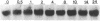Abstract
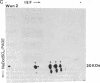
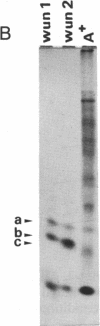
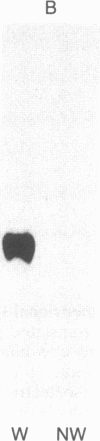
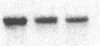
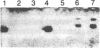
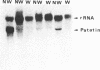
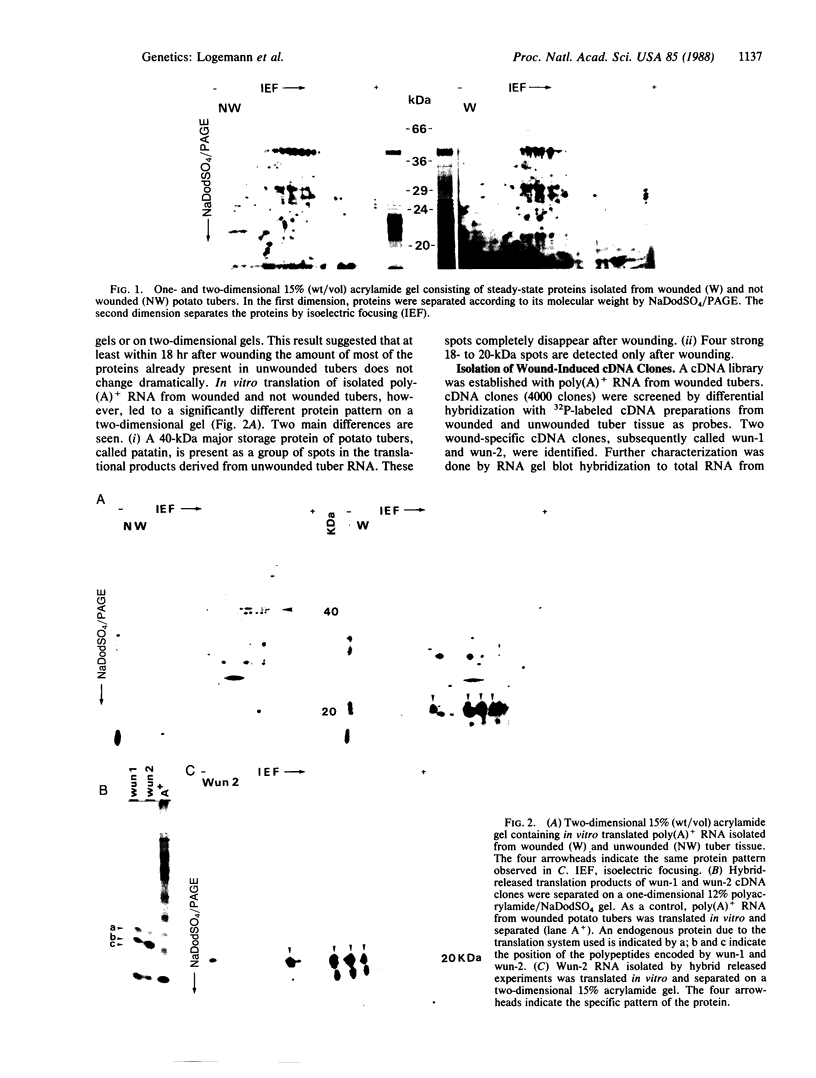
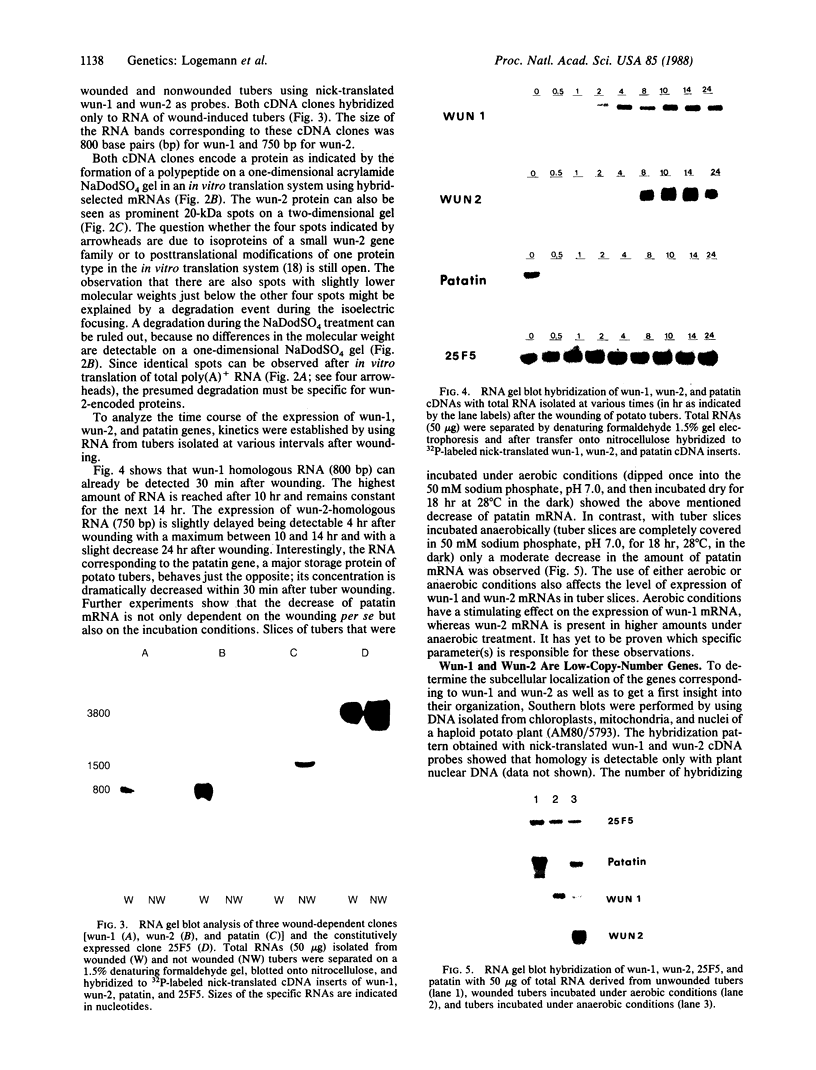
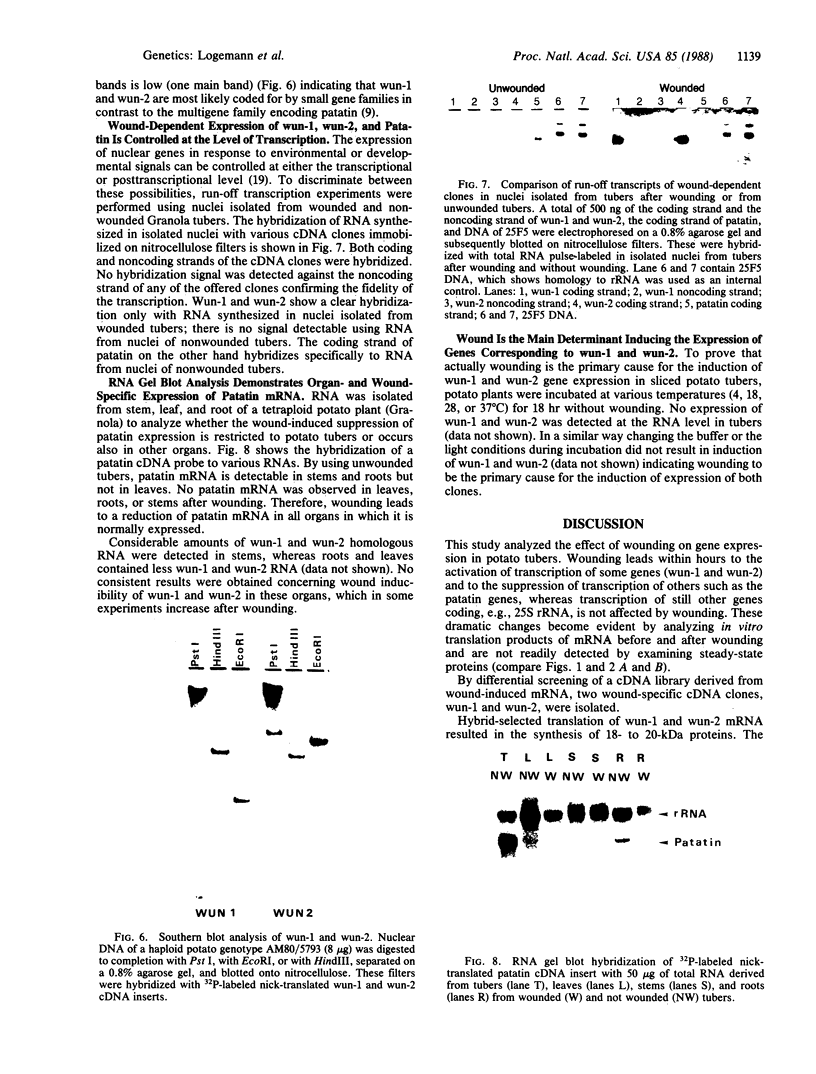
Tubers of a common tetraploid species of Solanum tuberosum (Granola) were mechanically wounded by slicing. After 18 hr only small differences were detectable in the pattern of the steady-state protein extracted from wounded versus unwounded tubers. In contrast the protein pattern obtained by in vitro translation of mRNA isolated from wounded and unwounded tubers differed significantly. A cDNA library was established and screened for wound-induced cDNA clones by differential hybridization. Two clones, wun-1 and wun-2, were found that corresponded to genes that were highly expressed in wounded potato tubers but were not expressed in unwounded tubers. The expression of the gene corresponding to wun-1 is detectable 30 min after wounding; the expression of the gene corresponding to wun-2 is detectable 4 hr after wounding. The expression of both genes (hereafter referred to as wun-1 and wun-2) remains constant for up to 24 hr after