Abstract
Background
Some anesthetics have been suggested to induce neurotoxicity including promotion of Alzheimer’s disease neuropathogenesis. Nitrous oxide and isoflurane are common anesthetics. Here, we set out to assess effects of nitrous oxide and/or isoflurane on apoptosis and β-amyloid (Aβ) levels in H4 human neuroglioma cells and primary neurons from naïve mice.
Methods
The cells or neurons were exposed to 70% nitrous oxide and/or 1% isoflurane for six hours. The cells or neurons and conditioned media were harvested at the end of the treatment. Caspase-3 activation, apoptosis, processing of amyloid precursor protein, and Aβ levels were determined.
Results
Treatment with a combination of 70% nitrous oxide and 1% isoflurane for six hours induced caspase-3 activation and apoptosis in H4 naïve cells and primary neurons from naïve mice. The 70% nitrous oxide plus 1% isoflurane, but neither alone, for six hours induced caspase-3 activation and apoptosis, and increased levels of β-site amyloid precursor protein-cleaving enzyme and Aβ in H4-amyloid precursor protein cells. In addition, the nitrous oxide plus isoflurane-induced Aβ generation was reduced by a broad caspase inhibitor Z-VAD. Finally, the nitrous oxide plus isoflurane-induced caspase-3 activation was attenuated by γ-secretase inhibitor L-685,458, but potentiated by exogenously added Aβ.
Conclusion
These results suggest that common anesthetics nitrous oxide plus isoflurane may promote neurotoxicity by inducing apoptosis and increasing Aβ levels. The generated Aβ may further potentiate apoptosis to form another round of apoptosis and Aβ generation. More studies, especially the in vivo confirmation of these in vitro findings, are needed.
Introduction
Alzheimer’s disease (AD), one of the most common forms of dementia, affects 4.5 million Americans and costs $100 billion a year on direct care alone, and its impact will only increase in the coming decades. Excessive production and/or accumulation of β-amyloid protein (Aβ), the major component of plaque in AD patient brain, play a fundamental role in the pathology of AD [1; reviewed by 2,3]. Aβ is produced via serial proteolysis of amyloid precursor protein (APP) by aspartyl protease β-site APP-cleaving enzyme (BACE), or β-secretase, and γ-secretase. BACE cleaves APP to generate a 99-residue membrane-associated C-terminus fragment (APP-C99). APP-C99 is further cleaved by γ-secretase to release 4-kDa Aβ and β-amyloid precursor protein intracellular domain 4–6. APP can also undergo caspase-mediated cleavage to generate a 90 kDa N-terminus APP caspase fragment (APP-N-caspase fragment) 7–9. Increasing evidence suggests a role for caspase activation and apoptosis in AD neuropathogenesis [8–24; reviewed in 25,26].
An estimated 200 million patients worldwide undergo anesthesia and surgery each year. Even though anesthesia and surgery may not increase the incidence of AD 27, it has been reported that age of onset of AD is inversely related to cumulative exposure to anesthesia and surgery before age 50 28. A recent study also reported that patients having coronary artery bypass graft surgery under general anesthesia are at increased risk for AD as compared to those having percutaneous transluminal coronary angioplasty under local anesthesia 29. However, other studies have suggested that there is little or no relationship between anesthesia and AD 30,31. More population studies, defining the role of anesthesia in AD, are necessary 32.
Nevertheless, perioperative factors, including hypoxia 33–37, hypocapnia 38, and anesthetics 7,24,39–46, have been reported to potentially contribute to AD neuropathogenesis in cultured cells and in animals. Nitrous oxide and isoflurane are common anesthetics for patients, however, the effects of nitrous oxide plus isoflurane on neurotoxicity such as AD neuropathogenesis, including caspase activation, apoptosis and Aβ levels, have not been assessed.
In the present studies, we set out to determine the effects of nitrous oxide plus isoflurane on caspase-3 activation, apoptosis, APP processing and Aβ levels in H4 human neuroglioma cells (H4 naïve cells), H4 naïve cells stably-transfected to express full-length (FL) APP (H4-APP cells) and primary neurons from naïve mice. We further studied the effects of the caspase inhibitor Z-VAD, γ-secretase inhibitor L-685,458, and Aβ on the nitrous oxide plus isoflurane-induced caspase-3 activation and Aβ accumulation.
Materials and Methods
Cell lines
We employed H4 human neuroglioma cells (H4 naïve cells) and H4 naïve cells stably-transfected to express full-length (FL) APP (H4-APP cells) in the experiments. All cell lines were cultured in Dulbecco's Modified Eagle Medium (high glucose) containing 9% heat-inactivated fetal calf serum, 100 units/ml penicillin, 100 µg/ml streptomycin, and 2 mM L-glutamine. Stably-transfected H4 cells were additionally supplemented with 200 µg/ml G418.
Primary neurons
This protocol was approved by Massachusetts General Hospital Standing Committee on Animals (Boston, Massachusetts) on the Use of Animals in Research and Teaching. Naïve (C57BL/6J) mice with gestation stage of day 18 were euthanized with carbon dioxide. We then performed a cesarean section to pull out the embryos and decapitate them in a 100 mm dish of phosphate buffered saline. We placed the head on the top of a 100 mm dish and dissected out the cortices, removed meninges and placed the neurons into another 100 mm dish of phosphate buffered saline. The neurons were dissociated by trypsinization and trituration. The dissociated neurons were re-suspended in serum-free B27/neurobasal medium and were placed into six well plates with confluent rate of 50%. Ten days after the harvest, the neurons were exposed to nitrous oxide plus isoflurane.
Cell treatment
Inhalation anesthetic nitrous oxide and/or isoflurane were delivered from an anesthesia machine to a sealed plastic box in a 37°C incubator containing six-well plates seeded with one million cells or 0.25 million neurons in 1.5 ml cell or neuron culture media. A Datex infrared gas analyzer (Puritan-Bennett, Tewksbury, MA) was used to continuously monitor the delivered concentrations of carbon dioxide, oxygen, nitrous oxide and/or isoflurane. The cells were treated for six hours with one of the following three conditions: nitrous oxide alone (21% O2, 5% CO2 and 70% nitrous oxide), isoflurane alone (21% O2, 5% CO2 and 1% isoflurane), or nitrous oxide plus isoflurane (21% O2, 5% CO2, 70% nitrous oxide and 1% isoflurane). The primary neurons were treated with the nitrous oxide plus isoflurane for six hours. In the interaction studies, the H4-APP cells were treated with Z-VAD (65 µM,), L-685,458 (0.65 µM), and Aβ42 (7.5 µM) one hour before nitrous oxide plus isoflurane treatment. The controls for Z-VAD and L685,458 were dimethyl sulfoxide, and the control for Aβ42 was saline. The control condition for nitrous oxide, isoflurane, and nitrous oxide plus isoflurane was 21% O2 plus 5% CO2, which did not affect caspase-3 activation, cell viability, APP processing, and Aβ generation 40.
Cell or neuron lysis and protein amount quantification
Cell or neuron pellets were detergent-extracted on ice using immunoprecipitation buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40) plus protease inhibitors (1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin A). The lysates were collected, centrifuged at 13,000 rpm for 15 min, and quantified for total proteins by a bicinchoninic acid protein assay kit (Pierce, Iselin, NJ).
Western blots analysis
The cells or neurons were harvested at the end of the experiments and were subjected to Western blot analyses as described by Xie et al. 47. A caspase-3 antibody (1:1,000 dilution; Cell Signaling Technology, Inc. Beverly, MA) was used to recognize FL-caspase-3 (35 – 40 kDa) and caspase-3 fragment (17 – 20 kDa) resulting from cleavage at asparate position 175. Rabbit polyclonal anti-BACE-1 antibody (1:1,000, Abcam, Cambridge, MA) was used to detect protein levels of BACE (65 kDa). Antibodies C66 (1:2,000, generous gift of Dora Kovacs (Ph.D., Associate Professor of Neurology at Harvard Medical School, Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA) was used to visualize FL-APP (110 kDa) and APP-N-caspase (90 kDa) fragments. Antibody anti-β-actin (1:10,000, Sigma, St. Louis, MO) was used to detect β-actin (42 kDa). Each band in the Western blot represents an independent experiment. We have averaged results from three to ten independent experiments. The quantification of Western blots was performed as described by Xie et al. 40. Briefly, intensity of signals was analyzed by using the National Institute of Health image program (National Institute of Health Image 1.62, Bethesda, MD). We quantified Western blots using two steps. First, we used levels of β-actin to normalize (e.g., determining ratio of FL-APP amount to β-actin amount) levels of FL-APP, APP-N-caspase fragment, FL-caspase-3, caspase-3 fragment and BACE to control for any loading differences in total protein amounts. Second, we presented changes in levels of FL-APP, APP-N-caspase fragment, FL-caspase-3, caspase-3 fragment and BACE in treated cells as percentages of those in cells treated with controls.
Quantification of Aβ using Sandwich ELISA assay
Secreted Aβ was measured with a Sandwich ELISA assay by using an Aβ measurement kit (Wako, Richmond, VA) as described by Xie et al. 48. The monoclonal antibody BAN50, the epitope of which is human Aβ(1–16), is coated on 96 well surfaces of a separable microplate and acts as a capture antibody for the N-terminal portion of human Aβ40. Captured human Aβ40 is recognized by antibody BA27, which specifically detects the C-terminal portion of Aβ40. Wells were incubated overnight at 4 °C with test samples of conditioned cell culture media, and then BA27 was added. Plates were then developed with tetramethylbenzidine reagent, and terminated by stop solution and well absorbance was measured at 450 nm. Aβ levels in the test samples were determined by comparing results with signals from unconditioned media spiked with known quantities of Aβ40.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
TMR red kit (Roche Diagnostics, Mannheim, Germany) was used for TUNEL staining. Specifically, cells were grown on coverslips overnight in the cell culture medium. The cells or neurons were fixed in 1% paraformaldehyde for 60 min following treatment of nitrous oxide plus isoflurane. The coverslips were rinsed with phosphate buffered saline three times for five minutes each time and then incubated in a permeabilization solution (0.1% TritonX -100 in 0.1% sodium citrate) at 4 °C for five minutes. Finally, the coverslips were incubated with a TUNEL reaction mixture for one hour at 37 °C in a humidified dark chamber. The negative control was prepared by incubating the coverslips in label solution only. The coverslips for the positive control were incubated with DNase I recombinant. Finally, the coverslips were incubated with 10μg/ml Hoechst 33342 in a humidified dark chamber for 10 minutes. Samples were then analyzed in mounting medium under a fluorescence microscope. The TUNEL-positive cells were counted manually in five randomly selected areas under a 20X objective microscope lens by an investigator who was blinded to the experiments.
Cell apoptosis assay
Cell apoptosis was assessed by a cell death detection ELISA kit (Roche, Palo Alto, CA), which assays cytoplasmic histone-associated DNA fragmentation associated with cellular apoptosis.
Statistics
Given the presence of background caspase-3 activation and apoptosis in the cells and neurons, we did not use absolute values to describe changes in caspase-3 activation and apoptosis. Instead, cell apoptosis and caspase-3 activation were presented as a percentage of those of the control group. 100% caspase-3 activation or apoptosis refers to control levels for purposes of comparison to experimental conditions. Then, we presented changes in levels of caspase-3 activation, apoptosis, FL-APP, APP-N-caspase fragment, BACE and Aβ in treated cells as percentages of those in cells treated with controls. Data were expressed as mean ± S.D.. The number of samples varied from 3 to 10, and the samples were normally distributed. ANOVA with repeated measurements or t-test was used to compare the difference from the control group. P-values less than 0.05 (* or #) and 0.01 (** or ##) were considered statistically significant. The significance testing was two-tailed, and we have used SAS software (Cary, NC) to analysis the data.
