Abstract
During breast cancer progression, transforming growth factor-β (TGF-β) switches from a tumor suppressor to a pro-metastatic molecule. Several recent studies suggest that this conversion in TGF-β function depends upon fundamental changes in the TGF-β signaling system. We show here that these changes in TGF-β signaling are concomitant with aberrant expression of the focal adhesion protein, p130Cas. Indeed, elevating expression of either the full-length (FL) or just the carboxyl terminus (CT) of p130Cas in mammary epithelial cells (MECs) diminished the ability of TGF-β1 to activate Smad2/3, but increased its coupling to p38 MAPK. This shift in TGF-β signaling evoked (i) resistance to TGF-β-induced growth arrest, and (ii) acinar filling upon three-dimensional organotypic cultures of p130Cas-FL or -CT expressing MECs. Furthermore, rendering metastatic MECs deficient in p130Cas enhanced TGF-β-stimulated Smad2/3 activity, which restored TGF-β-induced growth inhibition both in vitro and in mammary tumors produced in mice. Additionally, whereas elevating TβR-II expression in metastatic MECs had no affect on their phosphorylation of Smad2/3, this event markedly enhanced their activation of p38 MAPK, leading to increased MEC invasion and metastasis. Importantly, depleting p130Cas expression in TβR-II-expressing metastatic MECs significantly increased their activation of Smad2/3, which (i) reestablished the physiologic balance between canonical and noncanonical TGF-β signaling, and (ii) reversed cellular invasion and early mammary tumor cell dissemination stimulated by TGF-β. Collectively, our findings identify p130Cas as a molecular rheostat that regulates the delicate balance between canonical and noncanonical TGF-β signaling, a balance that is critical to maintaining the tumor suppressor function of TGF-β during breast cancer progression.
Introduction
Invasion and metastasis are the most lethal characteristics of breast cancer (1, 2). Transforming growth factor-β (TGF-β)2 is a powerful suppressor of mammary tumorigenesis, doing so through its ability to repress mammary epithelial cell (MEC) proliferation, as well as through its creation of cell microenvironments that inhibit MEC motility, invasion, and metastasis (2). During breast cancer progression, the tumor suppressing function of TGF-β is frequently subverted, thus transforming TGF-β from a suppressor of breast cancer formation to a promoter of its growth and metastasis (2–4). Unfortunately, how mammary tumorigenesis overcomes the cytostatic function of TGF-β remains incompletely understood, as does the manner in which developing breast cancers ultimately sense TGF-β as a pro-metastatic factor.
Transmembrane signaling by TGF-β commences upon binding to its type II receptor (TβR-II), which recruits and activates its type I receptor (TβR-I), which then phosphorylates and activates Smads 2 and 3. Following their activation, Smads 2 and 3 form heteromeric complexes with Smad4, which collectively translocate to the nucleus to regulate a multitude of transcriptional events and cellular responses (i.e. apoptosis, cytostasis, and homeostasis, (5, 6)). In addition to stimulating Smad2/3, TGF-β also activates several noncanonical signaling systems, including members of the MAP kinase family (e.g. ERK1/2, JNK, and p38 MAPK (7)). Interestingly, several studies suggest that genetic and epigenetic events cooperate with aberrant Smad2/3 activities and functions to facilitate the conversion of TGF-β from tumor suppressor to a tumor promoter (8, 9). However, these and other studies also present strong evidence implicating dysregulated activation of several noncanonical TGF-β effectors during this same switch in TGF-β function (10). Thus, deciphering the relative contribution of signaling imbalances that arise between Smad2/3-dependent and -independent TGF-β signaling systems is essential to enhancing our understanding of how TGF-β ultimately promotes the development and progression of mammary tumorigenesis.
Recently, we identified a critical αvβ3 integrin:pY284-TβR-II:Grb2 signaling axis that mediates TGF-β stimulation of MAP kinases in normal and malignant MECs, leading to their acquisition of epithelial-mesenchymal transition, invasive, and metastatic phenotypes both in vitro and in vivo (11–13). Moreover, activation of this oncogenic signaling axis by TGF-β requires β3 integrin to form complexes with TβR-II (11–13). Unfortunately, it remains uncertain as to whether this interaction is direct or facilitated through another scaffolding protein. As such, we sought to identify members of focal adhesion complexes as potential integrin effectors capable of contributing to altered TGF-β signaling.
p130Cas (Crk-associated substrate) functions as a molecular scaffold within focal adhesion complexes, and is readily phosphorylated by focal adhesion kinase (FAK) and Src (14). Additionally, p130Cas binds stably to a variety of signaling molecules, including the (i) protein-tyrosine kinases FAK, PYK2, Src, Fyn, and Abl; (ii) adaptor molecules Crk, CrkL, Trip6, and AJUBA; (iii) guanine nucleotide exchange factors AND34 and CG3; and (iv) the MAPK family member, JNK (15, 16). The extensive interactome of p130Cas ideally positions and enables this molecule to interpret and integrate a variety of signaling inputs arising from numerous receptor systems. Indeed, the biological importance of p130Cas is emphasized by studies showing that its genetic ablation in mice elicits embryonic lethality, whereas fibroblasts derived from p130Cas-deficient embryos exhibit drastically altered cytoskeletal architectures (17). Moreover, fibroblasts transformed by Src become significantly more invasive when engineered to simultaneously overexpress p130Cas (15). Patients with primary breast tumors expressing high levels of p130Cas (also known as breast cancer resistance-1) experience a more rapid disease recurrence and have a greater risk of resistance to tamoxifen therapy (18). Recent studies also indicate that specific overexpression of p130Cas/breast cancer resistance-1 expression can confer breast cancer resistance to adriamycin (19). Moreover, directed overexpression of p130Case in murine MECs significantly increased their proliferative and survival indices in vivo, as well as greatly reduced the latency of mammary tumors arising from murine mammary tumor virus-driven Her2/Neu expression in mice (20). This study also observed the expression of p130Cas to be up-regulated significantly in a subset of human breast cancer samples (20). Collectively, these findings highlight the critical roles played by p130Cas in regulating normal tissue morphogenesis, and in promoting breast cancer progression. With respect to TGF-β, a recent study identified p130Cas as a potential inhibitor of Smad3 function (21). However, the pathophysiological importance of this event, if any, in mediating the oncogenic activities of TGF-β and/or p130Cas during breast cancer progression remains to be established.
The objective of the present study was to determine the role of p130Cas in facilitating the acquisition of oncogenic signaling by TGF-β during breast progression. We show here that p130Cas expression is up-regulated significantly in metastatic breast cancer cells (murine 4T1/human MCF10A-Ca1a) as compared with their nonmetastatic counterparts (murine 67NR/human MCF10A). Moreover, increased p130Cas expression was consistent with a decrease in TGF-β1-induced Smad2/3 signaling. Indeed, overexpression of p130Cas in nonmetastatic MECs led to a decrease in Smad2/3 activity, whereas depletion of p130Cas in metastatic MECs increased Smad2/3 activity. Most importantly, we show for the first time that p130Cas is essential for TGF-β stimulation of breast cancer growth, invasion, and pulmonary dissemination in mice. Taken together, our findings establish p130Cas as a novel molecular rheostat that regulates the balance between canonical and noncanonical TGF-β signaling in developing mammary tumors, whose acquisition of metastatic phenotypes is potentiated by elevated p130Cas expression and its consequential disruption of homeostatic TGF-β signaling.
