jackson S. P. (2007) Blood 109, 5087–5095 [DOI] [PubMed] [Google Scholar]
- 5.Jandrot-Perrus M., Lagrue A. H., Okuma M., Bon C. (1997) J. Biol. Chem. 272, 27035–27041 [DOI] [PubMed] [Google Scholar]
- 6.Knight C. G., Morton L. F., Onley D. J., Peachey A. R., Ichinohe T., Okuma M., Farndale R. W., Barnes M. J. (1999) Cardiovasc. Res. 41, 450–457 [DOI] [PubMed] [Google Scholar]
- 7.Siljander P., Farndale R. W., Feijge M. A., Comfurius P., Kos S., Bevers E. M., Heemskerk J. W. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 618–627 [DOI] [PubMed] [Google Scholar]
- 8.Gibbins J. M., Briddon S., Shutes A., van Vugt M. J., van de Winkel J. G., Saito T., Watson S. P. (1998) J. Biol. Chem. 273, 34437–34443 [DOI] [PubMed] [Google Scholar]
- 9.Lagrue A. H., Francischetti I. M., Guimarães J. A., Jandrot-Perrus M. (1999) FEBS Lett. 448, 95–100 [DOI] [PubMed] [Google Scholar]
- 10.Pasquet J. M., Bobe R., Gross B., Gratacap M. P., Tomlinson M. G., Payrastre B., Watson S. P. (1999) Biochem. J. 342, 171–177 [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe N., Nakajima H., Suzuki H., Oda A., Matsubara Y., Moroi M., Terauchi Y., Kadowaki T., Suzuki H., Koyasu S., Ikeda Y., Handa M. (2003) Blood 102, 541–548 [DOI] [PubMed] [Google Scholar]
- 12.Stephens L., Williams R., Hawkins P. (2005) Curr. Opin. Pharmacol. 5, 357–365 [DOI] [PubMed] [Google Scholar]
- 13.Rückle T., Schwarz M. K., Rommel C. (2006) Nat. Rev. Drug Discov. 5, 913–918 [DOI] [PubMed] [Google Scholar]
- 14.Ali K., Bilancio A., Thomas M., Pearce W., Gilfillan A. M., Tkaczyk C., Kuehn N., Gray A., Giddings J., Peskett E., Fox R., Bruce I., Walker C., Sawyer C., Okkenhaug K., Finan P., Vanhaesebroeck B. (2004) Nature 431, 1007–1011 [DOI] [PubMed] [Google Scholar]
- 15.Rittenhouse S. E. (1996) Blood 88, 4401–4414 [PubMed] [Google Scholar]
- 16.Vanhaesebroeck B., Waterfield M. D. (1999) Exp. Cell Res. 253, 239–254 [DOI] [PubMed] [Google Scholar]
- 17.Jackson S. P., Schoenwaelder S. M., Goncalves I., Nesbitt W. S., Yap C. L., Wright C. E., Kenche V., Anderson K. E., Dopheide S. M., Yuan Y., Sturgeon S. A., Prabaharan H., Thompson P. E., Smith G. D., Shepherd P. R., Daniele N., Kulkarni S., Abbott B., Saylik D., Jones C., Lu L., Giuliano S., Hughan S. C., Angus J. A., Robertson A. D., Salem H. H. (2005) Nat. Med. 11, 507–514 [DOI] [PubMed] [Google Scholar]
- 18.Schlessinger J. (2000) Cell 103, 211–225 [DOI] [PubMed] [Google Scholar]
- 19.Hirsch E., Bosco O., Tropel P., Laffargue M., Calvez R., Altruda F., Wymann M., Montrucchio G. (2001) FASEB J. 15, 2019–2021 [DOI] [PubMed] [Google Scholar]
- 20.Cosemans J. M., Munnix I. C., Wetzker R., Heller R., Jackson S. P., Heemskerk J. W. (2006) Blood 108, 3045–3052 [DOI] [PubMed] [Google Scholar]
- 21.Schoenwaelder S. M., Ono A., Sturgeon S., Chan S. M., Mangin P., Maxwell M. J., Turnbull S., Mulchandani M., Anderson K., Kauffenstein G., Rewcastle G. W., Kendall J., Gachet C., Salem H. H., Jackson S. P. (2007) J. Biol. Chem. 282, 28648–28658 [DOI] [PubMed] [Google Scholar]
- 22.Lecut C., Schoolmeester A., Kuijpers M. J., Broers J. L., van Zandvoort M. A., Vanhoorelbeke K., Deckmyn H., Jandrot-Perrus M., Heemskerk J. W. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1727–1733 [DOI] [PubMed] [Google Scholar]
- 23.Pasquet J. M., Quek L., Stevens C., Bobe R., Huber M., Duronio V., Krystal G., Watson S. P. (2000) EMBO J. 19, 2793–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu P. J., Hsu A. L., Wang D. S., Chen C. S. (1998) Biochemistry 37, 9776–9783 [DOI] [PubMed] [Google Scholar]
- 25.Woulfe D., Jiang H., Morgans A., Monks R., Birnbaum M., Brass L. F. (2004) J. Clin. Invest. 113, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senis Y. A., Atkinson B. T., Pearce A. C., Wonerow P., Auger J. M., Okkenhaug K., Pearce W., Vigorito E., Vanhaesebroeck B., Turner M., Watson S. P. (2005) Platelets 16, 191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lova P., Paganini S., Hirsch E., Barberis L., Wymann M., Sinigaglia F., Balduini C., Torti M. (2003) J. Biol. Chem. 278, 131–138 [DOI] [PubMed] [Google Scholar]
- 28.Woulfe D., Jiang H., Mortensen R., Yang J., Brass L. F. (2002) J. Biol. Chem. 277, 23382–23390 [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H., Terauchi Y., Fujiwara M., Aizawa S., Yazaki Y., Kadowaki T., Koyasu S. (1999) Science 283, 390–392 [DOI] [PubMed] [Google Scholar]
