Abstract
Testosterone acting through the androgen receptor (AR) maintains the arrest of spermatogonial differentiation in juvenile spermatogonial depletion (jsd mutation in the Utp14b gene) mutant adult male mice. It is not known which of the somatic cell types expressing AR mediates this inhibition. To determine if Sertoli cells are responsible, we selectively eliminated AR in Sertoli cells in jsd mice containing a floxed-Ar gene and an anti-Müllerian hormone-Cre transgene. In these Sertoli AR-knockout (SCARKO)-jsd mice, spermatogonial differentiation did not recover. However, the normal organization of Sertoli cell nuclei was drastically disrupted in SCARKO-jsd mice, compared to SCARKO or jsd mice. In addition, the extent of ectoplasmic specializations was reduced, tight junctions were not found, vinculin, an anchoring protein found in ectoplasmic specializations, became uniformly distributed in the cytoplasm, and the adult Sertoli cells showed excess heterochromatin subjacent to their nuclear envelope. Despite the abnormalities in Sertoli cells in SCARKO-jsd mice, global suppression of testosterone action and levels was still effective in restoring the differentiated germ cells, and this was accompanied by an improved arrangement of Sertoli cell nuclei. We conclude that Sertoli cells are not targets for the testosterone-mediated inhibition of spermatogonial differentiation in jsd mice, and both AR in Sertoli cells and the presence of differentiated germ cells contribute to maintaining the organization of Sertoli cells within the seminiferous tubules.
Keywords: Testis, Spermatogonial differentiation, jsd, Azoospermia, AR, Vinculin
Introduction
Juvenile spermatogonial depletion (jsd) mice are spontaneous mutants (Beamer et al, 1988) in the Utp14b gene (Rohozinski and Bishop, 2004; Bradley et al, 2004). While homozygous females are fertile, the adult male homozygous jsd/jsd (“jsd” is used to indicate the Utp14bjsd mutation throughout the paper) mice (referred to as jsd mice) are sterile, which is their only phenotypic defect. In these males, there is a progressive loss of germ cells because the spermatogonial differentiation becomes arrested after the first several waves of spermatogenesis (Kojima et al, 1997). The only germ cells found in most seminiferous tubules of adult jsd mice are undifferentiated type A spermatogonia, which are able to proliferate, but instead of differentiating, they die through apoptosis (de Rooij et al, 1999). Spermatogonial transplantation experiments have shown that this arrest of spermatogonial differentiation is a results of intrinsic defects in spermatogonia (Boettger-Tong et al, 2000; Ohta et al, 2001) and gene expression studies showed that the Utp14b gene is almost exclusively expressed in germ cells, whereas a potentially compensating gene is more actively expressed in somatic cells(Zhao et al, 2007).
Previous studies (Matsumiya et al, 1999; Tohda et al, 2001) have demonstrated that suppression of testosterone by gonadotropin-releasing hormone (GnRH) analogs reverses the block of spermatogonial differentiation and consequently stimulates the recovery of spermatogenesis to the spermatocyte or early spermatid stage in jsd mice. Further studies (Shetty et al, 2001; Shetty et al, 2006) suggested that relatively high testosterone levels are required to maintain the arrest of spermatogonial differentiation in jsd mice and proved that the testosterone action is mediated by the androgen receptor (AR).
In the wild-type mouse testis, AR is localized to the somatic cells, including Sertoli, Leydig, peritubular myoid, and blood vessel smooth muscle, but is not expressed in germ cells (Zhou et al, 2002). The AR expression pattern in jsd mice has been shown to be the same as in wild-type testis (Meistrich et al, 2005). Thus, despite the fact that the primary defect in the jsd mice is in the germ cells, the action of testosterone in maintaining the block of spermatogonial differentiation in jsd mice would be mediated by AR in one or more types of somatic cells. The inhibition of spermatogonial differentiation by testosterone in irradiated rats (Kangasniemi et al, 1996) has also been shown to be due to damage to the somatic environment in the testis (Zhang et al, 2007). Recognition of the target cell responsible for the arrest of spermatogonial differentiation in jsd mice will be of importance in elucidation of the mechanism by which hormones mediate this arrest. In addition, the results may be applicable to reversal of toxicant-induced blocks in spermatogonial differentiation in rats, as well as for treatment of human oligospermia/azoospermia since transient suppression of intratesticular testosterone levels has been reported to facilitate sperm count recovery in human (Charny and Gordon, 1978).
Androgens and AR are absolutely essential for maintenance of spermatogenesis in normal testes. Global loss of functional AR leads to a testicular feminized phenotype (Lyon and Hawkes, 1970). In order to determine the relative roles of AR in different cell types in testis, it was necessary to develop cell-specific AR knockout mice. When Ar was ablated in Sertoli cells, germ cell development stopped at the spermatocyte (Chang et al, 2004; De Gendt et al, 2004) or early spermatid (Holdcraft and Braun, 2004) stages, suggesting that the AR in the Sertoli cells plays a critical role in maintaining the Sertoli cell's ability to support normal spermatogenesis. In contrast, deletion of Ar in peritubular cells had only small quantitative effects on spermatogenesis (Zhang et al, 2006) and germ cell Ar deletion had no effect on germ cell development (Tsai et al, 2006). Although the deletion of Ar in Leydig cells stopped germ cell development at the round spermatid stage (Xu et al, 2007), this effect of absence of AR in Leydig cells may only be a result of the low testosterone levels and not a result of absence of other Leydig cell androgen-dependent factors. Thus, the Sertoli cell appears to be the primary testicular cell that mediates androgen support of normal spermatogenesis.