EXPERIMENTAL PROCEDURES
Cell Lines and Reagents
Normal murine NMuMG and metastatic 4T1 cells were obtained from ATCC (Manassas, VA) and cultured as described previously (12). 4T1 cells were engineered to stably express firefly luciferase by transfection with pNifty-CMV-luciferase (22), followed by their selection and isolation with Zeocin (500 μg/ml; Invitrogen). The creation of 4T1 cells lacking p130Cas was accomplished by their transduction with lentiviral particles encoding either a scrambled (i.e. non-silencing shRNA) or murine-directed p130Cas shRNA (pLKO.1; Thermo Scientific, Huntsville, AL). The production of pLKO.1 lentiviral particles and their transduction into target cells was accomplished as described previously (23). In addition, NMuMG and 4T1 cells also were transduced with murine ecotropic retroviral particles that encoded for full-length p130Cas (pMSCV-Cas-FL), the carboxyl terminus of p130Cas (amino acids 544–874) (pMSCV-Cas-CT), or TβR-II (pMSCV-TβR-II), and the resulting polyclonal populations were selected by yellow fluorescence protein, or hygromycin resistance (200 μg/ml).
Cell Proliferation Assays
NMuMG and 4T1 cells were seeded in 96-well plates (10,000 cells/well) and allowed to adhere for 4 h, whereupon varying concentrations of TGF-β1 (0–5 ng/ml) were administered. Agonist stimulations were allowed to proceed for 48 h at 37 °C and cellular DNA was radiolabeled by inclusion of [3H]thymidine (1 μCi/well) during the final 6 h of TGF-β1 treatment. Afterward, the amount of [3H]thymidine incorporated into cellular DNA was quantified by scintillation counting.
Immunoblot Assays
NMuMG and 4T1 cells were lysed on ice in three-dimensional RIPA buffer (50 mm Tris, 150 mm NaCl, 0.25% (v/v) sodium deoxycholate, 0.1% SDS (v/v), pH 7.4) supplemented with (i) protease inhibitor mixture (Sigma), and (ii) the phosphatase inhibitors sodium orthovanadate (10 mm), β-glycerophosphate (40 mm), and sodium fluoride (20 mm). Afterward, the resulting whole cell extracts were clarified by microcentrifugation prior to being immunoblotted with the following primary antibodies (dilution): (a) anti-phospho-p38 MAPK (1:500; Cell Signaling, Danvers, MA); (b) anti-p38 MAPK (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA); (c) anti-phospho-Smad2 (1:1000; Cell Signaling); (d) anti-phospho-Smad3 (1:500; Cell Signaling); (e) anti-Smad 2/3 (1:1000; BD Biosciences); (f) anti-FAK (1:1000; Santa Cruz Biotechnology); (g) anti-p130Cas (1:1000; BD Biosciences); (h) phospho-p130Cas (1:1000; Cell Signaling); (i) anti-actin (1:1000; Santa Cruz Biotechnology); (j) lamin A/C (1:1000; Santa Cruz Biotechnology); and (k) E-Cadherin (1:2000; BD Biosciences).
Real-time PCR Analyses
Quiescent 4T1 cells were stimulated with TGF-β1 (5 ng/ml) for 24 h in the absence or presence of the p38 MAPK inhibitor SB208530 (10 μm) and total RNA was isolated using the RNeasy Plus Kit (Qiagen, Valencia, CA). Afterward, total RNA was reverse transcribed using the iScript cDNA Synthesis System (Bio-Rad) and semi-quantitative real-time PCR was conducted for PAI-1 using iQ SYBR Green (Bio-Rad) according to the manufacturer's recommendations and as described previously (23). Differences in RNA concentrations were controlled by normalizing individual gene signals to their corresponding glyceraldehyde-3-phosphate dehydrogenase signal.
Cell Fractionation Studies
Unstimulated and TGF-β1 (5 ng/ml)-stimulated NMuMG cells were lysed on ice in Buffer C (10 mm HEPES, 10 mm KCl, 0.1 mm EDTA, and 0.004% Nonidet P-40, pH 7.9) supplemented with protease inhibitor mixture (Sigma). Afterward, the resulting whole cell extract was subjected to a single freeze-thaw cycle, followed by microcentrifugation to yield a clarified cytoplasmic fraction. The remaining pellet was resuspended in Buffer N (20 mm HEPES, 400 mm NaCl, 1 mm EDTA, and 10% glycerol, pH 7.9) supplemented with protease inhibitor mixture, and shaken vigorously for 2 h at 4 °C. Afterward, this mixture was subjected to microcentrifugation to yield a clarified nuclear fraction.
Three-dimensional Culture Assays
NMuMG (1 × 104) and 4T1 (5 × 103) cells were diluted in complete medium supplemented with 5% Cultrex (R&D Systems, Minneapolis, MN), and subsequently seeded in 48-well plates on top of a Cultrex cushion. Where indicated, 4T1 cells were grown in the presence of TGF-β1 (5 ng/ml). The medium/Cultrex mixture was replaced at 7 days, and organoids were allowed to grow for a total length of 10 days, at which point they were monitored for hollowing by phase-contrast microscopy and quantified by three individuals who were blinded to the culture conditions. 4T1 acinar size was quantified using Image J software.
Cell Invasion Assays
The ability of TGF-β1 (5 ng/ml) to alter the invasion of 4T1 cells (50,000 cells/well) was analyzed using a modified Boyden Chamber assay as described previously (13).
Luciferase Reporter Gene Assays
NMuMG cells were transiently transfected overnight with LT1 liposomes (Mirus, Madison, WI) that contained 300 ng/well of pSBE-firefly luciferase (4X-CAGA) cDNA and 50 ng/well of pCMV-β-gal cDNA. Afterward, the cells were stimulated for 24 h with TGF-β1 (5 ng/ml), and subsequently harvested and assayed for firefly luciferase (Promega, Madison WI) and β-gal (Clontech, Mountain View, CA) activities. In addition, 4T1 cells that stably expressed firefly luciferase under control of the CMV promoter were similarly (i) transfected with 300 ng/well of pSBE-Renilla luciferase (4X-CAGA); (ii) stimulated with TGF-β1; and (iii) assayed for Renilla and firefly luciferase using the Dual-Glo Assay System as above (Promega).
Immunofluorescent Analyses
4T1 cells (25,000 cells/well) were allowed to adhere overnight to glass coverslips. Afterward, the cells were washed extensively in phosphate-buffered saline and immediately stimulated with TGF-β1 (5 ng/ml). Upon completion of agonist stimulation, the cells were (i) fixed in 4% paraformaldehyde; (ii) permeablized in 0.1% Triton X-100; (iii) stained with anti-Smad 2/3 antibodies (1:100; BD Biosciences); and (iv) visualized by addition of biotinylated anti-mouse antibodies (1:1000) in conjunction with the addition of rhodamine-conjugated streptavidin (1:2000).
Tumor Growth, in Vivo Bioluminescent Imaging, and Immunohistochemical Analyses
Control or various 4T1 derivatives engineered to stably express firefly luciferase were resuspended in sterile phosphate-buffered saline (50 μl) and injected orthotopically into the mammary fatpad (10,000 cells/injection) of 6-week-old female Balb/c mice (Jackson Laboratory, Bar Harbor, ME). Primary 4T1 tumor growth and metastasis development were assessed by (i) weekly bioluminescent imaging of tumor bearing animals on a Xenogen IVIS-200 (Xenogen Corporation, Hopkinton, MA); (ii) calculating primary tumor volumes using digital calipers and the equation digital V = (x2)(y)(0.5), where x is the tumor width and y is tumor length; and (iii) measuring primary tumor weights following their surgical excision on days 21 or 26 post-inoculation. Finally, serial histological sections of control and p130Cas-deficient 4T1 tumors were stained with Ki67 antibodies, and counterstained with hematoxylin as described previously (11). Data were quantified using Image J software. All animal studies were performed in accordance with the animal protocol procedures approved by the Institutional Animal Care and Use Committee of University of Colorado.
Statistical Analysis
Statistical values were defined using an unpaired Student's t test, where a p value < 0.05 was considered significant.
