Abstract
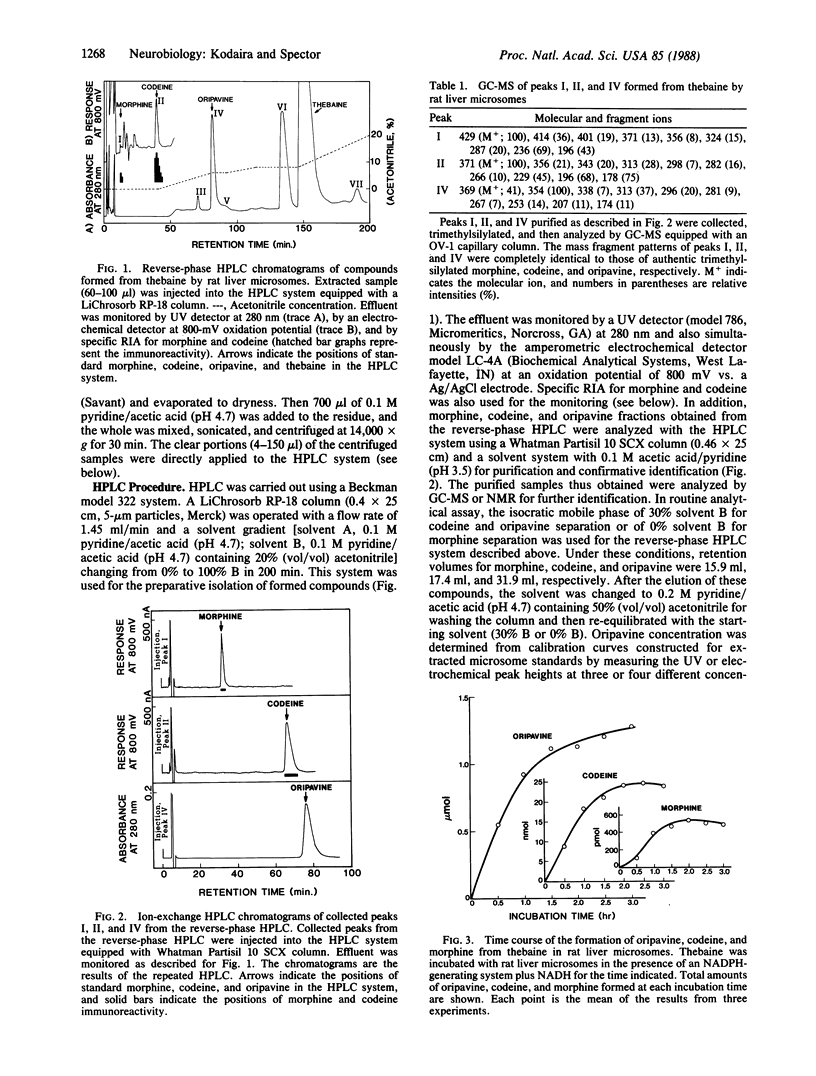
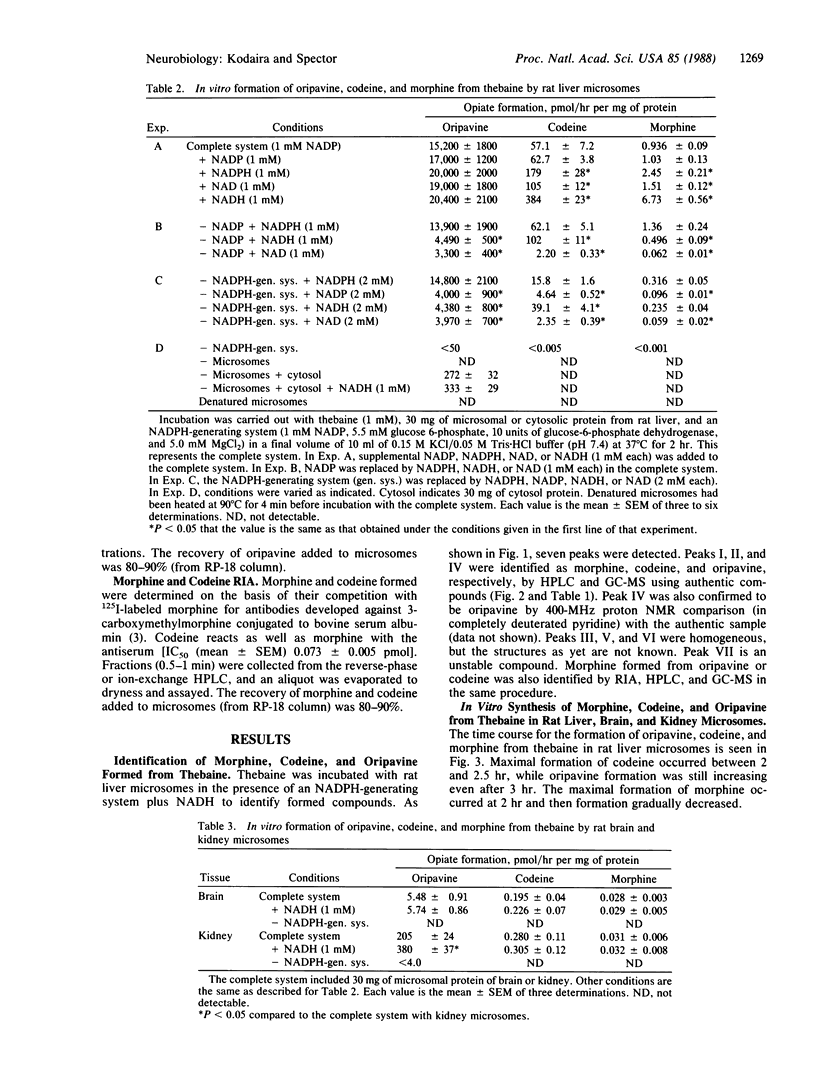
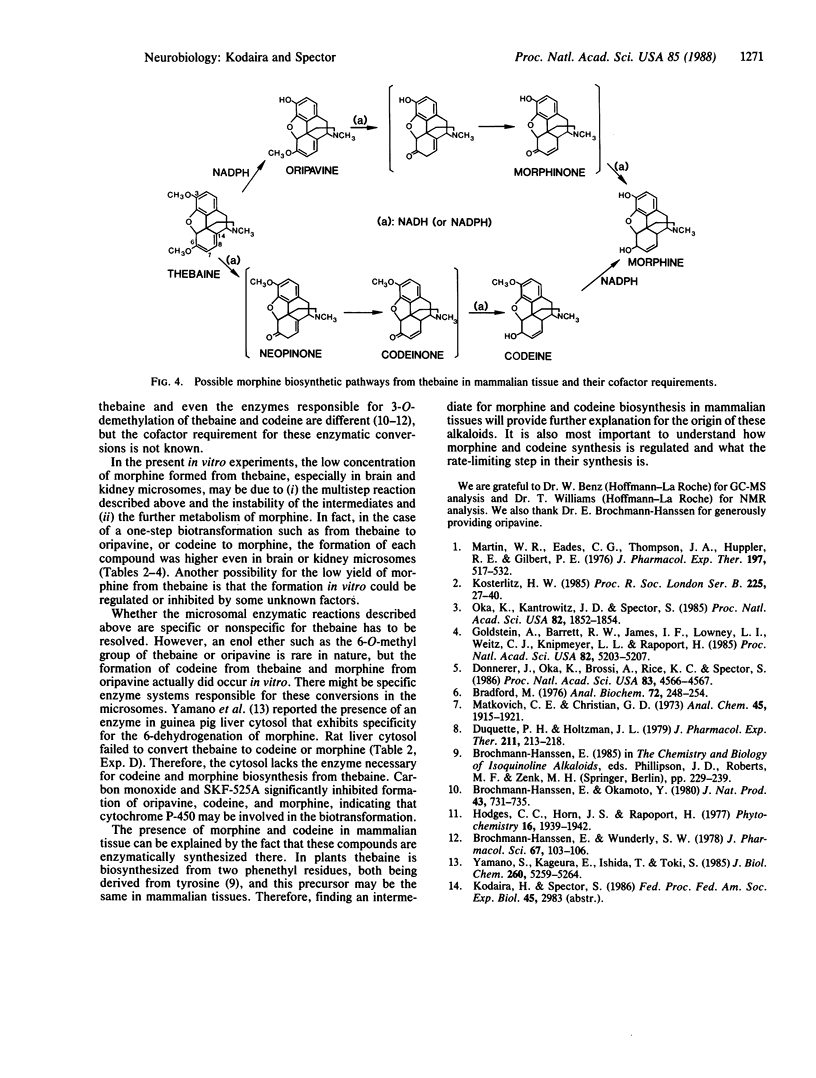
Thebaine, an intermediate of morphine biosynthesis in the poppy plant, Papaver somniferum, was transformed to oripavine, codeine, and morphine by rat liver, kidney, and brain microsomes in the presence of an NADPH-generating system. The formation of morphine, codeine, and oripavine was identified by a specific RIA, HPLC, and GCMS. Thebaine also gave rise to four other compounds, which for the moment are unidentified. NADH dramatically increased the formation of both codeine and morphine when used together with an NADPH-generating system, especially in liver microsomes. NADPH is essential in the formation of oripavine from thebaine and morphine from codeine, while NADH is critical in the conversion of thebaine to codeine and from oripavine to morphine. Carbon monoxide or SKF 525A inhibited the conversion, indicating a role of cytochrome P-450. These results provide evidence for the enzymatic in vitro conversion by mammalian tissues of thebaine to morphine. The pathway is similar to that which exists in plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brochmann-Hanssen E., Wunderly S. W. Biosynthesis of morphine alkaloids in Papaver bracteatum Lindl. J Pharm Sci. 1978 Jan;67(1):103–106. doi: 10.1002/jps.2600670126. [DOI] [PubMed] [Google Scholar]