In order to determine whether AR in Sertoli cells is also the target for testosterone inhibition of spermatogonial differentiation in jsd mice, we eliminated AR selectively in Sertoli cells (Sertoli cell AR knock-out, SCARKO) in jsd mouse testis by using a Cre-lox conditional knockout strategy (De Gendt et al, 2004; Holdcraft and Braun, 2004). There was no recovery of spermatogenesis in jsd mice lacking AR in their Sertoli cells, demonstrating that the Sertoli cell is not the target cell that mediates the androgen-dependent block of spermatogonial differentiation. In addition, we noted several abnormalities in Sertoli cells of SCARKO-jsd mice, particularly in the positioning of their nuclei, which were much more dramatic than those observed in either SCARKO or jsd mice.
Methods
Animals
Transgenic mice with floxed-Ar (Arflox) on a 129/Sv genetic background were obtained from the Catholic University of Leuven, Belgium (De Gendt et al, 2004). Both males and females were crossed with mice carrying the jsd mutation on a C3H-B6-129 (HB129) mixed background (Bolden-Tiller et al, 2007), and the offspring were intercrossed for several generations to generate Arflox and jsd homozygous double-mutant females (Arflox/flox, Utp14bjsd/jsd). C57BL/6 mice expressing Cre recombinase under the control of the anti-Mullerian hormone (Amh) promoter (Amh-Cre mice, Cre+/-) were obtained from the University of Washington (Holdcraft and Braun, 2004). Both males and females (Cre+/-, Utp14b+/+) were also crossed with mice carrying the jsd mutation for one or more generations to generate double-mutant males that were heterozygous for both Amh-Cre and jsd (Cre+/-, Utp14b+ /jsd). The Arflox/flox, Utp14bjsd /jsd females were then mated with Cre+/-, Utp14bjsd/+ males to produce the triple-mutant test model males (Arflox/Y, Cre+/-, Utp14bjsd/jsd, SCARKO-jsd). Since Amh is specifically expressed in the Sertoli cells in the testis, the Cre recombinase is expected to be expressed only in Sertoli cells and recombine the floxed sites in the Ar gene, leading to inactivation of the Ar gene selectively in Sertoli cells (Holdcraft and Braun, 2004). The wild-type, jsd, and SCARKO controls were chosen from littermates of the test models. The wild-type and jsd controls carried Arflox, and some of the wild-type and SCARKO controls were jsd heterozygotes. PCR genotyping of tail DNA was used to identify mice with Arflox (De Gendt et al, 2004), Amh-Cre transgenes (Primers: forward: 5 ’-tggtttcccgcagaacctgaag- 3’, reverse: 5’-gagcctgttttgcacgttcacc-3’), and Utp14bjsd mutants (Shetty et al, 2001). All animal studies were approved by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee.
Hormone and Hormone Antagonist Treatment
The hormone and hormone analogues were given as previously described (Shetty et al, 2006). Briefly, GnRH antagonist, acyline (obtained from the Contraceptive Development Branch of NICHD, North Bethesda, MD), was subcutaneously injected twice, at doses of 20 and 10 mg/kg-body weight at 8 and 10 weeks of age, respectively, and the mice were killed at 12 weeks of age. For treatment with flutamide (an AR antagonist) or testosterone, two 2-cm Silastic capsules (Dow Corning, Midland, MI), filled with flutamide (Sigma, St. Louis, MO), or one 2-cm capsule filled with testosterone (Sigma, St. Louis, MO) were implanted subcutaneously at 8 weeks of age and left in place until the mice were killed 4 weeks later.
Testicular Histology
After the mice were killed, testes were removed and fixed in Bouin's solution and embedded in methacrylate. The blocks were sectioned and stained either with hematoxylin only or hematoxylin and periodic acid Schiff's (PAS) reagent.
The recovery of spermatogenesis was evaluated by the tubule differentiation index (TDI) as previously described (Shetty et al, 2001). The TDI is defined as the percentage of tubules that contain three or more differentiating germ cells at the B spermatogonial stage or beyond.
For scoring the abnormal Sertoli cell nuclear organization, the tubules were placed into one of four categories: Tubules with all the Sertoli cell nuclei aligned along the basement membrane (normal tubules); tubules with a few (less than 5) Sertoli cell nuclei displaced from the basement membrane; tubules with 5 or more randomly positioned Sertoli cell nuclei displaced from the basement membrane; tubules with a cluster containing at least 5 (usually degenerating) Sertoli cell nuclei.
Immunostaining and confocal microscopy
Testes were removed and fixed in 4% paraformaldehyde for up to 24 hours. The fixed tissues were embedded in paraffin, and sectioned, and the sections were deparaffinized and subjected to antigen retrieval.
Sections were sequentially incubated with AR antibody (PG21, (Prins et al, 1991)) at a 1:200 dilution overnight at 4°C, biotinylated anti-rabbit IgG, and then with an avidin-biotin-peroxidase complex reagent (Vectastain Elite kit, Vector Laboratories, Burlingame, CA). The staining was developed by incubation with the peroxidase substrate diaminobenzidine (DAB) (Vector Laboratories, Burlingame, CA). The slides were counterstained with hematoxylin.
For immunofluorescent staining of vinculin and vimentin, the sections were incubated with monoclonal, mouse anti-human antibodies either to vimentin (1:200 dilution) or to vinculin (1:300 dilution) (Sigma, St. Louis, MO) overnight at 4°C. After washing in PBS, the slides were then incubated with Alexa Fluor 594-conjugated goat anti-mouse antiserum (IgM, 1:200, Moleculer Probes, Carlsbad, CA) for vimentin or Alexa Fluor 488-conjugated goat anti-mouse (IgG, 1:200, Molecular Probes, Carlsbad, CA) for vinculin. The testis sections were examined using a confocal microscopy system (LSM 510, Carl Zeiss, Oberkochen, Germany).