RESULTS
Elevated p130Cas Expression Inhibits TGF-β-mediated Smad2/3 Activation
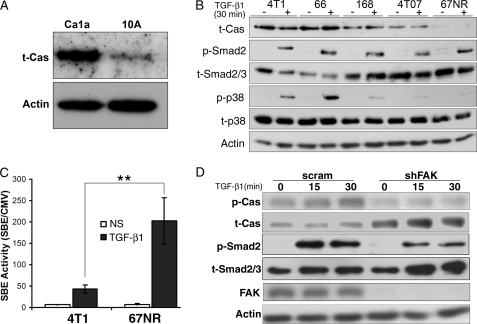
Elevated expression of p130Cas is associated with mammary tumor progression (20), and with the uncoupling of TGF-β to Smad3 activation in epithelial cells (21). Unfortunately, the pathophysiological importance of these events, if any, in mediating oncogenic TGF-β signaling in normal and malignant MECs remains unknown. As an initial measure of potential changes in p130Cas expression during mammary tumor progression, we monitored p130Cas protein levels by immunoblotting whole cell extracts prepared from nontransformed human MECs (MCF10A) and their corresponding metastatic derivatives (CA1a) (24–26). As shown in Fig. 1A, p130Cas expression was readily increased in human metastatic CAla cells as compared with nontransformed isogenic counterparts. Along these lines, we also observed p130Cas expression to be up-regulated dramatically in the murine 4T1 model of mammary tumor progression (Fig. 1B) (26, 27). Indeed, the highly metastatic 4T1 and 66c14 cells expressed significantly more p130Cas than did their moderately metastatic counterparts, 168-Farn and 4T07 (Fig. 1B). Consistent with this trend, we found nonmetastatic 67NR cells to express very little p130Cas (Fig. 1B). Importantly, the increased expression of p130Cas was consistent with a shift in the balance of TGF-β signaling from primarily that of canonical Smad2 phosphorylation in nonmetastatic 67NR cells to one that included a marked activation of p38 MAPK in metastatic 4T1 and 66c14 cells (Fig. 1B). Furthermore, using a measure of the downstream transcriptional activity of Smad2/3, we also observed a drastic diminution in the ability of TGF-β1 to activate Smad2/3 in 4T1 versus 67NR cells (Fig. 1C). We also monitored changes in the phosphorylation status of p130Cas upon stimulation with TGF-β. As shown in Fig. 1D, stimulating 4T1 cells with TGF-β not only readily induced the phosphorylation of Smad2, but also that of p130Cas (Fig. 1D). Moreover, the basal levels as well as the ability of TGF-β to induce phosphorylation of p130Cas were abrogated by rendering 4T1 cells deficient in its upstream kinase, FAK (Fig. 1D). Interestingly, depletion of FAK elicited a compensatory up-regulation of total p130Cas expression that was consistent with diminished coupling of TGF-β to Smad2 phosphorylation (Fig. 1D). Together, these findings are consistent with the notion that p130Cas, irrespective of its phosphorylation status, functions to shift the balance of TGF-β signaling during breast cancer progression by suppressing Smad2/3 activity and supporting p38 MAPK activation in response to TGF-β.
FIGURE 1.
Elevated p130Cas inhibits TGF-β-mediated Smad2/3 activation. A, normal human MECs (MCF-10A) and their metastatic derivatives (Ca1a) were immunoblotted for p130Cas expression. β-Actin (Actin) is shown as loading control. Data are representative images from a representative experiment that was performed two times with identical results. B, murine breast cancer cells derived from the same primary Balb/c tumor, including the highly metastatic 4T1 and 66c14 (66) cells, the partially metastatic 168Farn (168) and 4T07 cells, and the nonmetastatic 67NR cells were immunoblotted for p130Cas expression, and the phosphorylation of Smad2 (p-Smad2) and p38 MAPK (p-p38) in response to TGF-β1 stimulation (5 ng/ml). Total Smad2/3 (t-Smad2/3), p38 MAPK (t-p38), and β-actin (Actin) were analyzed as loading controls. Data are from a representative experiment that was performed two times with identical results. C, 4T1 and 67NR cells were transiently transfected with pSBE-luciferase and pCMV-β-gal plasmids, and subsequently stimulated with TGF-β1 (5 ng/ml) for 18 h prior to measuring luciferase and β-gal activities. NS, no stimulation. Data are the mean ± S.E. (n = 3) of luciferase/β-gal activity ratios. **, p = 0.01. D, quiescent 4T1 cells that expressed either a scrambled (scram) or FAK-specific (shFAK) shRNA were stimulated with TGF-β1 (5 ng/ml) for varying times, and subsequently immunoblotted with phospho-specific antibodies against p130Cas (p-Cas) and Smad2 (p-Smad2) as indicated. Membranes were stripped and reprobed with antibodies against p130Cas (t-Cas), Smad2/3 (t-Smad2/3), β-actin (Actin), and FAK as indicated. Data are from a representative experiment that was performed at least three times with similar results.
Overexpression of p130Cas Inhibits Smad2/3 Activity and Alters Normal Mammary Epithelial Acinar Formation
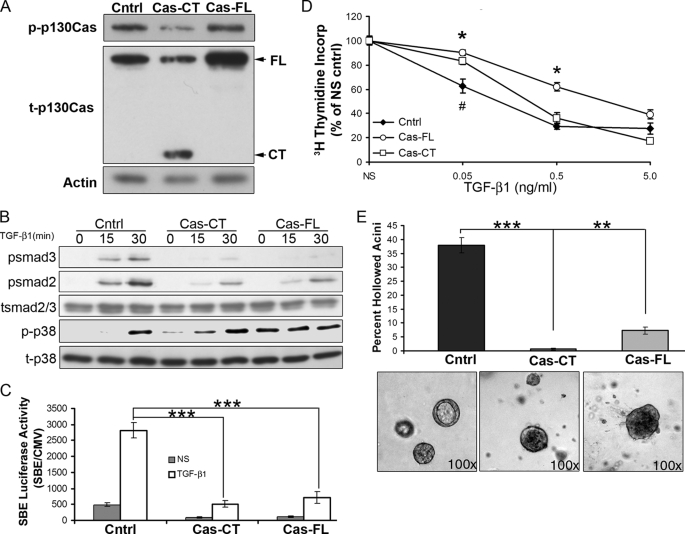
To address the role p130Cas in regulating TGF-β signaling, we overexpressed either the full-length protein (Cas-FL) or just the carboxyl terminus of p130Cas (Cas-CT) in normal murine mammary epithelial (NMuMG) cells (Fig. 2A). Indeed, overexpression of either Cas-CT or Cas-FL readily decreased the phosphorylation of Smad2 and Smad3 in response to TGF-β1 (Fig. 2B). In contrast, TGF-β-induced p38 MAPK phosphorylation was readily increased upon Cas-CT expression, whereas expression of Cas-FL was sufficient to induce the phosphorylation of p38 MAPK even in the absence of added TGF-β (Fig. 2B). Furthermore, overexpression of either Cas-CT or Cas-FL also dramatically decreased the extent of basal and TGF-β-induced Smad2/3-dependent transcription (Fig. 2C). Functionally, we observed the p130Cas-dependent reduction in Smad2/3 activity to significantly inhibit the cytostatic response of NMuMG cells to TGF-β (Fig. 2D). Finally, because TGF-β is critically involved regulating normal mammary gland development (28–30), we next sought to assess the affect of p130Cas overexpression on the formation of acini by NMuMG cells propagated in a three-dimensional organotypic culture system. Importantly, expression of either Cas-FL or Cas-CT readily invoked acinar filling, a phenotype that recapitulates in vivo mammary tumor progression (31). Taken together, these findings clearly indicate that inappropriate up-regulation (see Fig. 1) of p130Cas expression was sufficient to inhibit the physiologic activity of Smad2/3, thereby diminishing the tumor suppressive activities of TGF-β.
FIGURE 2.