Results
Nitrous oxide plus isoflurane induced caspase-3 activation and apoptosis, and increased levels of BACE and secreted Aβ in H4-APP cells
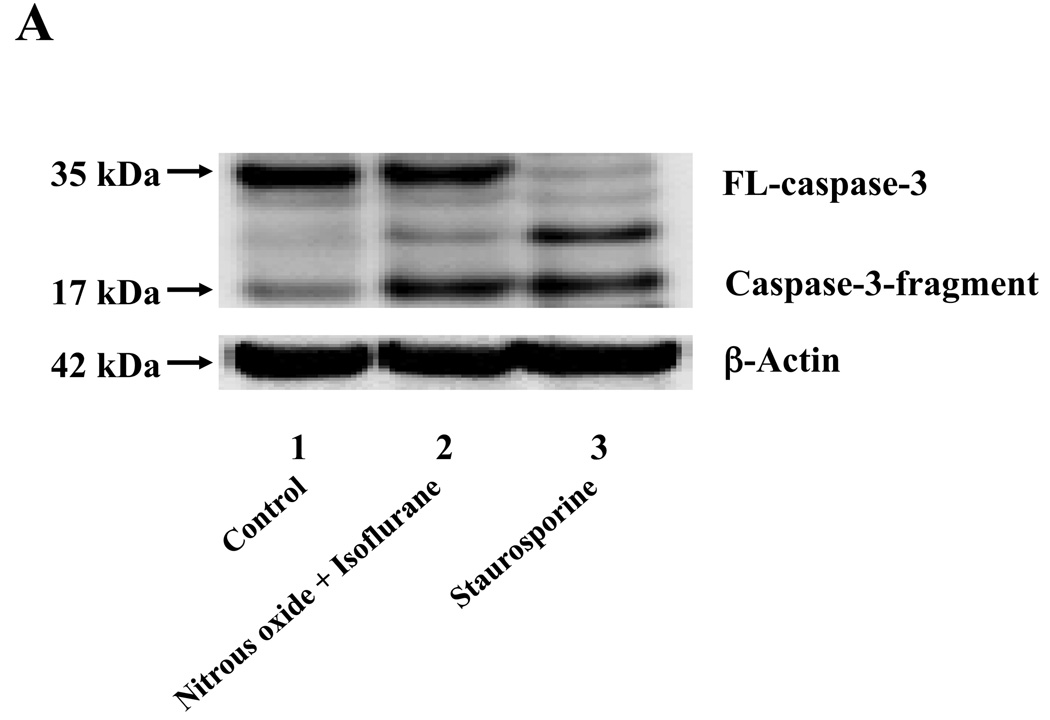
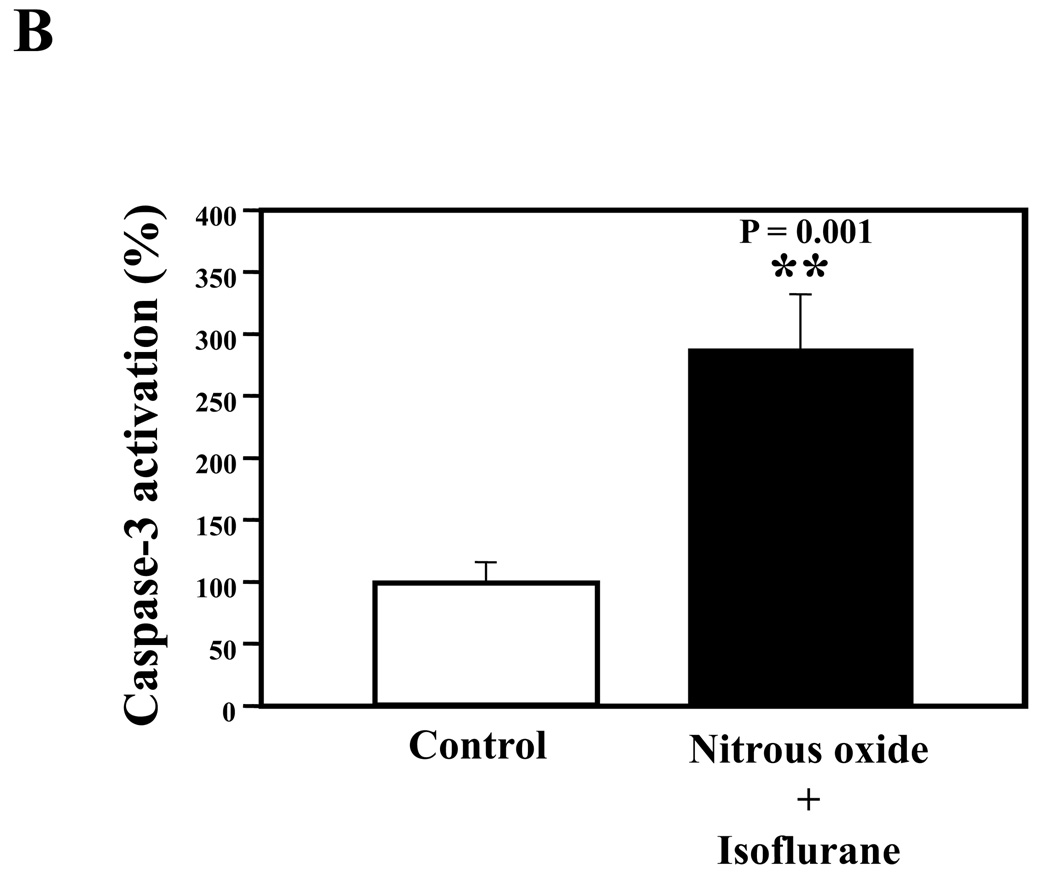
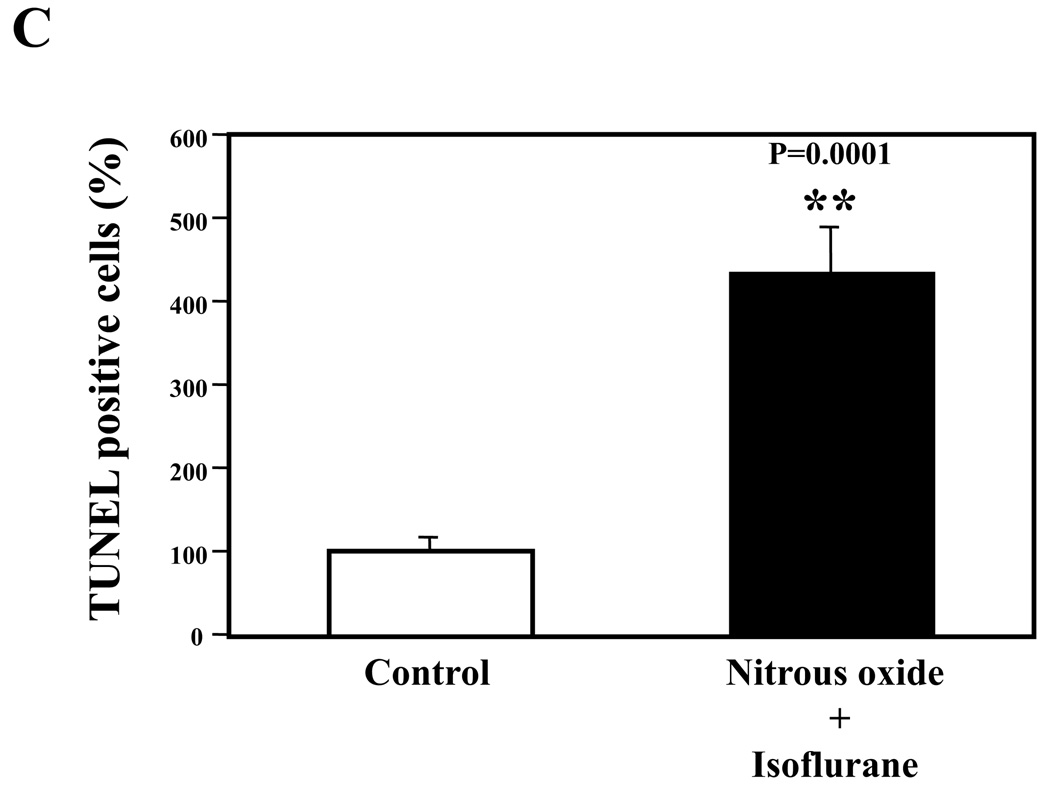
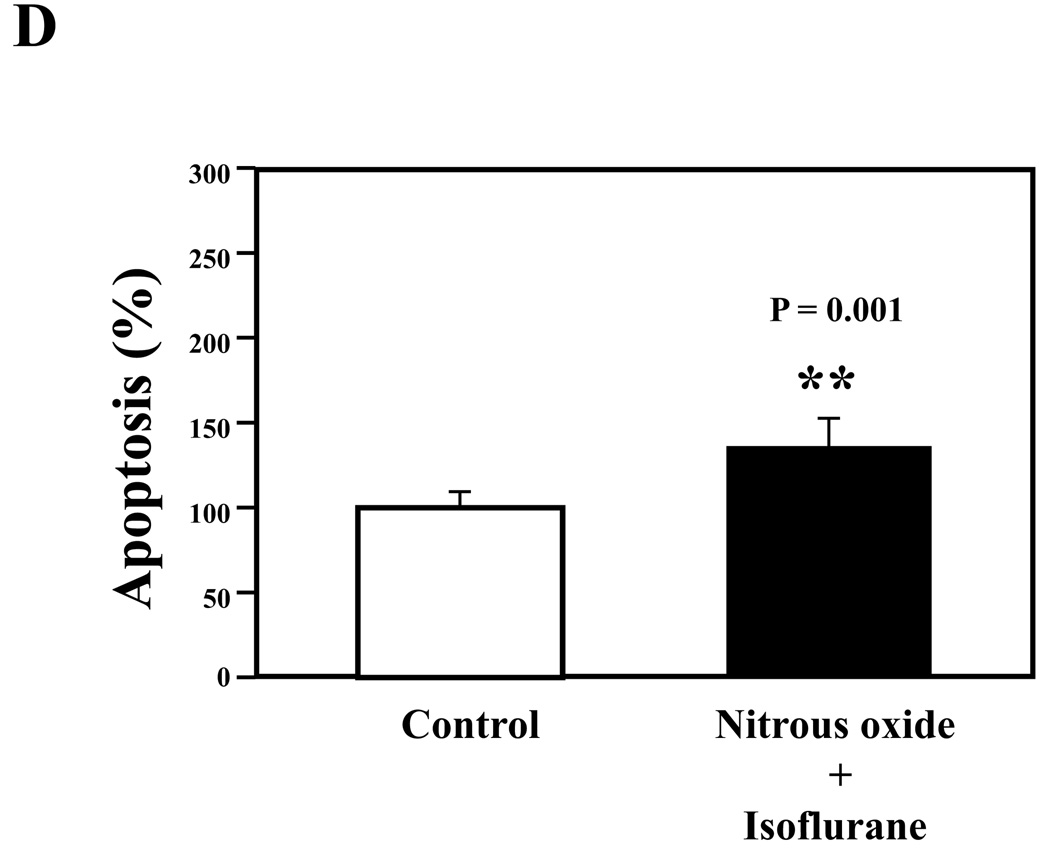
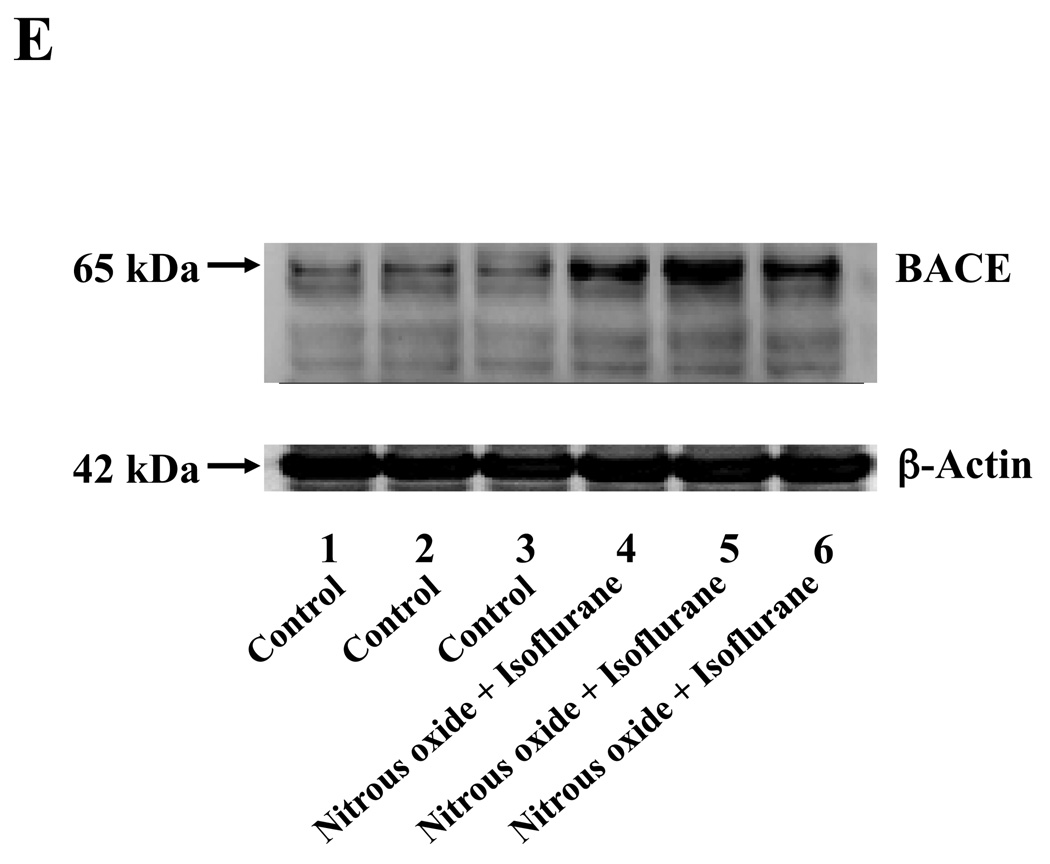
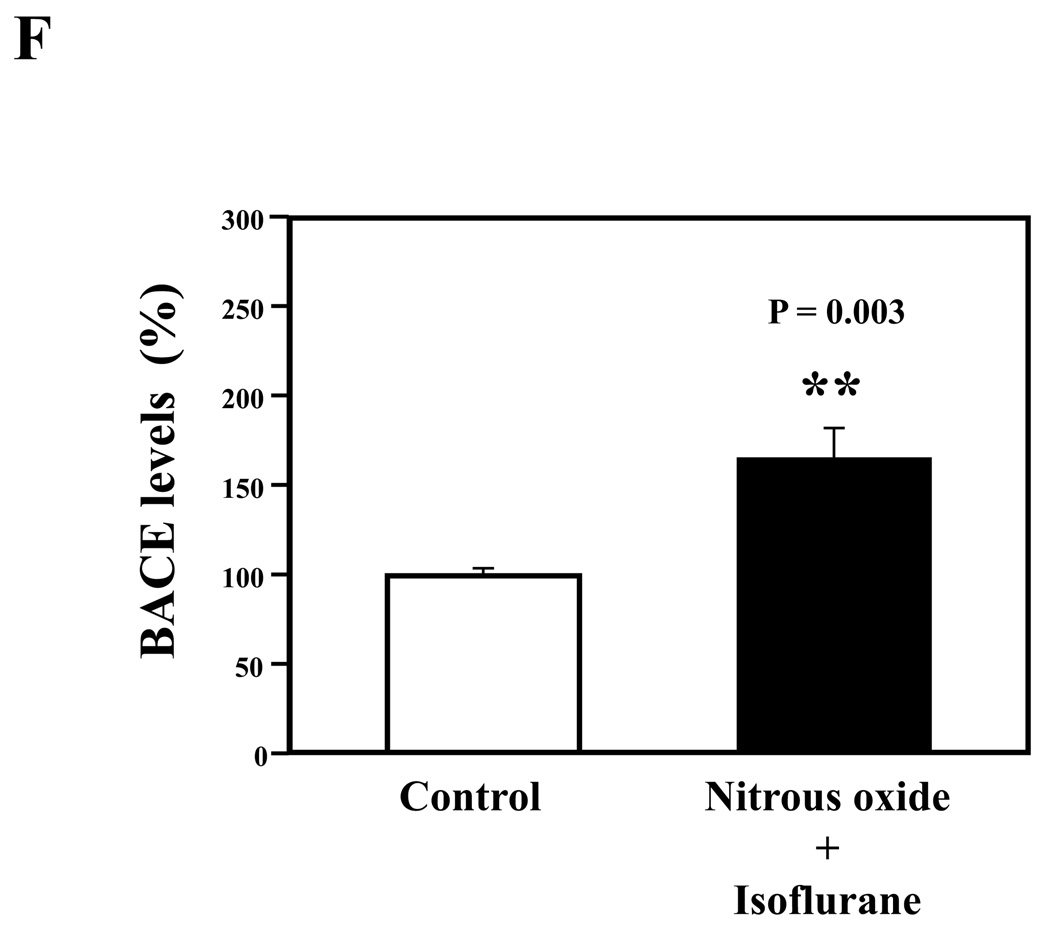
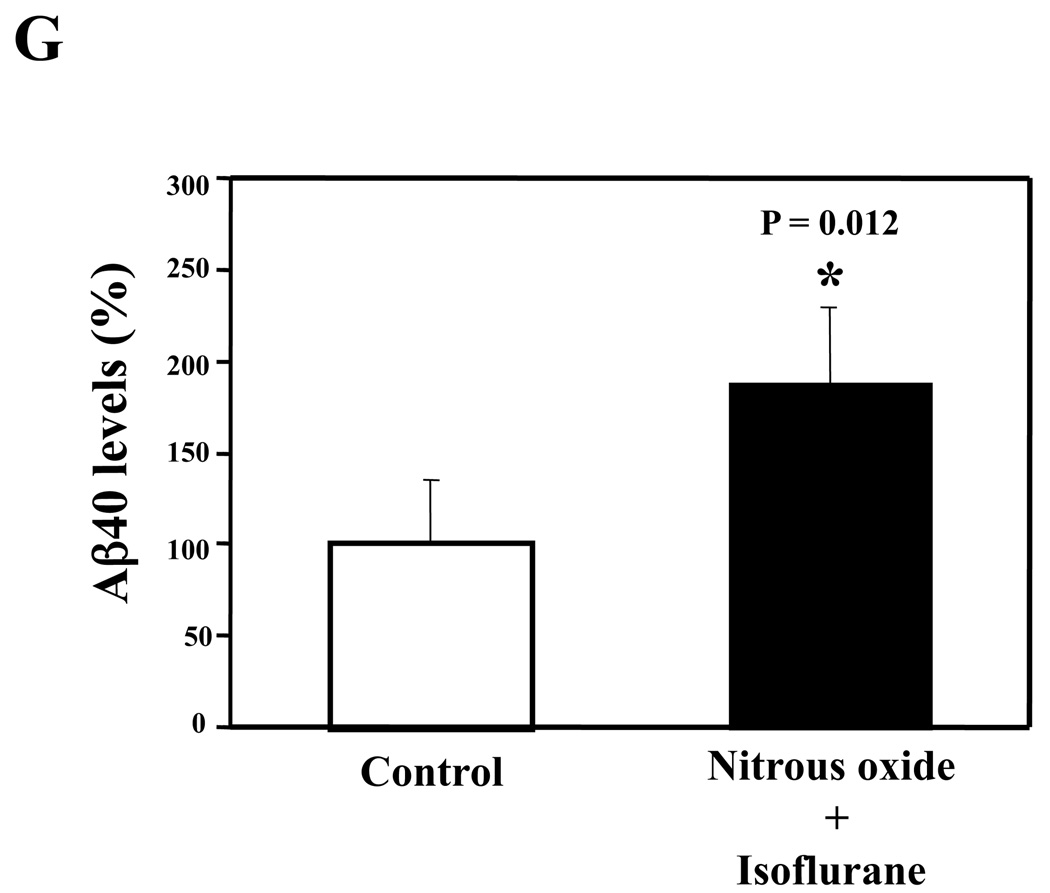
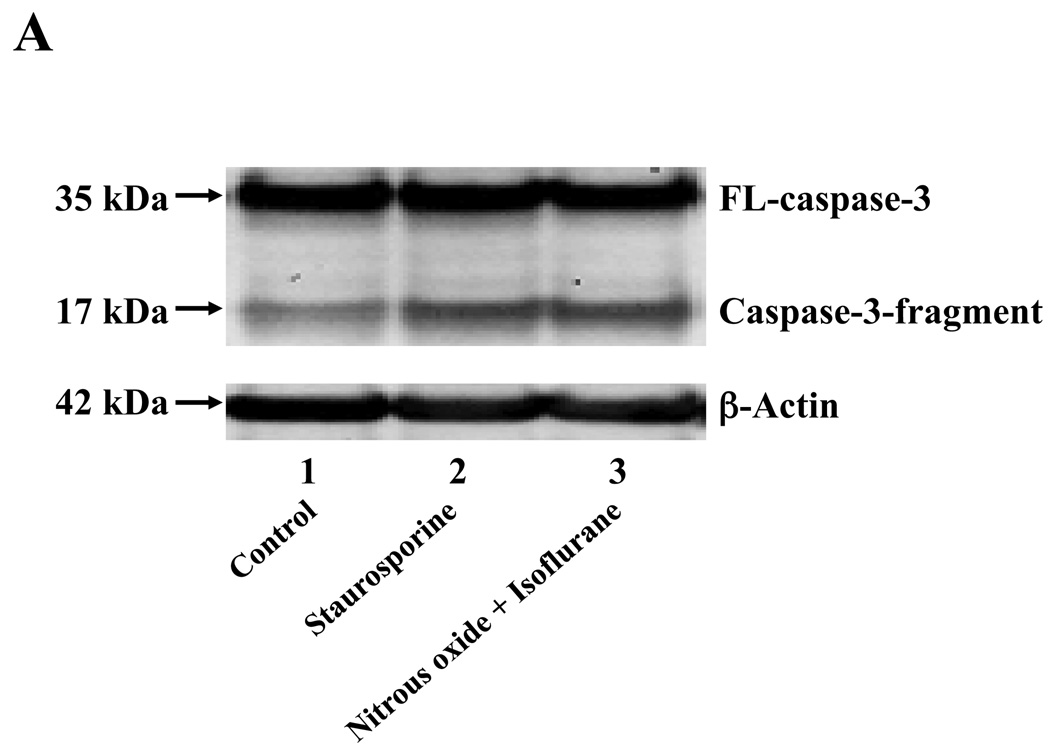
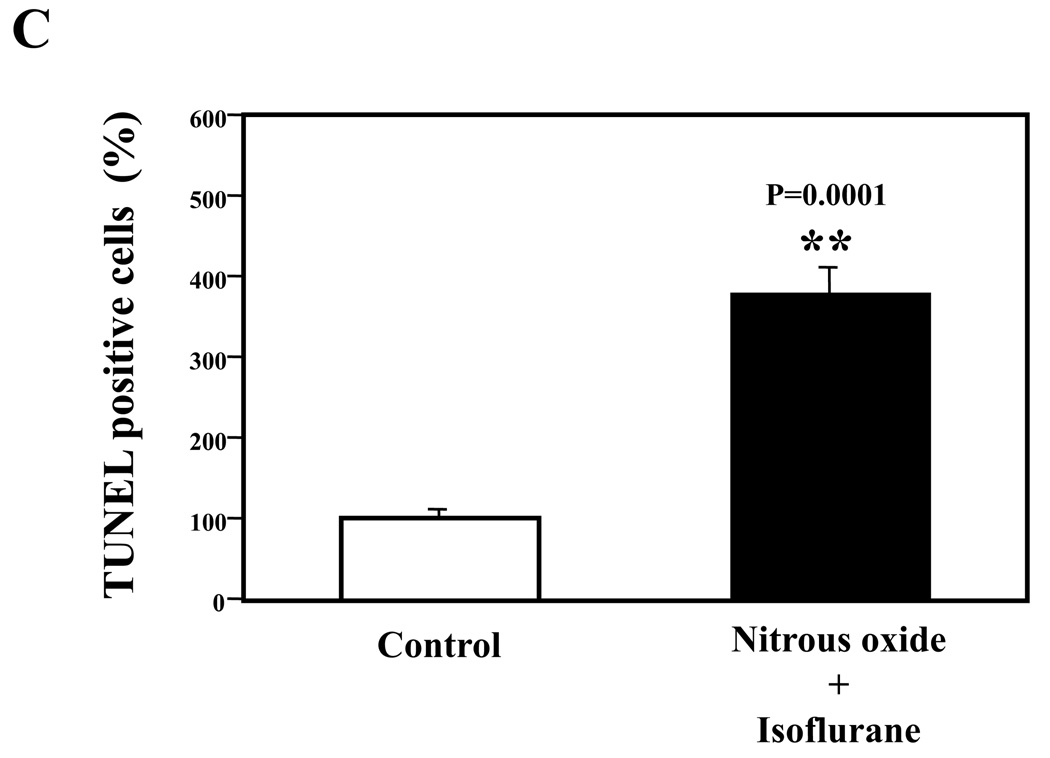
Nitrous oxide plus isoflurane are common anesthetics for patients. We, therefore, assessed the effects of nitrous oxide plus isoflurane on apoptosis and secreted Aβ levels in H4-APP cells. Since caspase-3 activation is one of the final steps of cellular apoptosis 49, we assessed the effects of nitrous oxide plus isoflurane on caspase-3 activation by quantitative Western blots analyses. Caspase-3 immunoblotting showed visible increases in protein levels of caspase-3 fragment following treatment with 70% nitrous oxide plus l% isoflurane for six hours as compared to the control condition (Figure 1A). We used staurosporine 50,51 in the experiments as a positive control to validate the caspase-3 antibody and to confirm that the visible band following the nitrous oxide plus isoflurane treatment is indeed the band representing cleaved caspase-3. Quantification of the Western blot, by determining ratio of cleaved (activated) caspase-3 fragment (17 – 19 kDa) to FL-caspase-3 (35 – 40 kDa), revealed that treatment of nitrous oxide plus isoflurane led to a 264% increase in caspase-3 cleavage (activation) as compared to the control condition (Figure 1B) (P = 0.001). Given that caspase-3 activation alone may not represent apoptotic cell