Real-time RT-PCR
Testis samples were collected from jsd and SCARKO-jsd mice (n = 4 for each group) and total RNA was extracted using RNeasy Mini kit (Qiagen, Valencia, CA), with DNase I treatment to digest genomic DNA. The cDNA was generated with a Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Sciences, Indianapolis, IN). Quantitative real-time PCR of the cDNA was performed using the Rotor-Gene 3000 thermocycler (Corbett Research, Sydney, Australia) and SYBR Green (JumpStart Taq Ready Mix, Sigma, St.Louis, MO) detection to determine the differential expression of the Rhox5 gene. The housekeeping gene, beta-actin, was used to normalize concentration values for each sample. The following primers were used: Rhox5: forward 5’- ggcccaagctcagaatc -3, reverse 5’- ctgaataggatcaatgatgaag -3’; beta actin: forward 5’- tgacaggatgcagaaggagat -3’, reverse 5’- tactcctgcttgctgatccac -3’. All samples were run in triplicate.
Electron Microscopy
Tissues were prepared as described previously (Chiarini-Garcia and Russell, 2001). Briefly, whole-body perfusion of mice was performed through the right atrium with 5% glutaraldehyde in 0.05 M cacodylate buffer for 15-20 minutes. Collected testes were fixed in glutaraldehyde overnight, and then stored in cacodylate buffer. After overnight postfixation with 1% buffered osmium tetroxide and 1.25% potassium ferrocyanide, samples were dehydrated and embedded in Araldite 502 medium. Ultrathin sections were cut and stained with uranyl acetate and lead citrate and examined by transmission electron microscopy.
Statistics
All quantitative data were represented as arithmetic means ± SEM. ANOVA and subsequent Student-Newman-Keuls post-hoc analysis for pairwise comparisons (Table 1, Table 2), and independent-samples t-tests (Figure 3) were performed to determine the significance of differences (P<0.05) between groups using the SPSS v 12.0 statistical software package (SPSS, Inc, Chicago, IL).
Table 1.
Testis and seminal vesicle weights of wild-type and various transgenic mice and effects of GnRH-antagonist and flutamide or testosterone treatment*
| Groups | Mouse genotype (Ar, Amh-Cre, Utp 14b) | Hormone treatment | Testis weight (mg) | Seminal Vesicle weight (mg) |
|---|---|---|---|---|
| Wild type | Arflox/Y, −/−, Utp 14b+/+ or Utp14bjsd/+ | None | 105.9 ± 3.1a,b | 294 ± 33 |
| SCARKO | Arflox/Y, −/Cre, Utp 14b+/+ or Utp14bjsd/+ | None | 25.9 ± 0.8 | 307 ± 34 |
| Jsd | Arflox/Y, −/−, Utp 14bjsd/jsd | None | 19.5 ± 0.5 | 302 ± 16 |
| SCARKO-jsd | Arflox/Y, -/Cre, Utp 14bjsd/jsd | None | 17.2 ± 0.6a | 286 ±16 |
| Jsd | Arflox/Y, −/−, Utp14bjsd/jsd | GnRH-antagonist + Flutamide | 11.4 ± 0.4c | 38 ± 5c |
| SCARKO-jsd | Arflox/Y, -/Cre, Utp 14bjsd/jsd | 10.8 ± 0.6c | 53 ± 7c | |
| Jsd | Arflox/Y, −/−, Utp14bjsd/jsd | GnRH-antagonist + Testosterone | 13.3 ± 0.4c | 385 ± 42c,d |
| SCARKO-jsd | Arflox/Y, -/Cre, Utp 14bjsd/jsd | 13.2 ± 0.5c | 399 ± 24c,d |
There were between 5 and 12 mice in each group
Untreated mice were significantly different from untreated SCARKO mice
Untreated mice were significantly different from untreated jsd mice
Treated mice were significantly different from untreated mice of same genotype
Mice treated with GnRH-antagonist + testosterone were significantly different from mice of same genotype treated with GnRH-antagonist + flutamide
Table 2.
Spermatogonial differentiation in testes of jsd and SCARKO-jsd mice as measured by the tubule differentiation index* (TDI) and the effects of hormone treatment on the TDI
| No treatment | GnRH-antagonist + Flutamide | GnRH-antagonist + Testosterone | |
|---|---|---|---|
| Jsd | 6.3 ± 4.8 | 34.1 ± 9.8a | 4.3 ± 0.6b |
| SCARKO-jsd | 7.0 ± 3.3 | 55.3 ± 7.7a | 5.9 ± 2.1b |
The TDI is defined as the percentage of tubules that contain three or more differentiating germ cells at the B spermatogonial stage or beyond.
Treated mice were significantly different from untreated mice of same genotype
Mice treated with GnRH-antagonist + testosterone were significantly different from mice of same genotype treated with GnRH-antagonist + Flutamide
Figure 3.
Disruption of Sertoli cell nuclear localization in testes of SCARKO-jsd mice. Localization of Sertoli cell nuclei was evaluated in cross sections of testes in SCARKO, jsd, and SCARKO-jsd mice. A Sertoli cluster is defined as containing more than five nuclei. a and b indicate values for SCARKO-jsd mice that are significantly different from SCARKO and jsd, respectively. Error bars represent MSE.