Overexpression of p130Cas inhibits Smad2/3 activity and alters normal mammary epithelial acinar formation. A, NMuMG cells were transduced with retroviral particles containing vector control (cntrl), full-length p130Cas (Cas-FL), or the carboxyl terminus of p130Cas (Cas-CT). Stable transgene expression was assessed by immunoblotting for phosphorylated (p-p130Cas) and total p130Cas (t-p130Cas). β-Actin (Actin) is shown as a loading control. B, the p130Cas-manipulated cell lines as described in A were stimulated with TGF-β1 (5 ng/ml) for the indicated times, and subsequently analyzed for the phosphorylation of Smad2 (psmad2), Smad3 (psmad3), and p38 MAPK (p-p38). Membranes were stripped and reprobed for total Smad2/3 (tsmad2/3) and p38 MAPK (t-p38) as loading controls. Data are from representative experiments that were performed at least three times with identical results. C, control (cntrl) and p130Cas-expressing (Cas-CT and Cas-FL) NMuMG cells were transiently co-transfected with pSBE-luciferase and pCMV-β-gal plasmids, and subsequently stimulated overnight with TGF-β1 (5 ng/ml) prior to measuring luciferase and β-gal activities. NS, no stimulation. Data are the mean ± S.E. of SBE/CMV activity ratios observed in three independent experiments completed in triplicate. ***, p < 0.001). D, control (cntrl) and p130Cas-expressing (Cas-CT and Cas-FL) NMuMG cells were stimulated with increasing concentrations of TGF-β1 (0–5 ng/ml) for 48 h, and subsequently assayed for [3H]thymidine incorporation into cellular DNA. Data are the mean ± S.E. quantities of incorporated [3H]thymidine normalized to unstimulated controls observed in three independent experiments completed in triplicate (*, p < 0.05 between Cntrl and Cas-FL; #, p < 0.05 between Cntrl and Cas-CT). E, the p130Cas-manipulated NMuMG cells described in A were grown in three-dimensional organotypic cultures for 10 days, at which point the percentage of hollowed acini were quantified by phase-contrast microscopy (**, p < 0.001; ***, p < 0.0001). Representative acini are shown.
p130Cas Deficiency Increases Smad2/3 Activity in Metastatic MECs
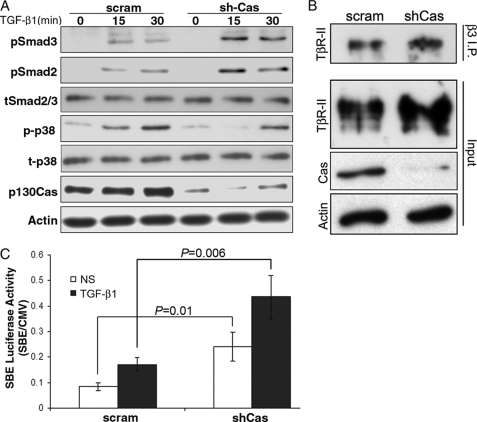
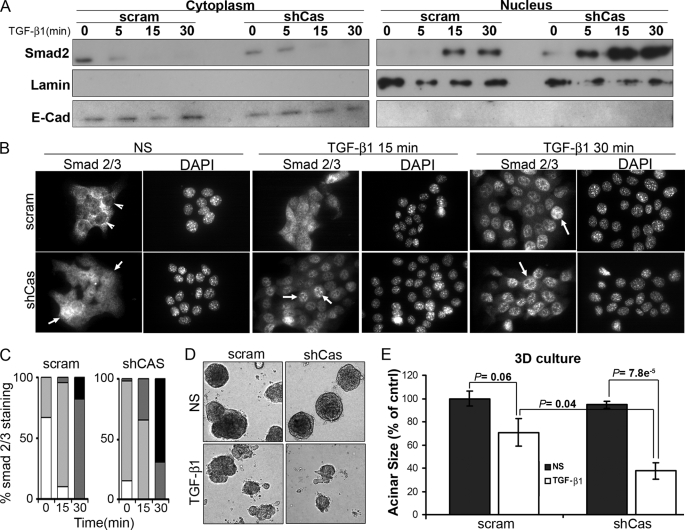
Given that elevating p130Cas expression was sufficient to inhibit Smad2/3 signaling stimulated by TGF-β, we next examined how p130Cas deficiency would affect the coupling of Smad2/3 to TGF-β in MECs. To this end, we expressed and screened five independent p130Cas-specific shRNA sequences in NMuMG cells. The general importance of p130Cas in maintaining normal MEC physiology and homeostasis was readily apparent as NMuMG cells that expressed shCas#5 shRNA, which elicited the greatest degree of p130Cas depletion (see supplemental Fig. S1A), failed to thrive and survive under extended culture conditions (data not shown). Overall, p130Cas deficiency led to decreased Smad2 expression in NMuMG cells (see supplemental Fig. S1A). These findings are consistent with the notion that p130Cas deficiency augments the activity of Smad2/3, which elicits proteosome-directed degradation of Smad2/3 (see supplemental Fig. S1B) (23, 32, 33). However, this decrease in Smad2 expression precluded a direct analysis of the affect p130Cas deficiency elicited on the activation of Smad2/3 by TGF-β in normal MECs. Despite this limitation, aberrant p130Cas expression has been associated with increased breast cancer progression and poorer clinical prognosis (18–20). Therefore, we sought to address the functional impact of p130Cas deficiency on the ability of TGF-β to initiate oncogenic signaling in the 4T1 metastatic model of breast cancer. Indeed, depletion of p130Cas greatly augmented the coupling of TGF-β to Smad2 and Smad3 in metastatic 4T1 cells without affecting total Smad2/3 levels (Fig. 3A). We previously demonstrated that the aberrant interaction of β3 integrin with TβR-II in post-epithelial-mesenchymal transition and malignant MECs is critical for the activation of p38 MAPK by TGF-β (11–13). Fig. 3B shows that p130Cas is not required for the formation of β3 integrin·TβR-II complexes in 4T1 cells, which serves to explain the slightly diminished coupling of TGF-β to p38 MAPK in p130Cas-depleted 4T1 cells (Fig. 3A). Furthermore, p130Cas deficiency not only elicited a significant increase in autocrine-driven SBE-luciferase activity in quiescent 4T1 cells (Fig. 3C), but also significantly augmented their induction of this Smad2/3-responsive reporter gene when stimulated by TGF-β (Fig. 3C). Accordingly, Smad2/3 localized primarily to the cytoplasm in quiescent parental 4T1 cells, as determined by (i) cellular fractionation coupled to Smad2/3 immunoblotting (Fig. 4A), and (ii) indirect Smad2/3 immunofluorescence (Fig. 4, B and C). In stark contrast, Smad2/3 was present in the cytoplasm and nuclear compartments in quiescent 4T1 cells that lacked p130Cas expression (Fig. 4, A–C), a finding consistent with their elevated basal levels of Smad2/3 activity. Moreover, p130Cas deficiency greatly accelerated the rate and extent of Smad2/3 that accumulated in the nucleus following TGF-β stimulation (Fig. 4, A–C). Collectively, these findings are consistent with the notion that aberrant p130Cas expression down-regulates the activity of Smad2/3 in metastatic breast cancer cells.
FIGURE 3.
p130Cas deficiency increases Smad2/3 activity in metastatic MECs. A, quiescent 4T1 cells that expressed either a scrambled (scram) or p130Cas-specific (shCas) shRNA were stimulated with TGF-β1 (5 ng/ml) as indicated, and subsequently immunoblotted with phosphospecific antibodies against Smad3 (pSmad3), Smad2 (pSmad2), or p38 MAPK (p-p38) as shown. Membranes were stripped and reprobed with antibodies against Smad2/3 (tSmad2/3), p38 MAPK (t-p38), β-actin (Actin), and p130Cas as loading controls. B, whole cell extracts prepared from control (scram) or p130Cas-deficient (shCas) 4T1 cells were incubated with β3 integrin antibodies (β3 I.P.), and the resulting immunocomplexes were isolated and immunoblotted for TβR-II. Immunoblotting aliquots of the prepared cell extracts (Input) served to monitor the levels of TβR-II, p130Cas, and β-actin (Actin). Data are representative of three independent experiments and show that p130Cas deficiency does not affect carcinoma-specific formation of β3 integrin·TβR-II complexes. C, control (scram) and p130Cas-deficient (shCas) 4T1 cells were transiently transfected with SBE-luciferase (Renilla), and subsequently stimulated overnight with TGF-β1 (5 ng/ml). NS, no stimulation. Data are the mean ± S.E. (n = 3) of Renilla/firefly activity ratios.