- 30.Chrzanowska-Wodnicka M., Smyth S. S., Schoenwaelder S. M., Fischer T. H., White G. C., 2nd (2005) J. Clin. Invest. 115, 680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condliffe A. M., Davidson K., Anderson K. E., Ellson C. D., Crabbe T., Okkenhaug K., Vanhaesebroeck B., Turner M., Webb L., Wymann M. P., Hirsch E., Ruckle T., Camps M., Rommel C., Jackson S. P., Chilvers E. R., Stephens L. R., Hawkins P. T. (2005) Blood 106, 1432–1440 [DOI] [PubMed] [Google Scholar]
- 32.Kuijpers M. J., Schulte V., Bergmeier W., Lindhout T., Brakebusch C., Offermanns S., Fässler R., Heemskerk J. W., Nieswandt B. (2003) FASEB J. 17, 685–687 [DOI] [PubMed] [Google Scholar]
- 33.Maxwell M. J., Yuan Y., Anderson K. E., Hibbs M. L., Salem H. H., Jackson S. P. (2004) J. Biol. Chem. 279, 32196–32204 [DOI] [PubMed] [Google Scholar]
- 34.Siljander P. R., Munnix I. C., Smethurst P. A., Deckmyn H., Lindhout T., Ouwehand W. H., Farndale R. W., Heemskerk J. W. (2004) Blood 103, 1333–1341 [DOI] [PubMed] [Google Scholar]
- 35.Feijge M. A., van Pampus E. C., Lacabaratz-Porret C., Hamulyàk K., Levy-Toledano S., Enouf J., Heemskerk J. W. (1998) Br. J. Haematol. 102, 850–859 [DOI] [PubMed] [Google Scholar]
- 36.Heemskerk J. W., Feijge M. A., Henneman L., Rosing J., Hemker H. C. (1997) Eur. J. Biochem. 249, 547–555 [DOI] [PubMed] [Google Scholar]
- 37.Cauwenberghs S., Feijge M. A., Theunissen E., Heemskerk J. W., van Pampus E. C., Curvers J. (2007) Br. J. Haematol. 136, 480–490 [DOI] [PubMed] [Google Scholar]
- 38.Munnix I. C., Kuijpers M. J., Auger J., Thomassen C. M., Panizzi P., van Zandvoort M. A., Rosing J., Bock P. E., Watson S. P., Heemskerk J. W. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 2484–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auger J. M., Kuijpers M. J., Senis Y. A., Watson S. P., Heemskerk J. W. (2005) FASEB J. 19, 825–827 [DOI] [PubMed] [Google Scholar]
- 40.Heemskerk J. W., Willems G. M., Rook M. B., Sage S. O. (2001) J. Physiol. 535, 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaussade C., Rewcastle G. W., Kendall J. D., Denny W. A., Cho K., Grønning L. M., Chong M. L., Anagnostou S. H., Jackson S. P., Daniele N., Shepherd P. R. (2007) Biochem. J. 404, 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S., Garcia A., Jackson S. P., Kunapuli S. P. (2007) Blood 110, 4206–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savage B., Ginsberg M. H., Ruggeri Z. M. (1999) Blood 94, 2704–2715 [PubMed] [Google Scholar]
- 44.Nesbitt W. S., Mangin P., Salem H. H., Jackson S. P. (2006) J. Mol. Med. 84, 989–995 [DOI] [PubMed] [Google Scholar]
- 45.Terauchi Y., Tsuji Y., Satoh S., Minoura H., Murakami K., Okuno A., Inukai K., Asano T., Kaburagi Y., Ueki K., Nakajima H., Hanafusa T., Matsuzawa Y., Sekihara H., Yin Y., Barrett J. C., Oda H., Ishikawa T., Akanuma Y., Komuro I., Suzuki M., Yamamura K., Kodama T., Suzuki H., Yamamura K., Kodama T., Suzuki H., Koyasu S., Aizawa S., Tobe K., Fukui Y., Yazaki Y., Kadowaki T. (1999) Nat. Genet. 21, 230–235 [DOI] [PubMed] [Google Scholar]
- 46.Falet H., Barkalow K. L., Pivniouk V. I., Barnes M. J., Geha R. S., Hartwig J. H. (2000) Blood 96, 3786–3792 [PubMed] [Google Scholar]
- 47.Nieswandt B., Bergmeier W., Eckly A., Schulte V., Ohlmann P., Cazenave J. P., Zirngibl H., Offermanns S., Gachet C. (2001) Blood 97, 3829–3835 [DOI] [PubMed] [Google Scholar]
- 48.Guillermet-Guibert J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., Meek S., Smith A. J., Okkenhaug K., Vanhaesebroeck B. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8292–8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suire S., Condliffe A. M., Ferguson G. J., Ellson C. D., Guillou H., Davidson K., Welch H., Coadwell J., Turner M., Chilvers E. R., Hawkins P. T., Stephens L. (2006) Nat. Cell Biol. 11, 1303–1309 [DOI] [PubMed] [Google Scholar]
- 50.Scharenberg A. M., Kinet J. P. (1998) Cell 94, 5–8 [DOI] [PubMed] [Google Scholar]
- 51.Vogt P. K. (2008) Cancer Cell 14, 107–108 [DOI] [PubMed] [Google Scholar]
- 52.Canobbio I., Stefanini L., Cipolla L., Ciraolo E., Gruppi C., Balduini C., Hirsch E., Torti M. (2009) Blood 114, 2193–2196 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.