Results
Characteristics of jsd mice with Ar deletion in Sertoli cells
The body weights of wild-type, SCARKO, jsd, and SCARKO-jsd mice were comparable (data not shown). The seminal vesicle weights were not significantly different among wild-type, SCARKO, jsd, and SCARKO-jsd mice (Table 1), indicating that circulating androgen levels were similar. The testis weights of SCARKO and jsd mice were both markedly reduced to 26% and 18%, respectively, of that of wild-type mice (Table 1). The testis weights of SCARKO-jsd mice were even lower than both those of jsd (P=0.054) and of SCARKO (P<0.05) mice.
To determine whether AR was selectively ablated in Sertoli cells in SCARKO-jsd mice, we examined testicular AR expression by immunohistochemical staining and compared it with that in wild-type, jsd, and SCARKO littermates. In wild-type and jsd mice (Figure 1 A, C), AR was detected in Sertoli cells, myoid peritubular cells, vascular smooth muscle cells, and Leydig cells. In SCARKO and SCARKO-jsd mice (Figure 1 B, D), while AR staining was still positive in the other testicular somatic cells, AR expression was eliminated in 99.5% of Sertoli cells of SCARKO-jsd mice.
Figure 1.
Immunohistochemical staining (brown) of androgen receptor in testes of wild-type (A), SCARKO (B), jsd (C), and SCARKO-jsd (D) mice. The sections were counterstained with hematoxylin (blue). GC, germ cells. SC, Sertoli cells. LC, Leydig cells. PMC, peritubular myoid cells. SMC, smooth muscle cells. Bars, 10 μm.
To confirm that AR function was absent in the Sertoli cells of SCARKO-jsd mice, the mRNA levels of Rhox5, a Sertoli cell-specific gene that is regulated by AR (Rao et al, 2003), were measured by real-time RT-PCR. The level of Rhox5 transcripts normalized to beta-actin in SCARKO-jsd testes was 0.034 ± 0.016 times the level of that in jsd testes, indicating a successful knock-out of AR function in the Sertoli cells.
Spermatogonial Differentiation in SCARKO-jsd Mice
If AR in Sertoli cells mediated the effect of testosterone on inhibition of spermatogonial differentiation in jsd mice, selective ablation of AR in Sertoli cells of jsd mice should reverse this inhibition. On other hand, if AR in Sertoli cells is not the mediator, spermatogonial differentiation should be blocked in SCARKO-jsd mice to the same extent as in jsd mice. The testes of SCARKO-jsd mice (Figure 2 B) contained type A spermatogonia, but almost no type B spermatogonia or spermatocytes, showing that, without AR in Sertoli cells, spermatogonial differentiation remained blocked.
Figure 2.
Histology of testis in jsd (A and C) and SCARKO-jsd (B and D) mice. Arrows indicate type A spermatogonia. N, Tubules with normal Sertoli cell nuclear localization. <5, Tubules with less than 5 Sertoli cell nuclei not on basement membrane. ≥5, Tubules with 5 or more Sertoli cell nuclei not on the basement membrane. C, Tubules with a cluster of Sertoli cell nuclei, usually in the center of the tubule. Bars, 20 μm.
Sertoli Cell Abnormalities in SCARKO-jsd Mice
We observed that in the SCARKO-jsd mouse testis, the positions of Sertoli cell nuclei were highly abnormal. Unlike wild-type (Figure 1A) and jsd mice (Figures 1 C, 2 A, C), in which most of the Sertoli cell nuclei were located in the basal area of tubules and aligned in a circle along the tubule basement membrane, many relatively normal Sertoli cell nuclei were displaced from the basal area and dispersed throughout the tubules in SCARKO-jsd mice (Figure 2 B, D). Clusters of abnormal Sertoli cell nuclei were also observed either near the basement membrane or in the center of the tubule (Figure 2 D).
To obtain a semiquantitative analysis of this phenomenon, we scored tubules as to whether they had fewer than 5 Sertoli nuclei not on basement membrane, more than 5 Sertoli nuclei not on basement membrane, or clusters of Sertoli cell (Figure 3). There were significantly more tubules with ≥5 Sertoli cell nuclei detached from the basement membrane and with Sertoli cell clusters in SCARKO-jsd mice than in SCARKO mice or in jsd mice. While 44% of tubules in jsd mice had all Sertoli cells arranged along the basement membrane, only 1% of tubules in SCARKO-jsd mice had normal Sertoli cell nuclear localization.
We next examined the morphology of these Sertoli cell nuclei by electron microscopy. There was a slight but consistent increase in the amount of heterochromatin associated with the nuclear membrane of Sertoli cells at the basement membrane in both SCARKO and SCARKO-jsd mice (Figure 4 B, D), compared to those found in wild-type and jsd mice (Figure 4 A, C). Such an increased level of heterochromatin is typical of immature, prepubertal Sertoli cells (Solari and Fritz, 1978) and Sertoli cells whose maturation is prevented by androgen deprivation (Chemes et al, 1979). Meanwhile, the displaced Sertoli cell nuclei, in both jsd and SCARKO-jsd mice, were highly abnormal, characterized by more prominent condensed chromatin deposits on nuclear membrane and irregular shapes (Figure 4 E, F). The abnormal chromatin distribution on nuclear membrane was more severe in clustered Sertoli cells than in the scattered Sertoli cells displaced from the basement membrane.
Figure 4.
Electron micrographs of Sertoli cell nuclei in testes of wild-type (A), SCARKO (B), jsd (C and E), and SCARKO-jsd (D and F) mice. Thin arrows indicate the Sertoli cell nuclei located in basal area. Arrowheads indicate some abnormal condensed chromatin deposits on the nuclear membrane of Sertoli cells near the basement membrane. Wide arrows indicate individual (filled arrows) or clustered (open arrows) Sertoli nuclei displaced from the basement membrane. The displaced nuclei had varying degree of abnormal condensed chromatin on the nuclear membrane and irregular nuclear outlines with invaginations. Bars, 2 μm (A, B, C and D) and 10 μm (E and F).