damage 52, we also assessed effects of 70% nitrous oxide plus 1% isoflurane on cellular apoptosis by TUNEL study and by cell death detection ELISA kit (detecting cytoplasmic histone-associated DNA fragmentation). We found that the treatment of nitrous oxide plus isoflurane increased TUNEL positive cells (apoptosis) as compared to the control condition (See Figure 1 in Supplemental Digital Content 1, which is histological image showing effects of nitrous oxide plus isoflurane on number of TUNEL positive H4-APP cells; and Figure 1C): 100% versus 431%, (P = 0.0001). Moreover, we were able to show that the treatment of nitrous oxide plus isoflurane (Figure 1D, black bar) increased cytoplasmic histone-associated DNA fragmentation (apoptosis) as compared to the control condition (Figure 1D, white bar): 100% versus 136%, (P = 0.001). BACE is one of the enzymes for Aβ generation, so we asked whether the treatment of nitrous oxide plus isoflurane can increase levels of BACE. We were able to show that treatment with 70% nitrous oxide plus 1% isoflurane for six hours caused visible increases in BACE levels as compared to the control condition (Figure 1E). Quantification of the Western blot showed that the nitrous oxide plus isoflurane treatment led to a 171% increase in BACE levels as compared to the control condition (P = 0.003, Figure 1F). Finally, the treatment of nitrous oxide plus isoflurane led to increases in secreted Aβ40 levels as compared to the control condition in H4-APP cells (Figure 1G): 100% versus 188%, P = 0.012. These findings suggest that the treatment of 70% nitrous oxide plus 1% isoflurane may induce apoptosis in H4-APP cells, which enhances BACE levels to facilitate APP processing, leading to increases in Aβ levels.