FIGURE 4.
p130Cas deficiency increases Smad2/3 nuclear localization and decreases the proliferation of metastatic MECs. A, quiescent control (scram) or p130Cas-deficient (shCas) 4T1 cells were stimulated with TGF-β1 (5 ng/ml) for the indicated times, and subsequently lysed, fractionated, and immunoblotted for Smad2/3. Membranes were stripped and reprobed with antibodies against E-cadherin (E-Cad) and lamin A/C (Lamin) to monitor the integrity of the cytoplasmic and nuclear preparations, respectively. Data are from a representative experiment that was performed three times with similar results. B, control (scram) and p130Cas-deficient (shCas) 4T1 cells were stimulated as described in A and subsequently fixed and processed for indirect Smad2/3 immunofluorescence, as well as counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize cell nuclei. Arrows indicate the distinct absence or presence of Smad2/3 in 4T1 cell nuclei. Data are representative images from four independent experiments. C, Smad2/3 immunofluorescence data in B was quantified as follows: white bars, nuclear exclusion; light gray bars, cellular diffuse; dark gray bars, weak nuclear; black bars, strong nuclear staining for Smad2/3. Data are from 10 randomly selected fields for each time point obtained in two independent experiments. D, control (scram) and p130Cas-deficient (shCas) 4T1 cells were grown in a three-dimensional organotypic culture for 10 days in the absence or presence of TGF-β1 (5 ng/ml). Representative acini from three independent experiments are shown. E, p130Cas depletion restored the cytostatic response of TGF-β in 4T1 cells, which were grown as in D prior to quantifying acinar size. Data are the mean ± S.E. of 9 randomly selected fields obtained from three independent experiments.
A characteristic phenotype of mammary carcinoma cells, including 4T1 cells, is their resistance to TGF-β-mediated growth arrest when grown in two-dimensional culture systems (12, 34, 35). Interestingly, the resultant increase in Smad2/3 activity elicited by p130Cas deficiency was unable to restore a strong cytostatic response in 4T1 cells upon TGF-β administration (see supplemental Fig. S2A). However, it is known that culturing cells on plastic can mask several cell signaling events, most notably those of TGF-β (31, 36, 37). As such, we propagated control and p130Cas-depleted 4T1 cells in three-dimensional organotypic cultures in the absence or presence of TGF-β1 (Fig. 4D). In addition to restoring a more rounded, normal acinar structure (Fig. 4D), depletion of p130Cas also significantly increased the growth inhibitory effects of TGF-β1 as compared their p130Cas-expressing counterparts (Fig. 4E). Taken together, these findings suggest that p130Cas functions to sequester Smad2/3 in the cytoplasm, thereby inhibiting their activity and the cytostatic function of TGF-β. Our findings also suggest that aberrant expression of p130Cas may elicit profound effects on mammary tumor growth regulated by TGF-β.
p130Cas Deficiency Inhibits Primary Mammary Tumor Growth and Cell Invasion
To further assess the role of p130Cas in TGF-β-mediated tumor progression, we engrafted parental and p130Cas-deficient 4T1 cells onto the mammary fat pads of syngeneic Balb/c mice. Indeed, orthotopic tumors lacking p130Cas clearly grew more slowly as compared with their parental counterparts (Fig. 5A). Consistent with a reduction in 4T1 tumor weights, we also observed p130Cas deficiency to elicit significantly impaired proliferative indices as determined by Ki67 immunohistochemistry of primary tumor sections (Fig. 5B). These data support our in vitro findings (Figs. 2D and 4E) and suggest that measures capable of inactivating p130Cas expression and/or function may provide a novel means to partially restore the tumor suppressive activity of TGF-β.
FIGURE 5.
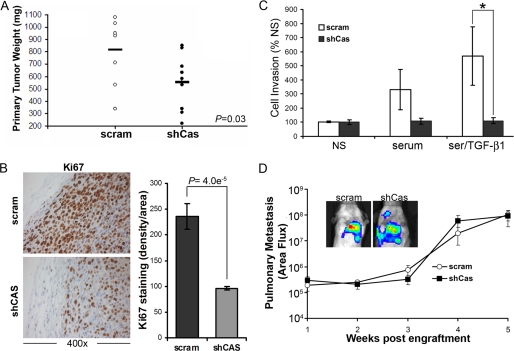
p130Cas deficiency inhibits primary tumor growth and cell invasion. A, control (scram) and p130Cas-deficient (shCas) 4T1 cells were engrafted onto the mammary fat pad of Balb/c mice. Primary tumors were removed surgically 21 days post-engraftment and weighed. Bar shows the mean tumor weights for each group (10 mice/group). p = 0.03. B, histological sections of primary tumors were stained with Ki67 to monitor the proliferative index of control (scram) and p130Cas-deficient (shCas) 4T1 tumors. Staining intensity was quantified over nine fields of view from three separate tumors/group and showed a decrease in the proliferative index at the invasive front of primary tumors upon p130Cas depletion. C, the invasion of control (scram) and p130Cas-deficient (shCas) 4T1 cells through Matrigel was stimulated by 2% fetal bovine serum (serum), or by 2% fetal bovine serum supplemented with TGF-β1 (5 ng/ml; Ser/TGF-β1). Data are the mean ± S.E. invasion to unstimulated MECs (NS) observed in three independent experiments completed in triplicate (*, p < 0.05). D, 4T1 cell derivatives were engrafted onto the mammary fat pads of female Balb/c mice as described in A and pulmonary photon flux readings were determined at the indicated time points post-engraftment. Inset shows representative bioluminescent signals of pulmonary metastases 4 weeks post-engraftment.
Through its inclusion in focal adhesion complexes, p130Cas has also been proposed to play a critical role in mediating cell migration and invasion (15). Indeed, p130Cas deficiency abrogated 4T1 cell invasion induced by TGF-β1 (Fig. 5C); however, this same cellular condition failed to impact the pulmonary metastasis of 4T1 cells engrafted onto the mammary fat pad of Balb/c mice (Fig. 5D). These findings underscore the complexities of carcinoma metastasis in vivo and point to the existence of alternative and TGF-β-independent pathways that can compensate for the loss of cellular invasion normally regulated by p130Cas. Indeed, it is tempting to speculate that pulmonary metastasis of p130Cas-deficient 4T1 cells reflects their maintenance of p38 MAPK activity (Fig. 3A), a signaling pathway in which we demonstrated previously to be necessary for TGF-β stimulation of 4T1 pulmonary metastasis (11).