Since the Sertoli cell nuclear position is determined by cytoskeletal attachments between the basal aspect of the cell membrane and nucleus, we examined the intermediate filaments surrounding the nuclei and their connection with hemidesmosomes along the basal aspect of the Sertoli cell membrane as well as the basement membrane structure. Vimentin is the major intermediate filament protein present in structures in mature Sertoli cells that extend from the nucleus to desmosome-like junctions at the surface of the cell (Vogl et al, 1993), and in wild-type mice, it is concentrated around the Sertoli cell nucleus and also in some stalks extending towards the lumen (Supplemental Figure 1 A). The primary localization of vimentin in Sertoli cells of SCARKO, jsd, and SCARKO-jsd mice (Supplemental Figure 1 B, C, D) was also perinuclear, but the extensions of the vimentin trunks towards the center of the tubule were reduced or absent. The nuclei displaced from the basement membrane and those found in clusters in the central region of the tubules in SCARKO-jsd testes also showed the perinuclear of vimentin localization (Supplemental Figure 1 D). By electron microscopy, the intermediate filaments were observed around the nucleus and extending to make contact with hemidesmosome-like junctions along the Sertoli cell membrane in contact with the basement membrane extracellular matrix (Supplemental Figure 2). These structures were comparable among the four genotypes. However in the basement membrane of the seminiferous tubules, which consists of the basal laminae and collagen fibers, the basal laminae in testes of SCARKO and SCARKO-jsd mice were consistently thicker than that in testes of wild-type and jsd mice.
In order to determine if the ectoplasmic specialization (ES) junctional complexes between Sertoli cells were affected in SCARKO-jsd mice, we first examined their ultrastructure in these mice, as well as in wild-type, SCARKO, and jsd mice. These complexes are characterized by a pair of parallel Sertoli cell membranes, with both adherens and tight junctions, which are sandwiched between layers of actin bundles and endoplasmic reticulum in wild-type (Supplemental Figure 3 A). These complete structures were also observed in SCARKO, and in jsd mice (Supplemental Figures 3 B - E), although in jsd mice the tight junctions were not readily found. In SCARKO-jsd mice, although some basal junctional complexes consisting of ES and adherens junctions were observed (Supplemental Figure 3 F), they appeared to be less common and/or less well-formed. In addition, we could not find a tight junction in areas we sampled in these mice. Next we examined the distribution of vinculin, a protein associated with actin filaments at adherens junctions and ES (Vogl et al, 1993). In wild-type, SCARKO, and jsd testes, vinculin was observed in the cytoplasm of Sertoli cells and was brightest in the basal region of the tubule (Figure 5 A - C). In SCARKO-jsd mice (Figure 5 D), however, the cytoplasmic distribution of vinculin in the Sertoli cells was more uniform, and since there was no lumen formed in the tubules, the staining of vinculin occupied the whole tubule.
Figure 5.
Localization of vinculin in testes of wild-type (A), SCARKO (B), jsd (C), and SCARKO-jsd (D) mice. Arrows indicate clusters of Sertoli cells displaced from the BM. Arrowheads indicate some staining of vinculin in the basal region of the tubules. Bar, 20 μm. All pictures were taken under the same conditions.
Support of Spermatogonial Differentiation in SCARKO-jsd Mice
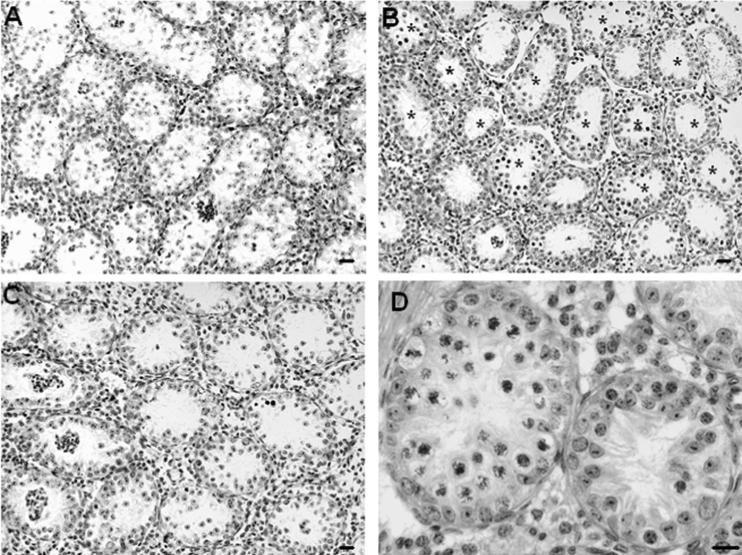
To determine whether failure of spermatogonial differentiation in SCARKO-jsd mice was indeed a result of testosterone action on cells other than Sertoli cells or whether Sertoli cell abnormalities were a contributing factor, we treated SCARKO-jsd mice with a GnRH-antagonist (acyline) and an AR antagonist (flutamide), since it had previously been shown that GnRH-antagonist treatment alone or with flutamide stimulated spermatogonial differentiation. The seminal vesicle and testis weights were markedly reduced (Table 1), demonstrating that testosterone action was blocked in these mice. As observed in jsd mice, treatment with GnRH-antagonist plus flutamide stimulated recovery of spermatogenesis up to the spermatocyte stage in SCARKO-jsd mice (Table 2, Figure 6 B). Interestingly, restoration of differentiating germ cells by suppression of testosterone greatly improved the organization of Sertoli cell nuclei in testes of SCARKO-jsd mice (Figure 6 D).
Figure 6.