Figure 1. Treatment of nitrous oxide plus isoflurane induces caspase-3 activation and apoptosis, and increases levels of BACE and Aβ in H4-APP cells.
A. Effects of 70% nitrous oxide plus 1% isoflurane for six hours, and staurosporine on caspase-3 activation. Each band in the Western blot represents an independent experiment. There is no significant difference in amounts of β-actin in the control condition-, staurosporine- or nitrous oxide plus isoflurane-treated H4-APP cells. B. Caspase-3 activation assessed by quantifying ratio of caspase-3 fragment to FL-caspase-3 in the Western blots, normalized to β-actin levels. We have averaged results from three independent experiments. C. Effects of the nitrous oxide plus isoflurane treatment on TUNEL positive cells. D. Effects of the nitrous oxide plus isoflurane treatment on apoptosis. E. Effects of the nitrous oxide plus isoflurane treatment on BACE levels. F. Quantification of the Western blot, normalized to β-actin levels. We have averaged results from three independent experiments. G. Effects of nitrous oxide plus isoflurane treatment on Aβ40 levels. We have averaged results from ten independent experiments. BACE, β-site amyloid precursor protein-cleaving enzyme; APP, amyloid precursor protein; Aβ, β-amyloid protein; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; FL, full length. * P < 0.05; ** P <0.01.
Nitrous oxide plus isoflurane induced caspase-3 activation and apoptosis in H4 naïve cells and primary neurons from naïve mice
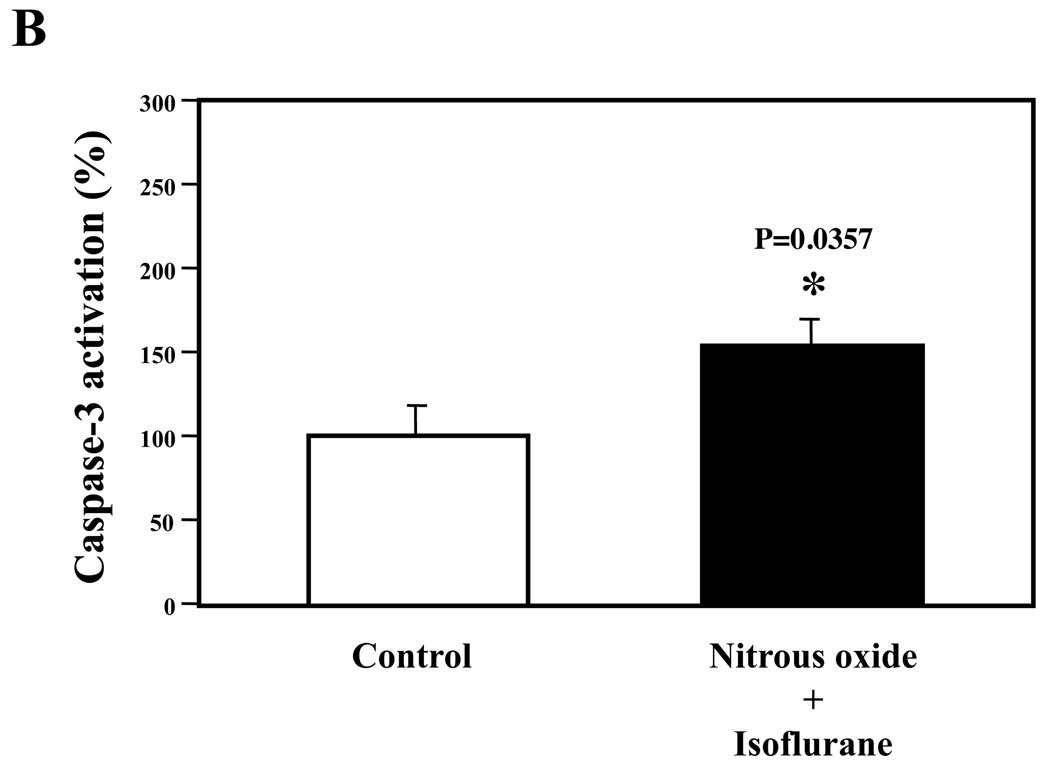
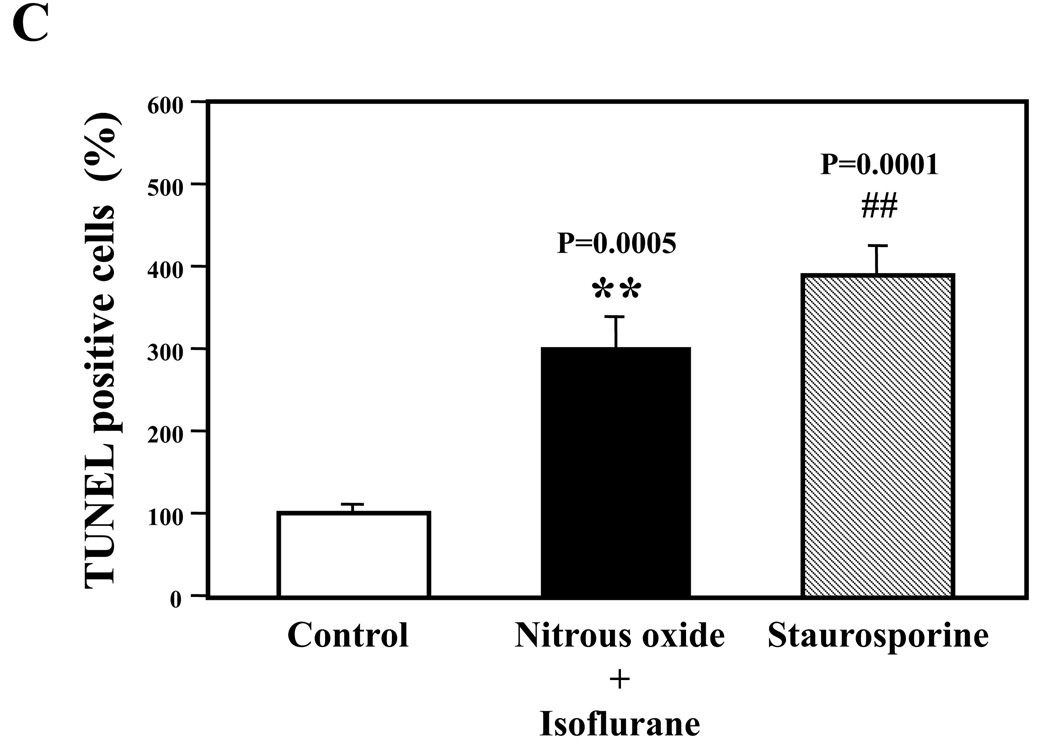
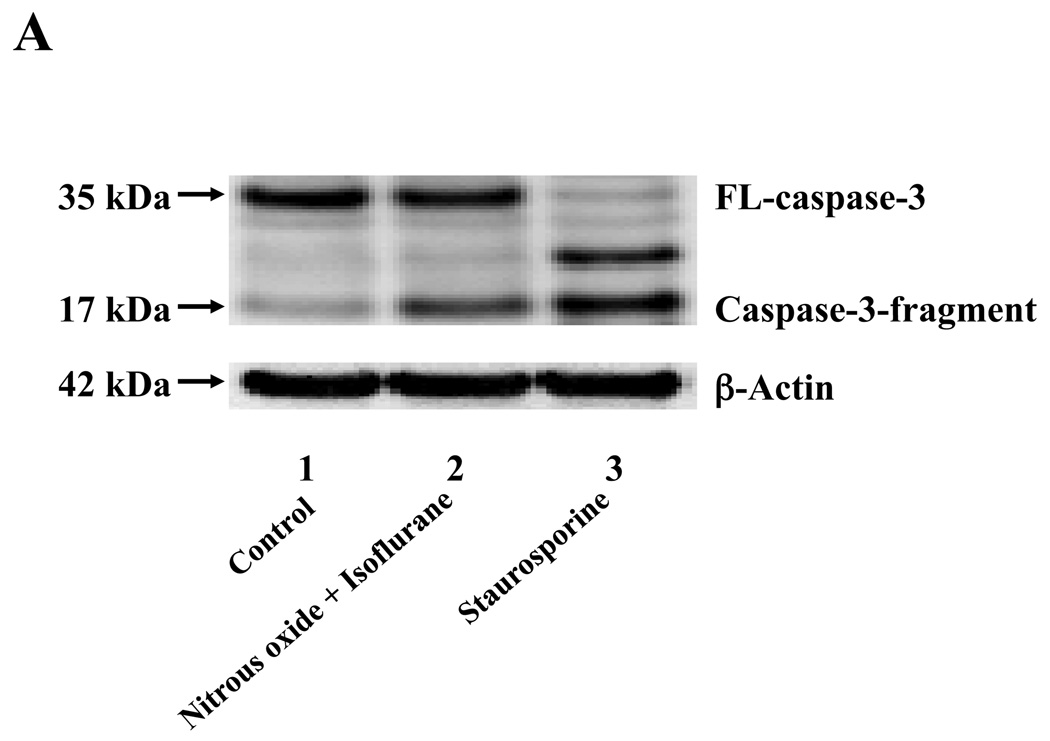
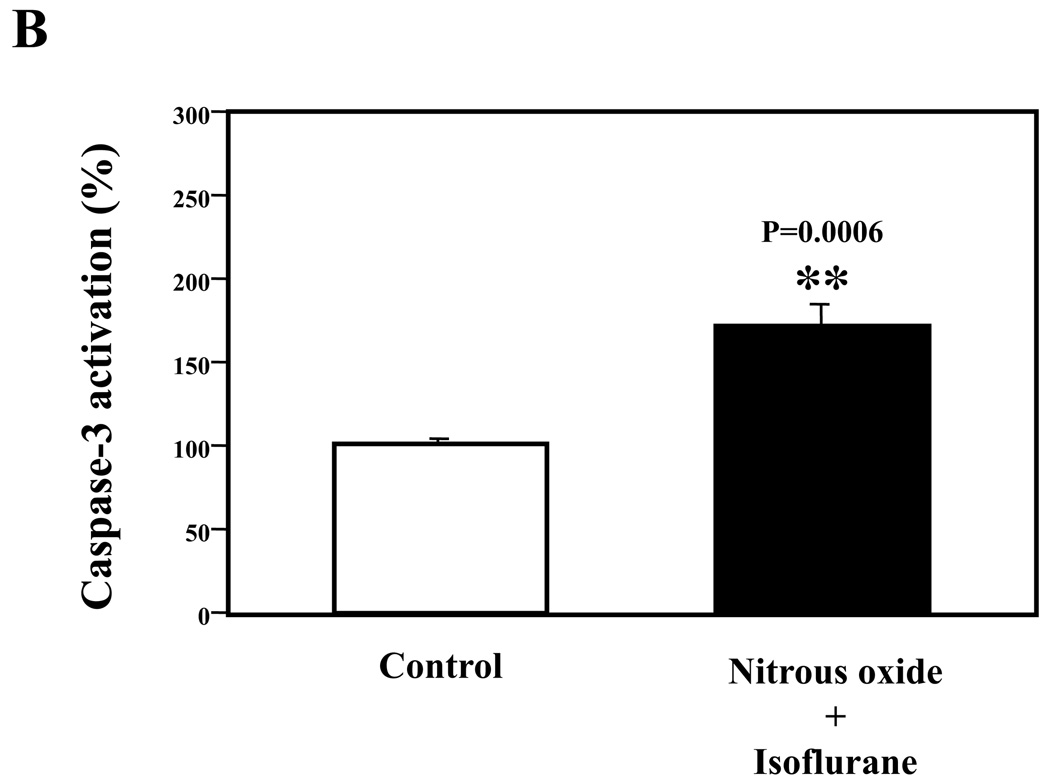
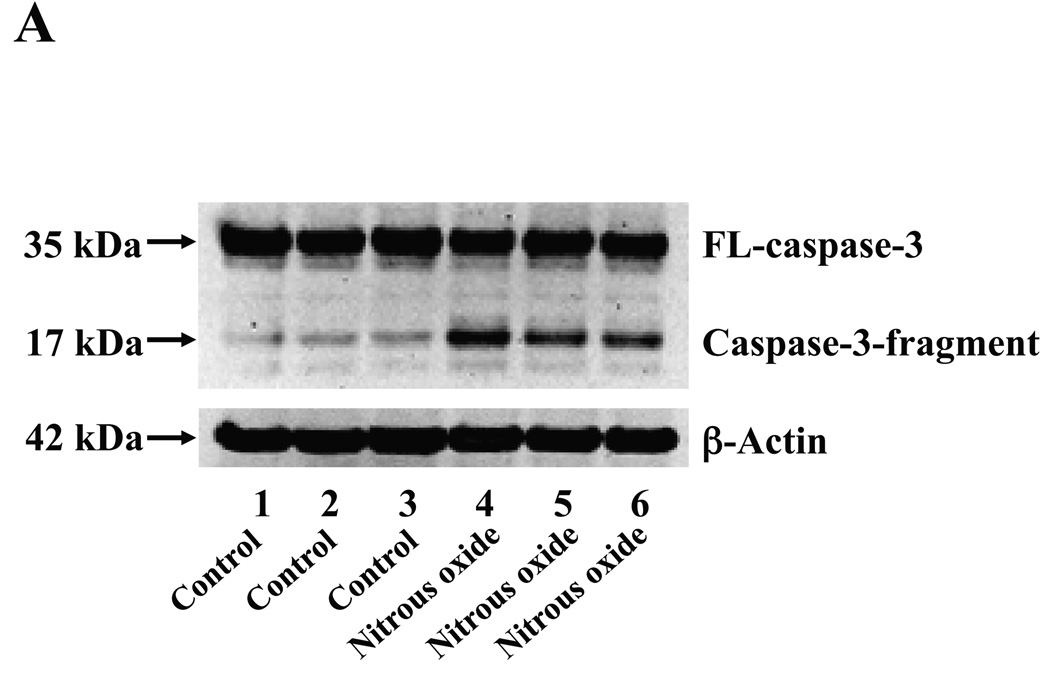
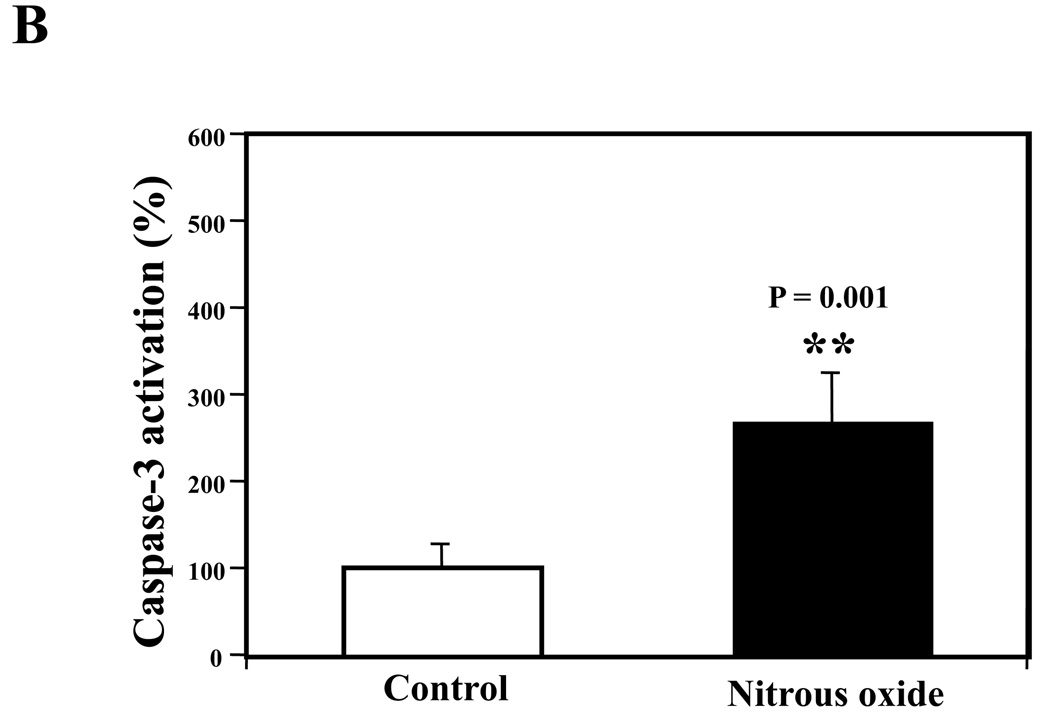
Next, we asked whether the nitrous oxide plus treatment can induce apoptosis in cells without overexpressing human APP or in healthy neurons. We were able to show that the treatment with 70% nitrous oxide plus l% isoflurane for six hours or staurosporine (the positive control in the experiments) induced caspase-3 activation as compared to control condition in H4 naïve cells (Figure 2A and 2B): 100% versus 172%, P = 0.0006, and primary neurons from naïve mice (Figure 3A and 3B): 100% versus 154%, P = 