p130Cas Balances Canonical and Noncanonical TGF-β Signaling
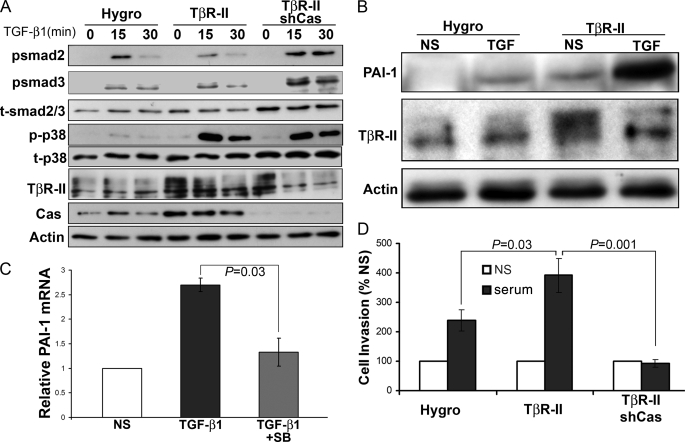
We previously demonstrated that transgenic expression of human TβR-II in 4T1 cells significantly enhances their invasion (12) and pulmonary metastasis (11) in mice. Therefore, we next sought to utilize this model to specifically address the role of p130Cas in mediating TGF-β-driven tumor progression and metastasis. Indeed, transgenic expression of TβR-II dramatically enhanced the coupling of TGF-β to the activation of p38 MAPK, but had little to no affect on Smad2 or Smad3 phosphorylation (Fig. 6A). This shift in TGF-β signaling was reflected by the increased basal and TGF-β-induced expression of the prometastatic factor, plasminogen activator inhibitor-1 (PAI-1; Fig. 6B). Moreover, pharmacological inhibition of p38 MAPK activity significantly impaired the ability of 4T1 cells to up-regulate PAI-1 in response to TGF-β1 (Fig. 6C). Importantly, rendering these “hyperinvasive” 4T1-TβR-II cells deficient in p130Cas had no appreciable effect on their enhanced ability to phosphorylate p38 MAPK in response to TGF-β; however, this same cellular condition did elicit elevated phosphorylation of both Smad2 and Smad3 (Fig. 6A). Thus, diminishing p130Cas expression in 4T1-TβR-II cells restores the physiologic balance between canonical and noncanonical TGF-β signaling (Fig. 6A). In accord with their increased p38 MAPK activity and PAI-1 secretion, 4T1-TβR-II cells are significantly more invasive compared with parental 4T1 cells (Fig. 6C) (11, 12). Importantly, depleting 4T1-TβR-II cells of p130Cas expression abrogated their enhanced invasiveness mediated by TβR-II expression and a serum stimulation (Fig. 6C). Taken together, these findings clearly show that elevated TβR-II expression enhances the coupling of TGF-β to its noncanonical effector, p38 MAPK, leading to augmented PAI-1 expression and cellular invasion. Furthermore, we show for the first time that p130Cas deficiency restores a physiologic balance between canonical and noncanonical TGF-β signaling, and as such, prevents breast cancer cell invasion.
FIGURE 6.
p130Cas balances canonical and noncanonical TGF-β signaling. A, control (Hygro), TβR-II- (TβR-II), or TβR-II-expressing 4T1 cells deficient in p130Cas expression (TβR-II/shCas) were stimulated with TGF-β1 (5 ng/ml) for varying times, and immunoblotted with phospho-specific antibodies against Smad2 (psmad2), Smad3 (psmad3), and p38 MAPK (p-p38) as indicated. Membranes were stripped and reprobed with antibodies against total Smad2/3 (tSmad2/3), p38 MAPK (t-p38), TβR-II, p130Cas (Cas), and β-actin (Actin) as loading controls. Data are from a representative experiment that was performed at least three times with similar results. B, quiescent control (Hygro) and TβR-II-expressing 4T1 cells were stimulated with TGF-β1 (5 ng/ml) for 24 h. NS, no stimulation. The resulting conditioned medium was collected, precipitated, and immunoblotted for PAI-1. The corresponding cell lysates were probed for TβR-II and β-actin (Actin) as loading controls. Data are from a representative experiment that was performed three times with similar results. C, 4T1 cells were stimulated with TGF-β1 (5 ng/ml) for 24 h in the absence or presence of the p38 MAPK inhibitor, SB208530 (10 μm), and analyzed by semi-quantitative reverse transcription-PCR for PAI-1 mRNA. Data are the mean ± S.E. induction of PAI-1 relative to unstimulated MECs (NS) observed in three independent experiments. D, 4T1-TβR-II cell variants described A were induced to invade synthetic basement membranes by 2% fetal bovine serum (serum). Data are the mean ± S.E. invasion relative to unstimulated (NS) controls of three independent experiments completed in triplicate.
p130Cas Deficiency Prevents Early TGF-β-driven Breast Cancer Dissemination
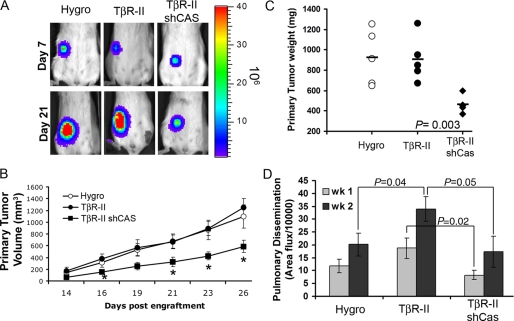
We next sought to utilize the 4T1-TβR-II model to define the specific role of p130Cas in mediating in vivo TGF-β-driven breast cancer progression. Bioluminescent imaging of tumor bearing Balb/c mice showed that parental, TβR-II-, and TβR-II-shCas-expressing 4T1 tumors exhibited similar rates of establishment (Fig. 7A, Day 7). However, their growth rates thereafter diverged rapidly due to the inability of TβR-II-shCas tumors to grow out as efficiently as the parental and TβR-II-expressing control cells (Fig. 7, A and B, Day 21). Importantly, combining TβR-II expression with p130Cas depletion significantly exacerbated (by 10-fold) the growth defects originally observed upon p130Cas depletion in wild-type 4T1 tumors (Fig. 7C). Thus, abrogating p130Cas was sufficient in restoring the tumor suppressing activities of TGF-β.
FIGURE 7.
Coupling TβR-II expression with p130Cas deficiency prevents TGF-β-driven breast cancer metastasis. A, control (Hygro), TβR-II- (TβR-II), and TβR-II-expressing 4T1 cells lacking expression of p130Cas (TβR-II/shCas) were engrafted onto the mammary fat pad of Balb/c mice. Bioluminescent visualization of primary 4T1 tumors showed an equal establishment at day 7 post-engraftment, but a significant growth defect at day 26 in TβR-II-expressing 4T1 tumors lacking p130Cas expression. B, data are the mean ± S.E. of tumor volumes measured for the indicated 4T1 tumor variants. *, p < 0.05, n = 5 mice/group. C, primary 4T1 tumors were removed surgically 26 days post-engraftment and weighed. Bar shows the mean tumor weights for each group (5 mice/group), p = 0.003. D, data are the mean ± S.E. (n = 5 mice/group) of pulmonary photon flux units measured at 1-week intervals following engraftment of the 4T1 variants onto the mammary fat pads.
Finally, we found that TβR-II expression elicited a dramatic increase in the early dissemination of 4T1 tumors to lungs as compared with parental cells (Fig. 7D). Importantly, this TGF-β/TβR-II-driven metastatic process was specifically inhibited by rendering TβR-II-expressing 4T1 cells deficient in p130Cas (Fig. 7D). Taken together, these findings show that p130Cas is critically involved in promoting primary mammary tumor growth, and is specifically required in facilitating early events in TGF-β-driven primary tumor dissemination.
DISCUSSION
TGF-β is a principal player involved in suppressing mammary tumorigenesis, doing so through its ability to maintain the composition of normal MEC microenvironments, and by inhibiting the aberrant proliferation of normal MECs (6, 38). Mammary tumorigenesis has evolved a variety of mechanisms that subvert the tumor suppressing functions of TGF-β, and in doing so, confer oncogenic and metastatic activities upon this multifunctional cytokine (34). Indeed, how TGF-β both suppresses and promotes mammary tumorigenesis remains a fundamental question that directly impacts the ability of science and medicine to effectively target the TGF-β signaling system during the treatment of breast cancer patients. Deciphering this paradox remains the most important question concerning the biological and pathological actions of this multifunctional cytokine (39).