Stimulation of recovery of spermatogenesis by suppression of testosterone in SCARKO- jsd mice. Hematoxylin-stained testis sections of SCARKO-jsd mice without treatment (A), treated with GnRH-antagonist plus flutamide (B, D), or treated with GnRH-antagonist plus testosterone (C). Asterisks indicate the tubules with differentiating germ cells. Sertoli cell arrangement was improved in the tubules in which differentiated germ cells were restored when treated with GnRH-antagonist plus flutamide (D). Bars, 20 μm.
Furthermore, treatment with testosterone, given in place of flutamide, reversed the stimulatory effect on spermatogonial differentiation in SCARKO-jsd mice because androgen levels were increased and androgen action on other cell types was no longer blocked (Table 2, Figure 6 C). The results clearly demonstrated that testosterone was still the factor maintaining arrest of spermatogonial differentiation in SCARKO-jsd mice and further confirmed that AR in Sertoli cells did not mediate this effect.
Discussion
Since AR in Sertoli cells mediates most of the indirect androgen action on germ cells (De Gendt et al, 2004) and specific ablation of Sertoli cell AR in jsd mice affected those cells, we initially expected that it would also mediate the testosterone-dependent block in spermatogonial differentiation in jsd mice. However, the block of spermatogonial cell differentiation was not reversed in SCARKO-jsd mice. Thus, the results of this study conclusively demonstrate that although androgen action on AR in Sertoli cells has significant effects on the structure of these cells, it does not mediate the action of testosterone in blocking spermatogonial differentiation in jsd mice.
We considered that the abnormalities in Sertoli cells structure in the absence of androgen might also contribute to their inability to support spermatogonial differentiation in SCARKO-jsd mice. Most prominently, the positioning of Sertoli nuclei was drastically disrupted in testes of these mice, compared to previous reports of minimal disorganization and dislocation Sertoli cell nuclei from the basement membrane in SCARKO mice (Tan et al, 2005; Wang et al, 2006). As the SCARKO-jsd mice can be a useful model for understanding the requirements for Sertoli cell organization, we further examined Sertoli cell structure in these mice.
The positions of the cell nucleus is believed to be controlled by cytoskeletal elements, which in Sertoli cells appear to be the vimentin intermediate filaments, and their attachment to junctional complexes at the cell membrane (Vogl et al, 1993). Although others have shown that elimination of androgen action on Sertoli cells up-regulated vimentin mRNA levels (Wang et al, 2006) and increased degradation of vimentin protein (Show et al, 2003), our results showed normal localization of vimentin protein and morphologically intact intermediate filament connections between the nuclei and the hemidesmosome junctions in the Sertoli cells with nuclei near the basement membrane in SCARKO-jsd mice. This result suggests that neither disruption of the vimentin filament arrangement nor the loss of anchoring of Sertoli cell nuclei to the basal side of its plasma membrane appear to be the primary reason for the displacement of Sertoli cell nuclei. The only abnormality within the anchoring structure we noted was the increased thickness of basal laminae in the testes of both SCARKO and SCARKO-jsd mice, suggesting that the regular structure of the basement membrane does not fully develop when androgenic signaling is disrupted in Sertoli cells, as has also been noted in SCARKO mice (Wang et al, 2006).
Junctions between Sertoli cells and between Sertoli and germ cells can also contribute to maintenance of the Sertoli cell structure. Indeed, in SCARKO-jsd mouse testes electron microscopy showed that typical ectoplasmic specialization structures were rare and tight junctions could not readily be found. This is consistent with observations showing that eliminating expression of AR in Sertoli cells increases the permeability of the Sertoli cell barrier and decreases the levels of expression of components of the occluding junctions, claudin 3, claudin 11, and occludin (Meng et al, 2005; Wang et al, 2006).
Consistent with ultrastructural observations, vinculin, one of the component proteins of the ectoplasmic specialization (Vogl et al, 1993) does not show its generally basal localization observed in wild-type, SCARKO, and jsd mice and was more uniformly distributed throughout the seminiferous tubule of SCARKO-jsd mice. Since vinculin functions in adherens junctions between cells and in focal contacts with the basal lamina, absence of concentrated distribution of vinculin in testes of SCARKO-jsd mice may contribute to the sloughing of Sertoli cells from the basal area. The formation of tight junctions and the localized vinculin distribution was, at least, partly dependent on androgen action in the Sertoli cells, although altered vinculin distribution could, in part, be a consequence of the redistribution of the Sertoli cell cytoplasm. Similarly, the localization of the cadherin-catenin complex, another component of adherens junctions, in the Sertoli cell ectoplasmic specialization, was also shown to be dependent on testosterone (Xia et al, 2005).
Although androgenic signaling in Sertoli cells is important to maintain their normal structure and nuclear positioning, presence of differentiated germ cells also appears to be involved. This conclusion is supported here by several lines of evidence. First, even with normal androgen action, jsd mice also showed mild disruption and sloughing of Sertoli cells. It should be noted that shrinkage of seminiferous tubules may also be a factor contributing to this abnormal organization. Second, dislocation of the nuclei and sloughing of Sertoli cells in SCARKO mice, which contain some differentiated germ cells, was much milder than that in SCARKO-jsd mice. In this case, the absence of androgen-dependent fluid secretion by Sertoli cells in SCARKO-jsd mice, which resulted in lack of lumen formation and loss of fluid pressure from the center of tubules, could also contribute to the disrupted organization of Sertoli cells and their nuclei. Third, the role of the differentiated germ cells in maintaining the positioning of the Sertoli cells and their nuclei was also strongly supported by the observation that restoration of germ cells by suppression of testosterone improved Sertoli cell organization in SCARKO-jsd mice.