0.0357. Moreover, the treatment of nitrous oxide plus isoflurane increased TUNEL positive cells (apoptosis) as compared to the control condition in H4 naïve cells (See Figure 2 in Supplemental Digital Content 1, which is histological image showing effects of nitrous oxide plus isoflurane on number of TUNEL positive H4 naïve cells; and Figure 2C): 100% versus 376%, P = 0.0001, and in primary neurons from naïve mice (See Figure 3 in Supplemental Digital Content 1, which is histological image showing effects of nitrous oxide plus isoflurane on number of TUNEL positive primary neurons; Figure 3C): 100% versus 298%, P = 0.0005. We were not able to detect alterations in secreted Aβ levels in H4 naïve cells or primary neurons from naïve mice because the endogenous levels of Aβ in the cells and neurons are low. Collectively, these results illustrate that treatment with 70% nitrous oxide plus l% isoflurane for six hours can induce caspase-3 activation and increase TUNEL positive cells (apoptosis) in H4 naïve cells (without APP overload) and in primary neurons from naïve mice (healthy neurons), which suggest that the nitrous oxide plus isoflurane treatment can induce apoptosis in healthy neurons and independent of APP overexpressing.
Figure 3. Treatment of nitrous oxide plus isoflurane induces caspase-3 activation and apoptosis in primary neurons from naïve mice.
A. Effects of 70% nitrous oxide plus 1% isoflurane for six hours, and staurosporine on caspase-3 activation. Each band in the Western blot represents an independent experiment. B. Quantification of the Western blot, normalized to β-actin levels. We have averaged results from three independent experiments. C. Effects of the nitrous oxide plus isoflurane treatment, and staurosporine treatment on TUNEL positive cells. We have averaged results from four independent experiments. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; FL, full length. * P < 0.05; ** P <0.01; ## P < 0.01.
Figure 2. Treatment of nitrous oxide plus isoflurane induces caspase-3 activation and apoptosis in H4 naïve cells.