We previously established the importance of aberrant interactions between β3 integrin and TβR-II to promote Src-mediated phosphorylation of TβR-II, which then recruits and binds Grb2. Once bound to phospho-Tyr-284 in TβR-II, Grb2 facilitates TGF-β-mediated activation of noncanonical MAP kinase signaling without affecting the coupling of TGF-β to Smad2/3 (12, 13). Importantly, measures capable of disrupting this signaling axis readily prevent TGF-β from driving breast cancer invasion and metastasis (11, 40). Thus, in addition to establishing the critical importance of p38 MAPK activation in mediating breast cancer metastasis stimulated by TGF-β, these studies also suggested that inappropriate imbalances between canonical and noncanonical TGF-β signaling systems may in fact underlie its prometastatic activities in breast cancer cells. Our findings herein provide the first definitive evidence that (i) canonical and noncanonical signaling imbalances do indeed dictate MEC response to TGF-β, and (ii) p130Cas functions as a novel molecular rheostat that governs the delicate balance between canonical and noncanonical TGF-β effectors. Indeed, overexpression of either full-length or the carboxyl terminus of p130Cas was sufficient to decrease TGF-β-induced Smad2/3 phosphorylation while simultaneously increasing that of p38 MAPK. Moreover, depleting p130Cas significantly increased the activity of Smad2/3 and concomitantly decreased that of p38 MAPK induced by TGF-β, and finally, elevating TβR-II expression amplified the activation of p38 MAPK by TGF-β, which significantly enhanced early metastatic progression of mammary tumors in mice (Fig. 7) (11). In “hypermetastatic” TβR-II-expressing cells, p130Cas deficiency similarly increased the coupling of TGF-β to Smad2/3, an event that negated the proinvasive and prometastatic activities of p38 MAPK in developing 4T1 tumors. Thus, p130Cas functions in balancing the activation status of canonical and noncanonical effectors targeted by TGF-β, findings that are clinically and medically relevant to the development and progression of mammary tumors regulated by TGF-β.
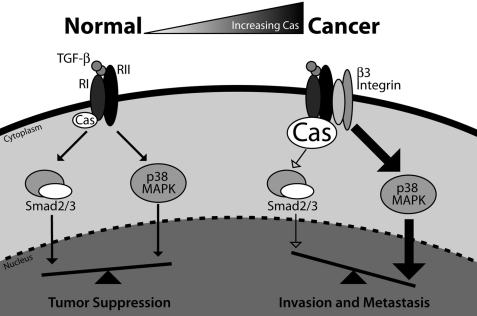
A schematic depicting the function of p130Cas in TGF-β signaling is presented in Fig. 8. Indeed, in normal MECs, TGF-β receptors fail to interact significantly with integrins, which limits TGF-β stimulation of p38 MAPK and the initiation of oncogenic signaling by TGF-β (13, 40–42). The net effect of these signaling events results in tumor suppression by TGF-β. However, during mammary tumorigenesis, p130Cas expression is up-regulated dramatically, as is the aberrant formation of integrin and TGF-β receptor complexes (11–13, 40), which collectively decrease the activity of Smad2/3 and increase that of p38 MAPK and other noncanonical effectors that promote breast cancer metastasis stimulated by TGF-β (11, 40). This signaling imbalance can be potentiated by elevated TβR-II expression and its consequential enhancement of p38 MAPK activation and metastasis (11, 34, 40, 43). In all cases, these various signaling inputs are critically balanced and influenced by the level of p130Cas expression. Indeed, we (see Fig. 1) and others (20) find mammary tumorigenesis to dramatically increase the expression of p130Cas. Based on our findings presented herein, we suggest that this event limits TGF-β stimulation of Smad2/3, which (i) diminishes MEC responsiveness to the cytostatic activities of TGF-β (44); and (ii) promotes amplified coupling of TGF-β to its noncanonical effectors, leading to breast cancer invasion and metastasis. In fact, our findings strongly support the progressive hypothesis that inappropriate imbalances between canonical and noncanonical TGF-β signaling systems underlies the acquisition of metastatic phenotypes in mammary carcinomas, as well as facilitates the oncogenic switch of TGF-β from a tumor suppressor to a prometastatic molecule.
FIGURE 8.
p130Cas functions as a molecular rheostat that maintains the balance between canonical and noncanonical TGF-β signaling. In normal MECs, physiologic expression levels of integrins and p130Cas maintain the balance between Smad2/3 and p38 MAPK signaling, which collectively support the tumor suppressing and cytostatic functions of TGF-β. During breast cancer progression, aberrant up-regulation of p130Cas expression inhibits Smad2/3 activation in a manner that parallels the inappropriate formation of β3 integrin·TβR-II complexes, which promotes increased coupling of TGF-β to p38 MAPK. Overall, these untoward events shift the balance of TGF-β signaling to favor activation of noncanonical effectors, particularly p38 MAPK, during the acquisition of metastatic phenotypes by breast cancer cells. Importantly, rendering late-stage breast cancer cells deficient in p130Cas enhances the activation of Smad2/3 by TGF-β, which thereby restores its ability to suppress the growth and pulmonary metastasis of breast cancer cells in mice.
Along these lines, a recent report suggests that murine mammary tumor virus-driven p130Cas expression in mice is sufficient to induce mammary gland hyperplasia (20). However, it was necessary to combine transgenic p130Cas expression with that of HER2 to enhance formation of mammary tumors (20). Although specific effects on TGF-β activity and signaling were not examined in this mouse model, these findings do suggest that the tumor promoting properties of p130Cas only manifest in the face of additional oncogenic signaling inputs (i.e. elevated HER2 expression), which mirrors our own results showing that heightened TGF-β signaling (TβR-II expression) requires p130Cas to induce pulmonary dissemination. Moreover, we show that transgenic TβR-II expression led to increased basal and TGF-β-induced production of the prometastatic protein, PAI1, without impacting the phosphorylation of Smad2/3. These findings suggest that (i) p130Cas specifically regulates the activity of Smad2/3 as opposed to that of the TGF-β receptors, and (ii) Smad2/3 expression levels, not those of TGF-β receptors, are rate-limiting during the activation of canonical TGF-β signaling. Thus, p130Cas acts as a molecular rheostat of canonical Smad2/3 and noncanonical p38 MAPK signaling stimulated by TGF-β, and disruption of the balance between these two pathways has dramatic affects on breast cancer growth and progression.
In summary, we demonstrated that p130Cas functions to regulate the balance between TGF-β-mediated activation of Smad2/3 and p38 MAPK in normal and metastatic MECs. Moreover, we provide compelling evidence that p130Cas is both necessary and sufficient to drive the oncogenic activities of TGF-β, including its regulation of mammary tumor growth and the initiation of early steps in the metastatic dissemination of breast cancer cells. Collectively, our findings establish p130Cas as an essential mediator that underlies the oncogenic conversion of TGF-β function, thereby enhancing its ability to promote the progression of mammary carcinomas.
Supplementary Material
Acknowledgments
We thank members of the Schiemann Laboratory for critical reading of the manuscript. We also thank Dr. Amy Bouton for providing the p130Cas-CT and -FL cDNA constructs.
This work was supported, in whole or in part, by National Institutes of Health Grant CA129359, Komen Foundation Grant BCTR0706967, Department of Defense Grant BC084651 (to W. P. S.), and American Cancer Society Grant PF-09-120-01-CSM (to M. K. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- TGF-β
- transforming growth factor-β
- FAK
- focal adhesion kinase
- MEC
- mammary epithelial cell
- TβR-I
- TGF-β type I receptor
- TβR-II
- TGF-β type II receptor
- p130Cas
- Crk-associated substrate
- PAI-1
- plasminogen activator inhibitor-1
- NmuMG
- normal murine mammary epithelial
- MAPK
- mitogen-activated protein kinase
- ERK
- extracellular signal-regulated kinase
- JNK
- c-Jun N-terminal kinase
- CMV
- cytomegalovirus
- shRNA
- short hairpin RNA
- β-gal
- β-galactosidase
- FL
- full-length
- CT
- carboxyl-terminal.