- Donnerer J., Oka K., Brossi A., Rice K. C., Spector S. Presence and formation of codeine and morphine in the rat. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4566–4567. doi: 10.1073/pnas.83.12.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette P. H., Holtzman J. L. Studies on the N-demethylation and O-de-ethylation of ethylmorphine[6-3H] by male rat hepatic microsomes. J Pharmacol Exp Ther. 1979 Oct;211(1):213–218. [PubMed] [Google Scholar]
- Goldstein A., Barrett R. W., James I. F., Lowney L. I., Weitz C. J., Knipmeyer L. L., Rapoport H. Morphine and other opiates from beef brain and adrenal. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5203–5207. doi: 10.1073/pnas.82.15.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W. The Wellcome Foundation lecture, 1982. Opioid peptides and their receptors. Proc R Soc Lond B Biol Sci. 1985 Jul 22;225(1238):27–40. doi: 10.1098/rspb.1985.0048. [DOI] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- Oka K., Kantrowitz J. D., Spector S. Isolation of morphine from toad skin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1852–1854. doi: 10.1073/pnas.82.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano S., Kageura E., Ishida T., Toki S. Purification and characterization of guinea pig liver morphine 6-dehydrogenase. J Biol Chem. 1985 May 10;260(9):5259–5264. [PubMed] [Google Scholar]