A. Effects of 70% nitrous oxide plus 1% isoflurane for six hours, and staurosporine on caspase-3 activation. Each band in the Western blot represents an independent experiment. B. Quantification of the Western blot, normalized to β-actin levels. We have averaged results from three independent experiments. C. Effects of the nitrous oxide plus isoflurane treatment on TUNEL positive cells. We have averaged results from four independent experiments. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; FL, full length. ** P <0.01
Nitrous oxide or isoflurane alone induced neither apoptosis nor increases in Aβ levels in H4-APP cells
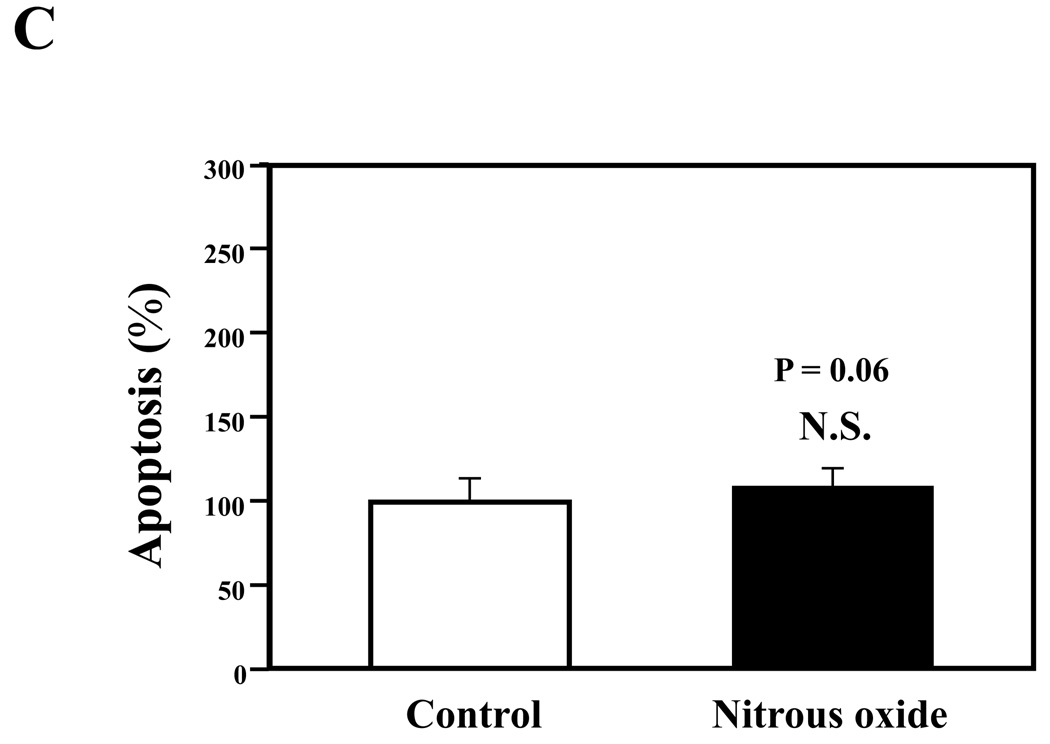
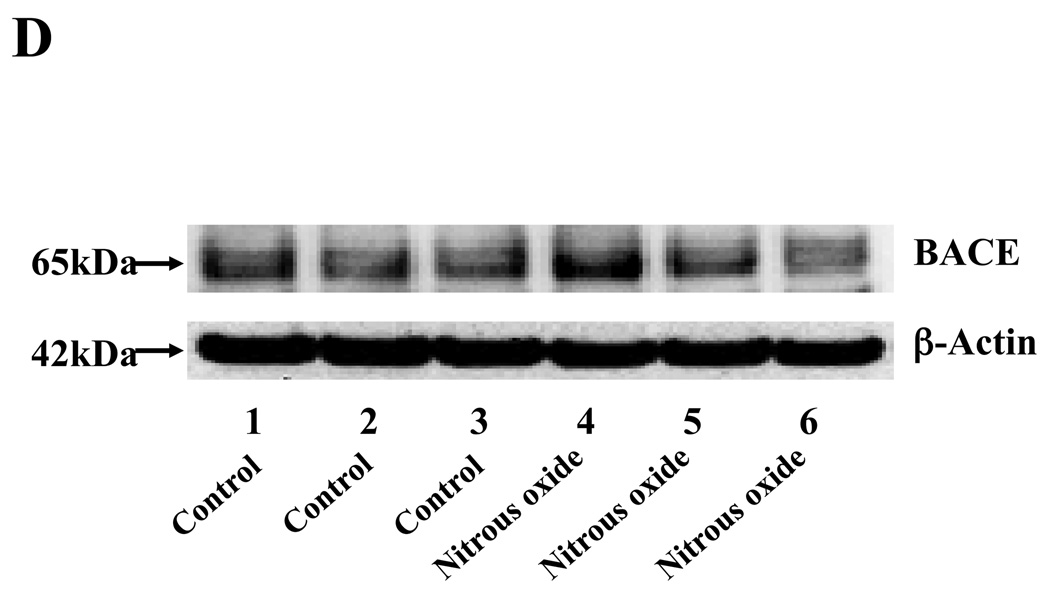
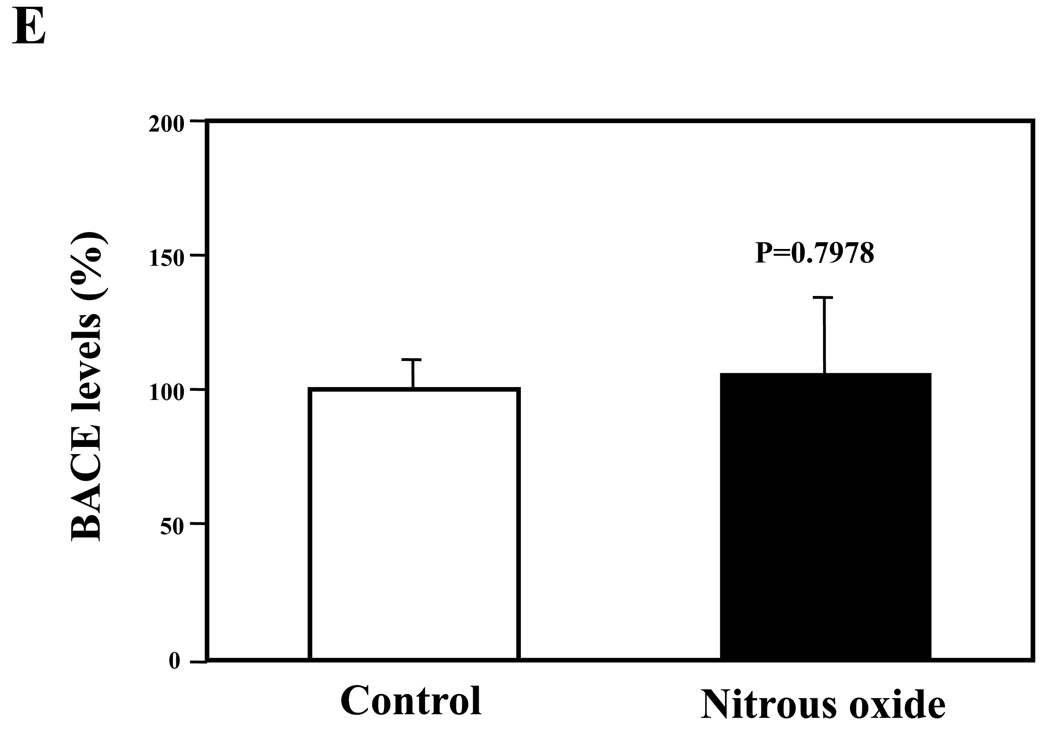
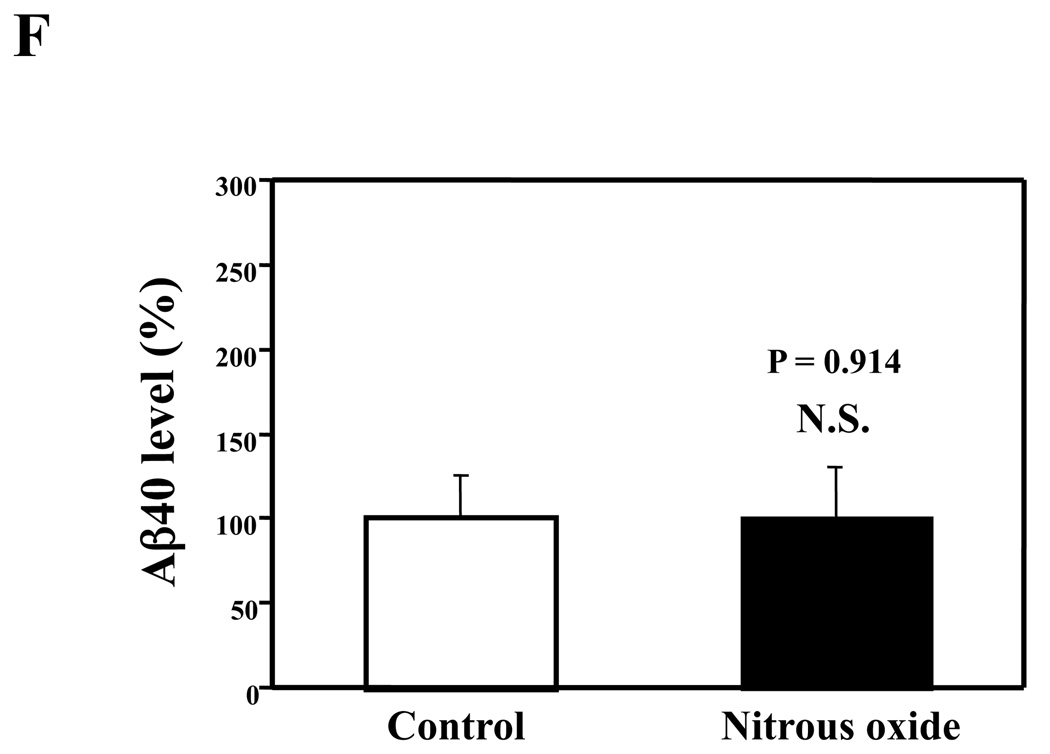
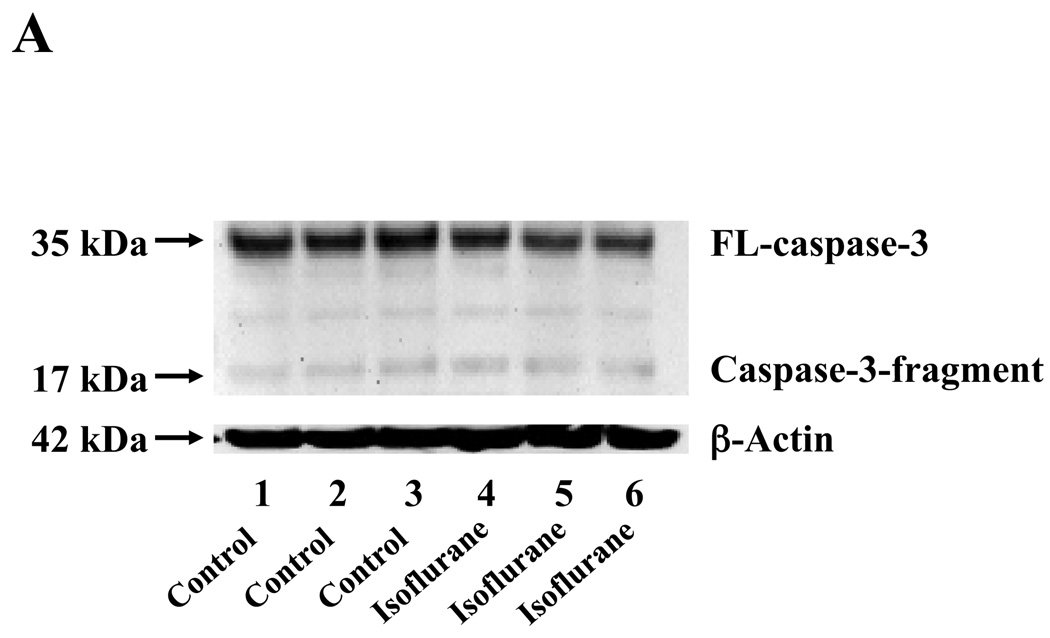
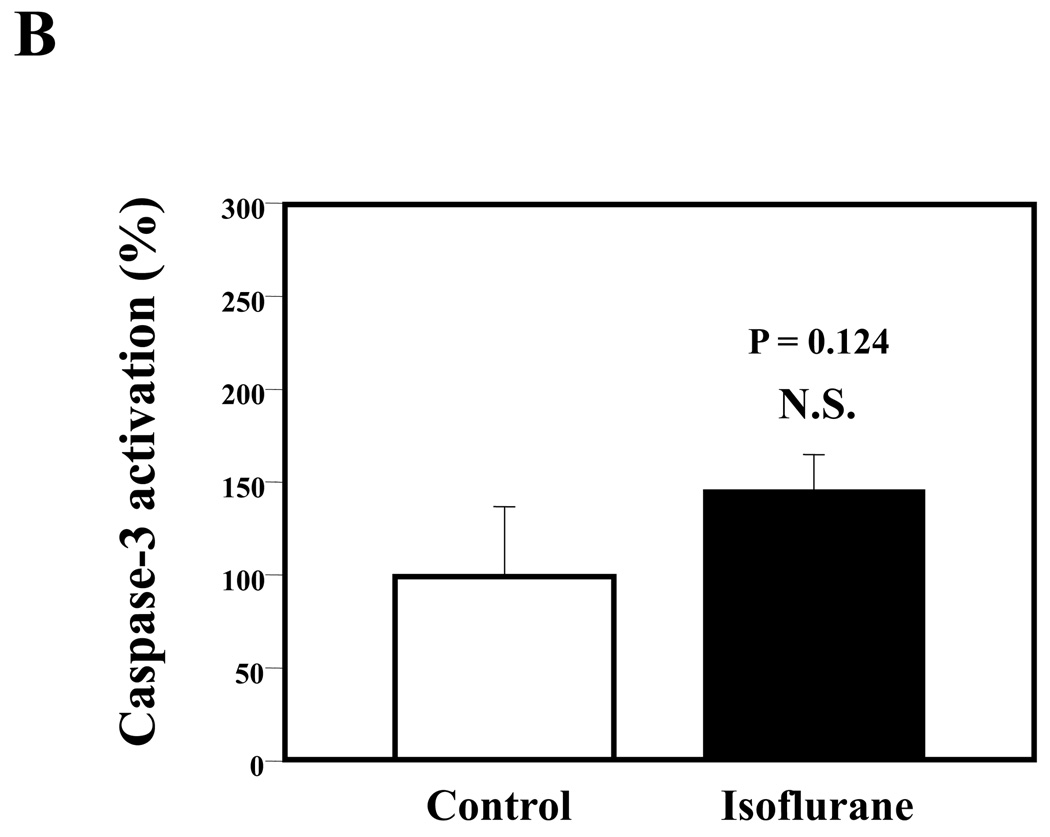
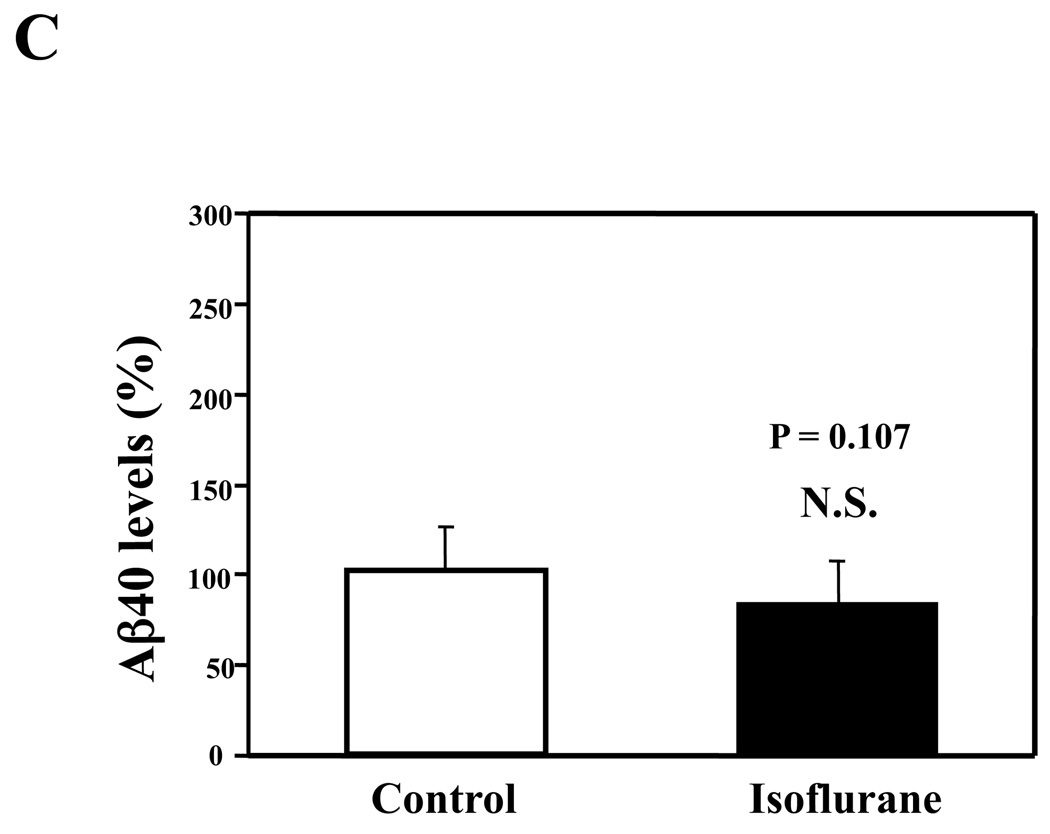
Given that the treatment of 70% nitrous oxide plus 1% isoflurane can induce caspase-3 activation and apoptosis, and can increase levels of BACE and secreted Aβ in H4-APP cells, we next asked whether treatment with 70% nitrous oxide or 1% isoflurane alone can cause the same effects. In the present experiment, treatment with 70% nitrous oxide for six hours induced caspase-3 cleavage (activation) (Figure 4A and Figure 4B): 100% versus 279% (P = 0.001). However, the nitrous oxide treatment induced neither apoptosis, assessed by detecting cytoplasmic histone-associated DNA fragmentation (Figure 4C, 100% versus 109%, P = 0.06, N.S.), nor increases in BACE levels (Figure 4D and 4E, 100% versus 105%, P = 0.7978, N.S.) as compared to control condition. Finally, the nitrous oxide treatment did not increase secreted Aβ40 levels as compared to the control condition (Figure 4F): 100% versus 101% (P = 0.914, N.S.). These results indicate that even 70% nitrous oxide induces caspase-3 activation; the nitrous oxide treatment alone induces neither apoptosis nor increases in BACE and Aβ levels. Next, we assessed whether 1% isoflurane alone can increase Aβ levels in H4-APP cells. Treatment with 1% isoflurane for six hours did not induce caspase-3 activation (Figure 5A and 5B) and did not increase Aβ levels (Figure 5C). Taken together, these findings suggest that the apoptosis and Aβ level increase induced by 70% nitrous oxide plus 1% isoflurane are likely resulted from the synergistic effects of nitrous oxide plus isoflurane.