wounding. Interestingly the RNA corresponding to patatin, a major storage protein of potato tubers, behaves in the opposite way; it decreases dramatically in tubers within 30 min after wounding. The low level of patatin mRNA observed in unwounded roots and stems also disappears after wounding. Run-off transcription experiments, performed with isolated nuclei, indicate that the activation of the wound-induced genes as well as the inhibition of the patatin gene are controlled at the transcriptional level.
Keywords: patatin, gene regulation, two-dimensional gel electrophoresis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borchert R. Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol. 1978 Nov;62(5):789–793. doi: 10.1104/pp.62.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman H., Meincke G., Havsteen B. Microheterogeneity of Immunoglobulin G from plasmocytomas. Identification of two types of IgG by isoelectric focusing. Z Immunitatsforsch Exp Klin Immunol. 1975 Oct;150(4):370–377. [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L. H., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. II. The cDNA-deduced primary structure of pre-inhibitor II. J Biol Chem. 1985 Jun 10;260(11):6561–6564. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gustafson G., Ryan C. A. Specificity of protein turnover in tomato leaves. Accumulation of proteinase inhibitors, induced with the wound hormone, PIIF. J Biol Chem. 1976 Nov 25;251(22):7004–7010. [PubMed] [Google Scholar]
- Kamalay J. C., Goldberg R. B. Organ-specific nuclear RNAs in tobacco. Proc Natl Acad Sci U S A. 1984 May;81(9):2801–2805. doi: 10.1073/pnas.81.9.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Pikaard C. S., Brusca J. S., Hannapel D. J., Park W. D. The two classes of genes for the major potato tuber protein, patatin, are differentially expressed in tubers and roots. Nucleic Acids Res. 1987 Mar 11;15(5):1979–1994. doi: 10.1093/nar/15.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner D., Chemla Y., Herzberg M. Isolation of poly(A)+ RNA by paper affinity chromatography. Anal Biochem. 1984 Sep;141(2):329–336. doi: 10.1016/0003-2697(84)90050-2. [DOI] [PubMed] [Google Scholar]
- Willemot C., Stumpf P. K. Fat metabolism in higher plants. XXXIV. Development of fatty acid synthetase as a function of protein synthesis in aging potato tuber slices. Plant Physiol. 1967 Mar;42(3):391–397. doi: 10.1104/pp.42.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Schmalenbach W., Schell J. Transcription of T-DNA in octopine and nopaline crown gall tumours is inhibited by low concentrations of alpha-amanitin. Nucleic Acids Res. 1981 Oct 10;9(19):4801–4812. doi: 10.1093/nar/9.19.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]