We also are in the process of examining the overall changes in Sertoli cell gene expression in SCARKO-jsd by microarray comparison of these testes with jsd mice. Preliminary data suggest that the Sertoli cells in these SCARKO-jsd mice have equivalent expression of most markers of Sertoli cell maturation as jsd mice (Sharpe et al, 2003). Markers of mature Sertoli cells, SGP2 (Clu), GATA-1, and p27-Kip1 (Cdkn1b) were expressed at equivalent levels. Markers of immature Sertoli cells, AMH and aromatase (Cyp19a1), were not expressed. However there was a 4-fold higher expression of cytokeratin 1-18 (Krt18), a marker of immature Sertoli cells in the SCARKO-jsd mice than in jsd mice indicating that Sertoli cell maturation may be somewhat abnormal.
Despite the disrupted organization and some structural and molecular defects, the absence of AR in the Sertoli cells of SCARKO-jsd mice cannot be the cause of the inability of spermatogonial differentiation to proceed. These testes are indeed capable of supporting spermatogonial differentiation when the testosterone levels and action were globally blocked. Thus, the target of testosterone producing the spermatogonial block in jsd mice must be some other AR-positive somatic cell within or outside the testis. In a related study, we have shown that increased temperature of testis alone is able to stimulate the spermatogonial differentiation in jsd mice even more effectively than did suppression of testosterone (Shetty and Weng, 2004). The association between testosterone levels, testicular temperature, and spermatogonial differentiation is currently under further investigation.
Supplementary Material
Acknowledgements
The authors are very grateful to the following people: Dr. Gunapala Shetty for contributing his ideas to some of these experiments; Mr. Kenneth Dunner at the M. D. Anderson Cancer Center High Resolution Electron Microscopy Facility for processing the samples and performing the electron microscopy; Dr. Miles F. Wilkinson for providing primer information for Rhox5; Dr. Gail Prins at the University of Illinois at Chicago for generously providing AR antibody; Mr. Kuriakose Abraham for his help with the histological preparations; and Mr. Walter Pagel for his editorial assistance. Also, one co-author, Dr. De Gendt, is the recipient of a postdoctoral fellowship from the Fund for Scientific Research Flanders in Belgium.
Supported by NIH research grant R01 HD-40397 to M.L.M. and NIH Cancer Center Support grant CA 16672 to M.D. Anderson Cancer Center
Footnotes
DISCLOSURE STATEMENT: The authors have no conflicts of interest to declare
References
- Beamer WG, Cunliffe-Beamer TL, Shultz KL, Langley SH, Roderick TH. Juvenile spermatogonial depletion (jsd): A genetic defect of germ cell proliferations of male mice. Biol Reprod. 1988;38:899–908. doi: 10.1095/biolreprod38.4.899. [DOI] [PubMed] [Google Scholar]
- Boettger-Tong HL, Johnston DS, Russell LD, Griswold MD, Bishop CE. Juvenile spermatogonial depletion (jsd) mutant seminiferous tubules are capable of supporting transplanted spermatogenesis. Biol Reprod. 2000;63:1185–1191. doi: 10.1095/biolreprod63.4.1185. [DOI] [PubMed] [Google Scholar]
- Bolden-Tiller OU, Chiarini-Garcia H, Poirier C, Alves-Feitas D, Weng CC, Shetty G, Meistrich M. Genetic factors contributing to defective spermatogonial differentiation in juvenile spermatogonial depletion (Utp14b/jsd) mice. Biol Reprod. 2007;77:237–246. doi: 10.1095/biolreprod.107.060087. [DOI] [PubMed] [Google Scholar]
- Bradley J, Baltus A, Skaletsky H, Royce-Tolland M, Dewar K, Page DC. An X-to-autosome retrogene is required for spermatogenesis in mice. Nat Genet. 2004;36:872–876. doi: 10.1038/ng1390. [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charny CW, Gordon JA. Testosterone rebound therapy: a neglected modality. Fertil Steril. 1978;29:64–68. [PubMed] [Google Scholar]
- Chemes HE, Dym M, Raj HG. Hormonal regulation of Sertoli cell differentiation. Biol Reprod. 1979;21:251–262. doi: 10.1095/biolreprod21.1.251. [DOI] [PubMed] [Google Scholar]
- Chiarini-Garcia H, Russell LD. High-resolution light microscopic characterization of mouse spermatogonia. Biol Reprod. 2001;65:1170–1178. doi: 10.1095/biolreprod65.4.1170. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG, Okabe M, Nishimune Y. Arrest of spermatogonial differentiation in jsd/jsd, Sl17H/Sl17H, and cryptorchid mice. Biol Reprod. 1999;61:842–847. doi: 10.1095/biolreprod61.3.842. [DOI] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- Kangasniemi M, Huhtaniemi I, Meistrich ML. Failure of spermatogenesis to recover despite the presence of A spermatogonia in the irradiated LBNF1 rat. Biol Reprod. 1996;54:1200–1208. doi: 10.1095/biolreprod54.6.1200. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Kominami K, Dohmae K, Nonomura N, Miki T, Okuyama A, Nishimune Y, Okabe M. Cessation of spermatogenesis in juvenile spermatogonial depletion (jsd/jsd) mice. Int J Urol. 1997;4:500–507. doi: 10.1111/j.1442-2042.1997.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- Matsumiya K, Meistrich ML, Shetty G, Dohmae K, Tohda A, Okuyama A, Nishimune Y. Stimulation of spermatogonial differentiation in juvenile spermatogonial depletion (jsd) mutant mice by gonadotropin-releasing hormone antagonist treatment. Endocrinology. 