Figure 4. Nitrous oxide treatment alone induces neither apoptosis nor increases in levels of BACE and Aβ in H4-APP cells.
A. Effects of 70% nitrous oxide on caspase-3 activation. Each band in the Western blot represents an independent experiment. B. Quantification of Western blot, normalized to β-actin levels. We have averaged results from three independent experiments. C. Effects of the nitrous oxide treatment on apoptosis. We have averaged results from six independent experiments. D. Effects of the nitrous oxide treatment on BACE levels. Each band in the Western blot represents an independent experiment. E. Quantification of the Western blot, normalized to β-actin levels. We have averaged results from three independent experiments. F. Effects of the nitrous oxide treatment on Aβ levels. We have averaged results from six independent experiments. BACE, β-site amyloid precursor protein-cleaving enzyme; APP, amyloid precursor protein; Aβ, β-amyloid protein; FL, full length. N.S., not significant; ** P <0.01
Figure 5. Isoflurane treatment alone induces neither caspase-3 activation nor Aβ accumulation in H4-APP cells.
A. Effects of 1% isoflurane for six hours on caspase-3 activation. Each band in the estern blot represents an independent experiment. B. Quantification of Western blot, normalized to β-actin levels. We have averaged results from three independent experiments. C. Effects of the isoflurane treatment on Aβ levels. We have averaged results from three independent experiments. APP, amyloid precursor protein; Aβ, β-amyloid protein; FL, full length. N.S., not significant.
Caspase inhibitor Z-VAD attenuated the nitrous oxide plus isoflurane-induced caspase-3 activation
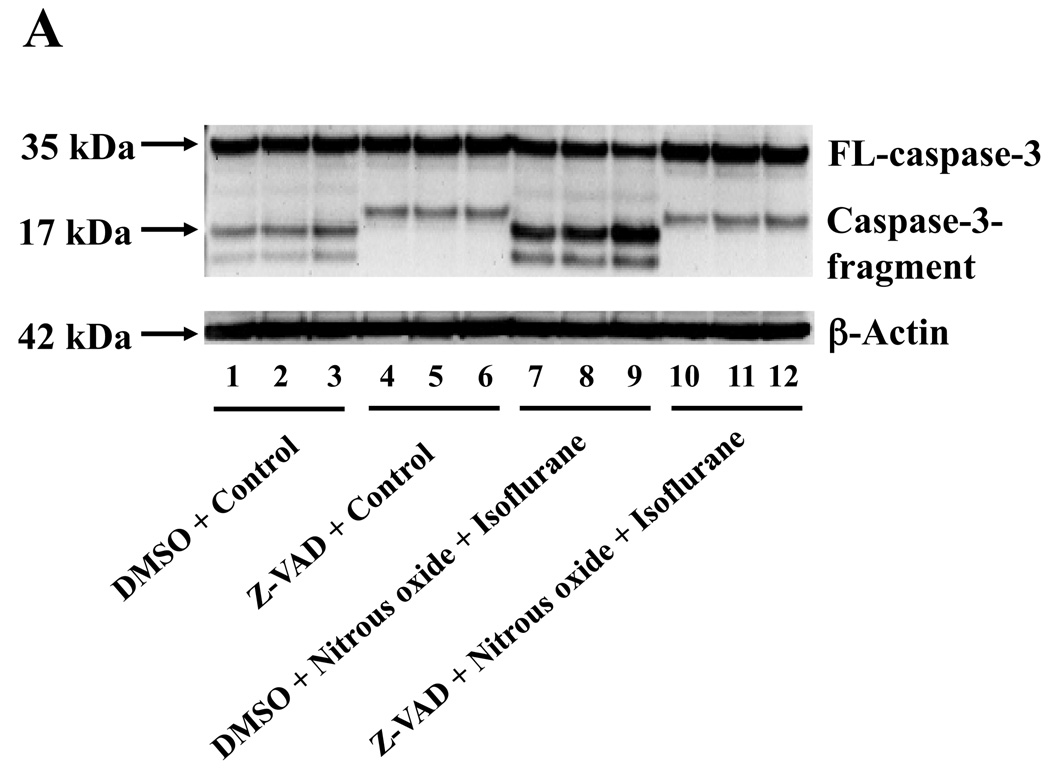
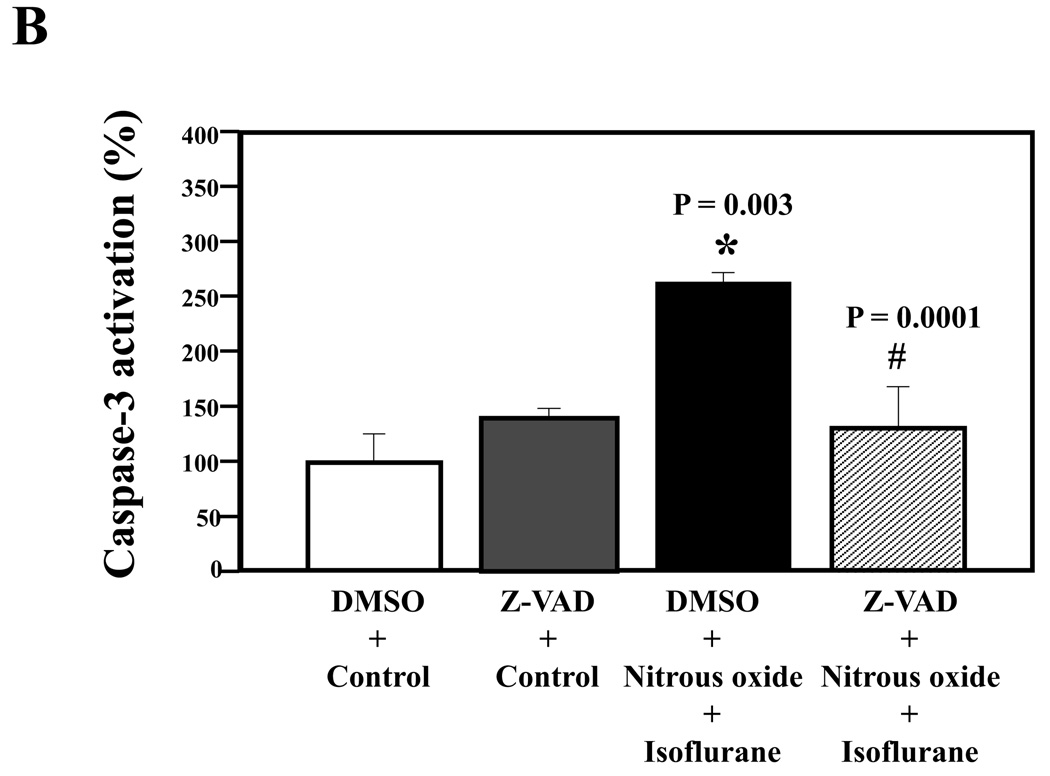
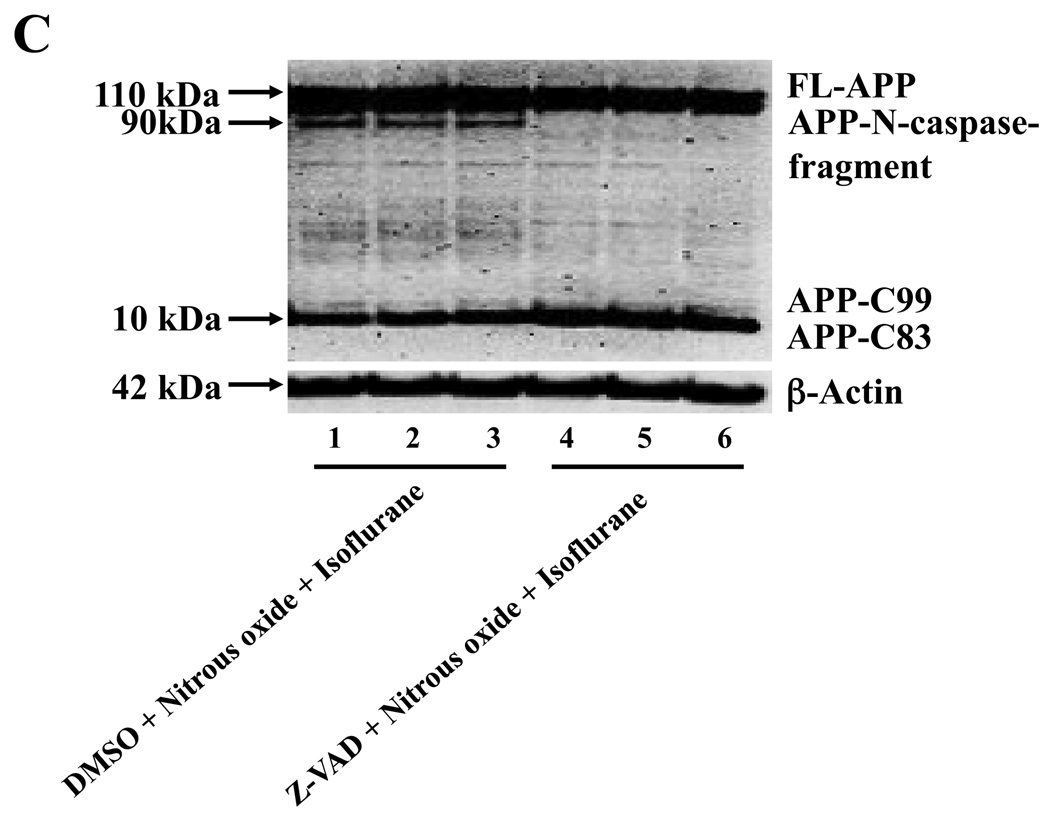
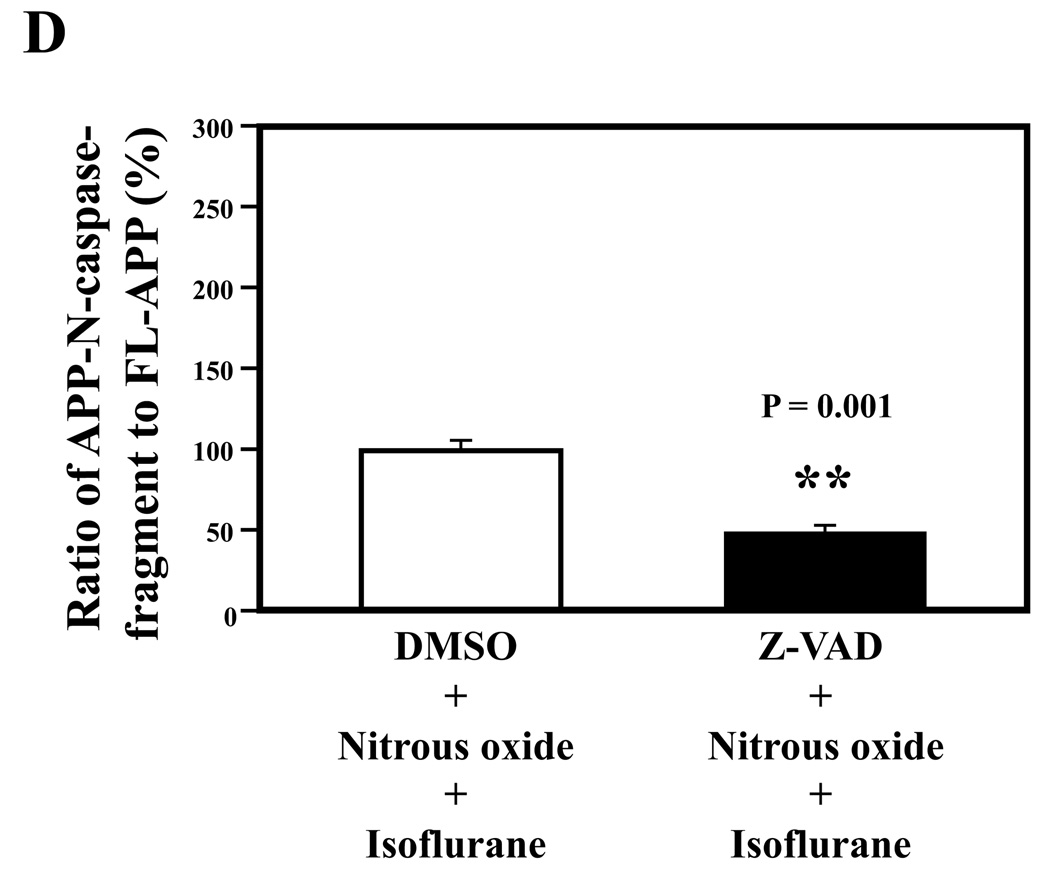
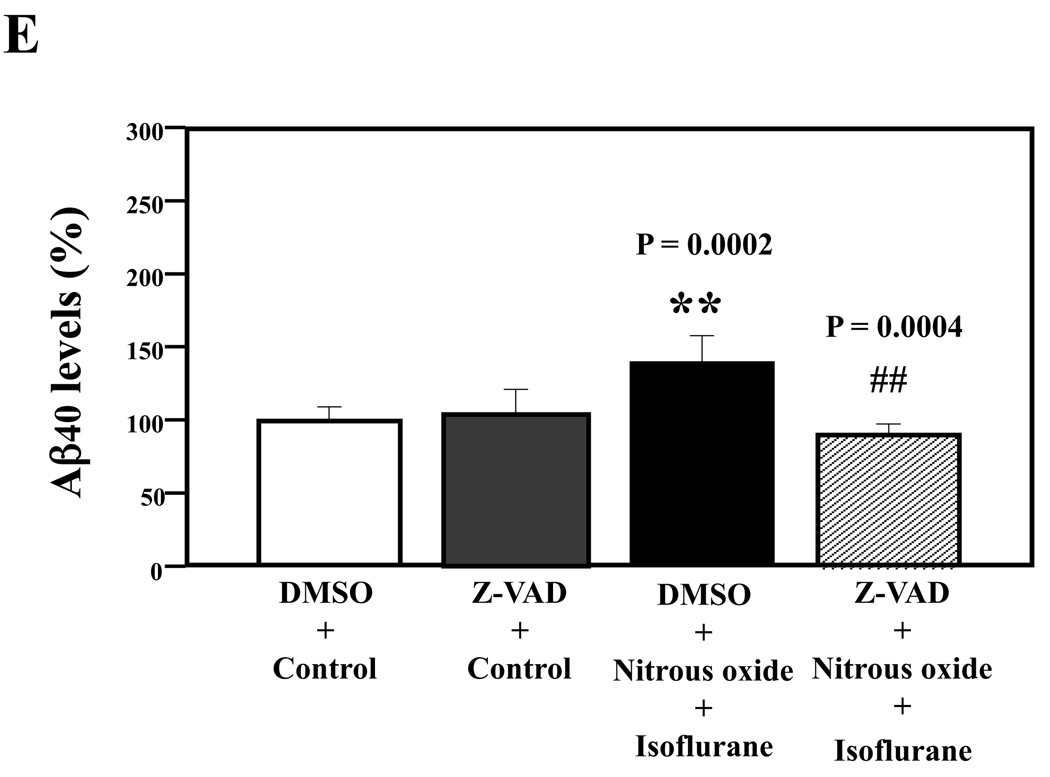
Given the findings that nitrous oxide plus isoflurane can induce apoptosis, increase levels of BACE and Aβ, we next asked whether the nitrous oxide plus isoflurane-induced alteration in APP processing and Aβ levels is dependent on the ability of nitrous oxide plus isoflurane to induce caspase-3 activation and apoptosis. For this purpose, we set out to assess the effects of the caspase inhibitor Z-VAD on nitrous oxide plus isoflurane-induced alterations in caspase-3 activation, APP processing and Aβ levels in H4-APP cells. Whereas Z-VAD alone