REFERENCES
- 1.Yoshida B. A., Sokoloff M. M., Welch D. R., Rinker-Schaeffer C. W. (2000) J. Natl. Cancer Inst. 92, 1717–1730 [DOI] [PubMed] [Google Scholar]
- 2.Wakefield L. M., Piek E., Böttinger E. P. (2001) J. Mammary Gland Biol. Neoplasia 6, 67–82 [DOI] [PubMed] [Google Scholar]
- 3.Buck M. B., Knabbe C. (2006) Ann. N. Y. Acad. Sci. 1089, 119–126 [DOI] [PubMed] [Google Scholar]
- 4.Benson J. R. (2004) Lancet Oncol. 5, 229–239 [DOI] [PubMed] [Google Scholar]
- 5.Chen R. H., Chang T. Y. (1997) Cell Growth Differ. 8, 821–827 [PubMed] [Google Scholar]
- 6.Blobe G. C., Schiemann W. P., Lodish H. F. (2000) N. Engl. J. Med. 342, 1350–1358 [DOI] [PubMed] [Google Scholar]
- 7.Galliher A. J., Neil J. R., Schiemann W. P. (2006) Future Oncol. 2, 743–763 [DOI] [PubMed] [Google Scholar]
- 8.Gomis R. R., Alarcón C., Nadal C., Van Poznak C., Massagué J. (2006) Cancer Cell 10, 203–214 [DOI] [PubMed] [Google Scholar]
- 9.Adorno M., Cordenonsi M., Montagner M., Dupont S., Wong C., Hann B., Solari A., Bobisse S., Rondina M. B., Guzzardo V., Parenti A. R., Rosato A., Bicciato S., Balmain A., Piccolo S. (2009) Cell 137, 87–98 [DOI] [PubMed] [Google Scholar]
- 10.Tian M., Schiemann W. P. (2009) Future Oncol. 5, 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galliher-Beckley A. J., Schiemann W. P. (2008) Carcinogenesis 29, 244–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galliher A. J., Schiemann W. P. (2007) Cancer Res. 67, 3752–3758 [DOI] [PubMed] [Google Scholar]
- 13.Galliher A. J., Schiemann W. P. (2006) Breast Cancer Res. 8, R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlaepfer D. D., Hauck C. R., Sieg D. J. (1999) Prog. Biophys. Mol. Biol. 71, 435–478 [DOI] [PubMed] [Google Scholar]
- 15.Brábek J., Constancio S. S., Shin N. Y., Pozzi A., Weaver A. M., Hanks S. K. (2004) Oncogene 23, 7406–7415 [DOI] [PubMed] [Google Scholar]
- 16.Geiger B. (2006) Cell 127, 879–881 [DOI] [PubMed] [Google Scholar]
- 17.Honda H., Oda H., Nakamoto T., Honda Z., Sakai R., Suzuki T., Saito T., Nakamura K., Nakao K., Ishikawa T., Katsuki M., Yazaki Y., Hirai H. (1998) Nat. Genet. 19, 361–365 [DOI] [PubMed] [Google Scholar]
- 18.van der Flier S., Chan C. M., Brinkman A., Smid M., Johnston S. R., Dorssers L. C., Dowsett M. (2000) Int. J. Cancer 89, 465–468 [DOI] [PubMed] [Google Scholar]
- 19.Ta H. Q., Thomas K. S., Schrecengost R. S., Bouton A. H. (2008) Cancer Res. 68, 8796–8804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabodi S., Tinnirello A., Di Stefano P., Bisarò B., Ambrosino E., Castellano I., Sapino A., Arisio R., Cavallo F., Forni G., Glukhova M., Silengo L., Altruda F., Turco E., Tarone G., Defilippi P. (2006) Cancer Res. 66, 4672–4680 [DOI] [PubMed] [Google Scholar]
- 21.Kim W., Seok Kang Y., Soo Kim J., Shin N. Y., Hanks S. K., Song W. K. (2008) Mol. Biol. Cell 19, 2135–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wendt M. K., Cooper A. N., Dwinell M. B. (2008) Oncogene 27, 1461–1471 [DOI] [PubMed] [Google Scholar]
- 23.Neil J. R., Johnson K. M., Nemenoff R. A., Schiemann W. P. (2008) Carcinogenesis 29, 2227–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santner S. J., Dawson P. J., Tait L., Soule H. D., Eliason J., Mohamed A. N., Wolman S. R., Heppner G. H., Miller F. R. (2001) Breast Cancer Res. Treat. 65, 101–110 [DOI] [PubMed] [Google Scholar]
- 25.Dawson P. J., Wolman S. R., Tait L., Heppner G. H., Miller F. R. (1996) Am. J. Pathol. 148, 313–319 [PMC free article] [PubMed] [Google Scholar]
- 26.Aslakson C. J., Miller F. R. (1992) Cancer Res. 52, 1399–1405 [PubMed] [Google Scholar]
- 27.Jin W., Yun C., Kwak M. K., Kim T. A., Kim S. J. (2007) Oncogene 26, 7684–7691 [DOI] [PubMed] [Google Scholar]
- 28.Ingman W. V., Robertson S. A. (2008) Biol. Reprod. 79, 711–717 [DOI] [PubMed] [Google Scholar]
- 29.Nelson C. M., Vanduijn M. M., Inman J. L., Fletcher D. A., Bissell M. J. (2006) Science 314, 298–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silberstein G. B., Daniel C. W. (1987) Science 237, 291–293 [DOI] [PubMed] [Google Scholar]
- 31.Weaver V. M., Fischer A. H., Peterson O. W., Bissell M. J. (1996) Biochem. Cell Biol. 74, 833–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin X., Liang M., Feng X. H. (2000) J. Biol. Chem. 275, 36818–36822 [DOI] [PubMed] [Google Scholar]
- 33.Brown K. A., Pietenpol J. A., Moses H. L. (2007) J. Cell. Biochem. 101, 9–33 [DOI] [PubMed] [Google Scholar]
- 34.Tang B., Vu M., Booker T., Santner S. J., Miller F. R., Anver M. R., Wakefield L. M. (2003) J. Clin. Invest. 112, 1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nam J. S., Terabe M., Mamura M., Kang M. J., Chae H., Stuelten C., Kohn E., Tang B., Sabzevari H., Anver M. R., Lawrence S., Danielpour D., Lonning S., Berzofsky J. A., Wakefield L. M. (2008) Cancer Res. 68, 3835–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenny P. A., Lee G. Y., Myers C. A., Neve R. M., Semeiks J. R., Spellman P. T., Lorenz K., Lee E. H., Barcellos-Hoff M. H., Petersen O. W., Gray J. W., Bissell M. J. (2007) Mol. Oncol. 1, 84–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mani S. A., Guo W., Liao M. J., Eaton E. N., Ayyanan A., Zhou A. Y., Brooks M., Reinhard F., Zhang C. C., Shipitsin M., Campbell L. L., Polyak K., Brisken C., Yang J., Weinberg R. A. (2008) Cell 133, 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegel P. M., Massagué J. (2003) Nat. Rev. Cancer 3, 807–821 [DOI] [PubMed] [Google Scholar]
- 39.Schiemann W. P. (2007) Expert Rev. Anticancer Ther. 7, 609–611 [DOI] [PubMed] [Google Scholar]
- 40.Wendt M. K., Schiemann W. P. (2009) Breast Cancer Res. 11, R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamashita M., Fatyol K., Jin C., Wang X., Liu Z., Zhang Y. E. (2008) Mol. Cell 31, 918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorrentino A., Thakur N., Grimsby S., Marcusson A., von Bulow V., Schuster N., Zhang S., Heldin C. H., Landström M. (2008) Nat. Cell Biol. 10, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 43.Muraoka R. S., Dumont N., Ritter C. A., Dugger T. C., Brantley D. M., Chen J., Easterly E., Roebuck L. R., Ryan S., Gotwals P. J., Koteliansky V., Arteaga C. L. (2002) J. Clin. Invest. 109, 1551–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X., Sun Y., Constantinescu S. N., Karam E., Weinberg R. A., Lodish H. F. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 10669–10674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.