1999;140:4912–4915. doi: 10.1210/endo.140.10.7026. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Shetty G, Bolden-Tiller OU, Porter KL. Hormones and spermatogonial development. In: Skinner MK, Griswold MD, editors. Sertoli Cell Biology. Elsevier Academic Press; San Diego: 2005. pp. 437–448. [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Yomogida K, Tadokoro Y, Tohda A, Dohmae K, Nishimune Y. Defect in germ cells, not in supporting cells, is the cause of male infertility in the jsd mutant mouse: proliferation of spermatogonial stem cells without differentiation. Int J Androl. 2001;24:15–23. doi: 10.1046/j.1365-2605.2001.00257.x. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Rao M, Wayne CM, Meistrich ML, Wilkinson MF. Pem homebox gene promoter sequences that direct transcription in stage-specific, and androgen-dependent manner in the testis in vivo. Molec Endocrinol. 2003;17:223–233. doi: 10.1210/me.2002-0232. [DOI] [PubMed] [Google Scholar]
- Rohozinski J, Bishop C. The mouse juvenile spermatogonial depletion (jsd) phenotype is due to a mutation in the X-derived retrogene, mUtp14b. Proc Natl Acad Sci USA. 2004;101:11695–11700. doi: 10.1073/pnas.0401130101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Shetty G, Wilson G, Huhtaniemi I, Boettger-Tong H, Meistrich ML. Testosterone inhibits spermatogonial differentiation in juvenile spermatogonial depletion mice. Endocrinology. 2001;142:2789–2795. doi: 10.1210/endo.142.7.8237. [DOI] [PubMed] [Google Scholar]
- Shetty G, Weng CCY. Cryptorchidism rescues spermatogonial differentiation in juvenile spermatogonial depletion (jsd) mice. Endocrinology. 2004;145:126–133. doi: 10.1210/en.2003-0928. [DOI] [PubMed] [Google Scholar]
- Shetty G, Weng CC, Porter KL, Zhang Z, Pakarinen P, Kumar TR, Meistrich ML. Spermatogonial differentiation in juvenile spermatogonial depletion (jsd) mice with androgen receptor or follicle stimulating hormone mutations. Endocrinology. 2006;147:3563–3570. doi: 10.1210/en.2006-0159. [DOI] [PubMed] [Google Scholar]
- Show MD, Anway MD, Folmer JS, Zirkin BR. Reduced intratesticular testosterone concentration alters the polymerization state of the Sertoli cell intermediate filament cytoskeleton by degradation of vimentin. Endocrinology. 2003;144:5530–5536. doi: 10.1210/en.2003-0735. [DOI] [PubMed] [Google Scholar]
- Solari AJ, Fritz IB. The ultrastructure of immature Sertoli cells. Maturation-like changes during culture and the maintenance of mitotic potentiality. Biol Reprod. 1978;18:329–345. doi: 10.1095/biolreprod18.3.329. [DOI] [PubMed] [Google Scholar]
- Tan KA, Turner KJ, Saunders PT, Verhoeven G, De Gendt K, Atanassova N, Sharpe RM. Androgen regulation of stage-dependent cyclin D2 expression in Sertoli cells suggests a role in modulating androgen action on spermatogenesis. Biol Reprod. 2005;72:1151–1160. doi: 10.1095/biolreprod.104.037689. [DOI] [PubMed] [Google Scholar]
- Tohda A, Matsumiya K, Tadokoro Y, Yomogida K, Miyagawa Y, Dohmae K, Okuyama A, Nishimune Y. Testosterone suppresses spermatogenesis in juvenile spermatogonial depletion (jsd) mice. Biol Reprod. 2001;65:532–537. doi: 10.1095/biolreprod65.2.532. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Yeh SD, Wang RS, Yeh S, Zhang C, Lin HY, Tzeng CR, Chang C. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci USA. 2006;103:18975–18980. doi: 10.1073/pnas.0608565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl AW, Grove BD, Pfeiffer DC, Redenbach DM. The Sertoli Cell cytoskeleton. In: Russell LD, Griswald MD, Clearwater FL, editors. The Sertoli Cell. Cache River Press; Clearwater, Florida: 1993. pp. 39–86. [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant'Agnese PA, deMesy-Bentley KL, Tzeng CR, Chang C. Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- Xia W, Wong CH, Lee NP, Lee WM, Cheng CY. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: an in vivo study using an androgen suppression model. J Cell Physiol. 2005;205:141–157. doi: 10.1002/jcp.20377. [DOI] [PubMed] [Google Scholar]
- Xu Q, Lin HY, Yeh SD, Yu IC, Wang RS, Chen YT, Zhang C, Altuwaijri S, Chen LM, Chuang KH, Chiang HS, Yeh S, Chang C. Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine. 2007;32:96–106. doi: 10.1007/s12020-007-9015-0. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yeh S, Chen YT, Wu CC, Chuang KH, Lin HY, Wang RS, Chang YJ, Mendis-Handagama C, Hu L, Lardy H, Chang C. Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells. Proc Natl Acad Sci USA. 2006;103:17718–17723. doi: 10.1073/pnas.0608556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shao S, Meistrich M. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J Cell Physiol. 2007;211:149–158. doi: 10.1002/jcp.20910. [DOI] [PubMed] [Google Scholar]
- Zhao M, Rohozinski J, Sharma M, Ju J, Braun RE, Bishop CE, Meistrich ML. Utp14b: A unique retrogene within a gene that has acquired multiple promoters and a specific function in spermatogenesis. Dev Biol. 2007;304:848–859. doi: 10.1016/j.ydbio.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins G, Saunders P, Katzenellenbogen B, Hess R. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23:870–881. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.