did not affect caspase-3 activation, the Z-VAD treatment attenuated the nitrous oxide plus isoflurane-induced caspase-3 activation as compared to the control condition (Figure 6A). The observed ~20 kDa bands that only appeared in the Z-VAD-treated cells lysates in the Western blot were most likely caspase-dependent, non-specific, cross-reacting bands. However, the identity of the bands is unknown. Quantification of the Western blots revealed that Z-VAD attenuated nitrous oxide plus isoflurane-induced caspase-3 activation: 263% versus 131%, P = 0.0001 (Figure 6B). Furthermore, Z-VAD attenuated the nitrous oxide plus isoflurane-induced increases in levels of caspase-cleaved APP N-terminus fragment (Figure 6C and 6D), P = 0.001. Finally, Z-VAD reduced nitrous oxide plus isoflurane-induced increases in secreted Aβ40 levels in H4-APP cells, P = 0.0004 (Figure 6E). Collectively, these findings suggest that nitrous oxide plus isoflurane-induced APP processing and Aβ generation is dependent on the nitrous oxide plus isoflurane-induced caspase activation and apoptosis.
Figure 6. Caspase inhibitor Z-VAD attenuates the nitrous oxide plus isoflurane-induced caspase-3 activation and Aβ generation in H4-APP cells.
A. Effects of Z-VAD on the nitrous oxide plus isoflurane-induced caspase-3 activation in H4-APP cells. Each band in the Western blot represents an independent experiment. B. Quantification of the Western blot, normalized to β-actin levels. We have averaged results from three independent experiments. C. Nitrous oxide plus isoflurane (lanes 1–3) increases levels of APP-N-caspase fragment, which is attenuated by Z-VAD treatment (lanes 4–6) in H4-APP cells. Each band in the Western blot represents an independent experiment. D. Quantification of the Western blot shows that Z-VAD (black bar) decreases nitrous oxide plus isoflurane-induced increases in the ratio of APP-N-caspase-fragment to FL-APP as compared to nitrous oxide plus isoflurane treatment (white bar), normalized to β-actin levels. We have averaged results from three independent experiments. E. Z-VAD (net bar) reduces the nitrous oxide plus isoflurane-induced increases in Aβ40 levels in H4-APP cells. We have averaged results from six independent experiments. APP, amyloid precursor protein. DMSO, dimethyl sulfoxide; FL, full length. * P < 0.05; ** P <0.01; # P < 0.05; ## P < 0.01.
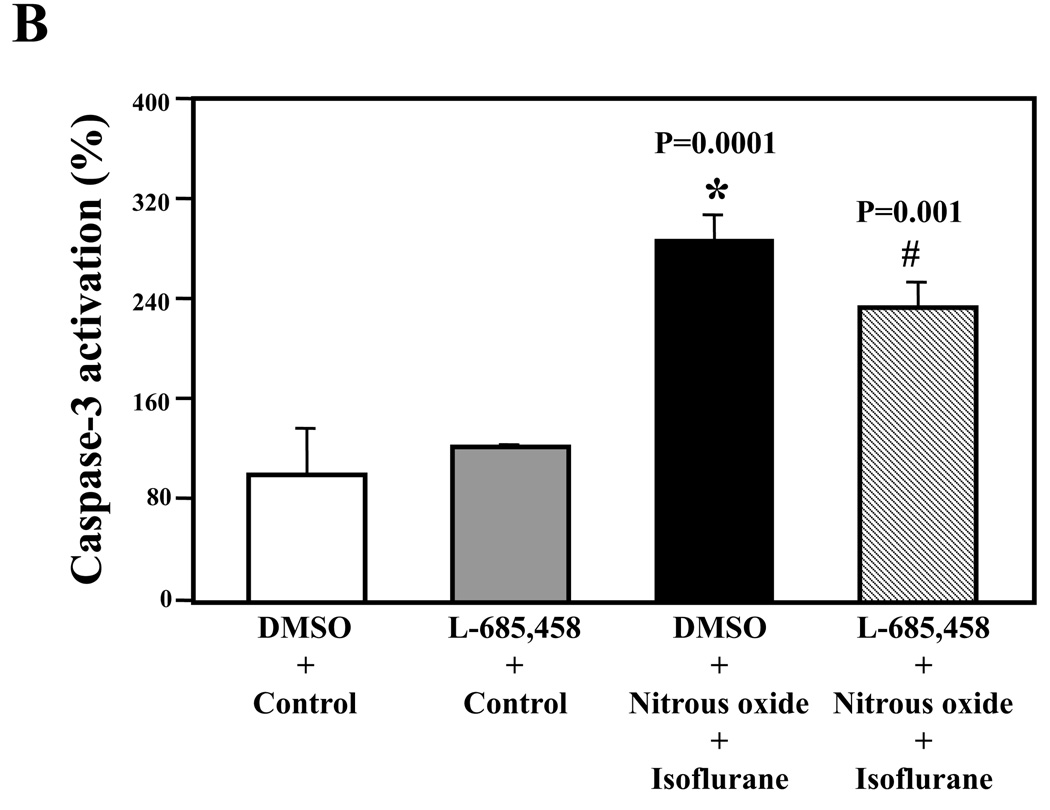
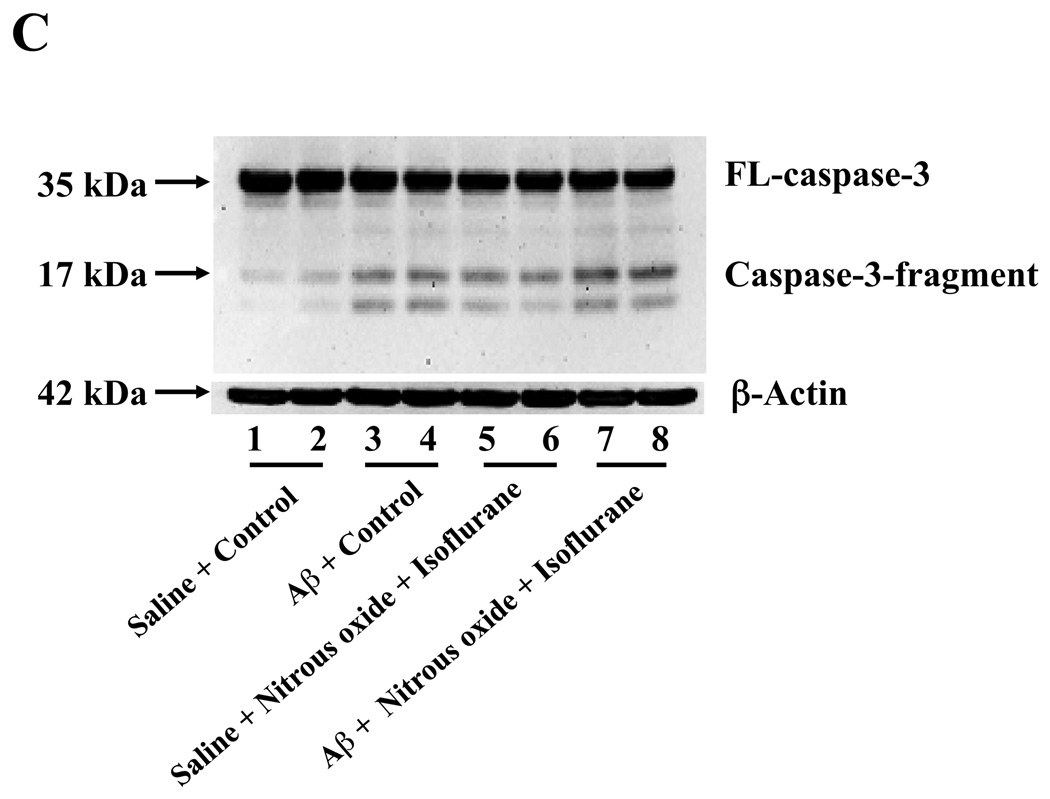
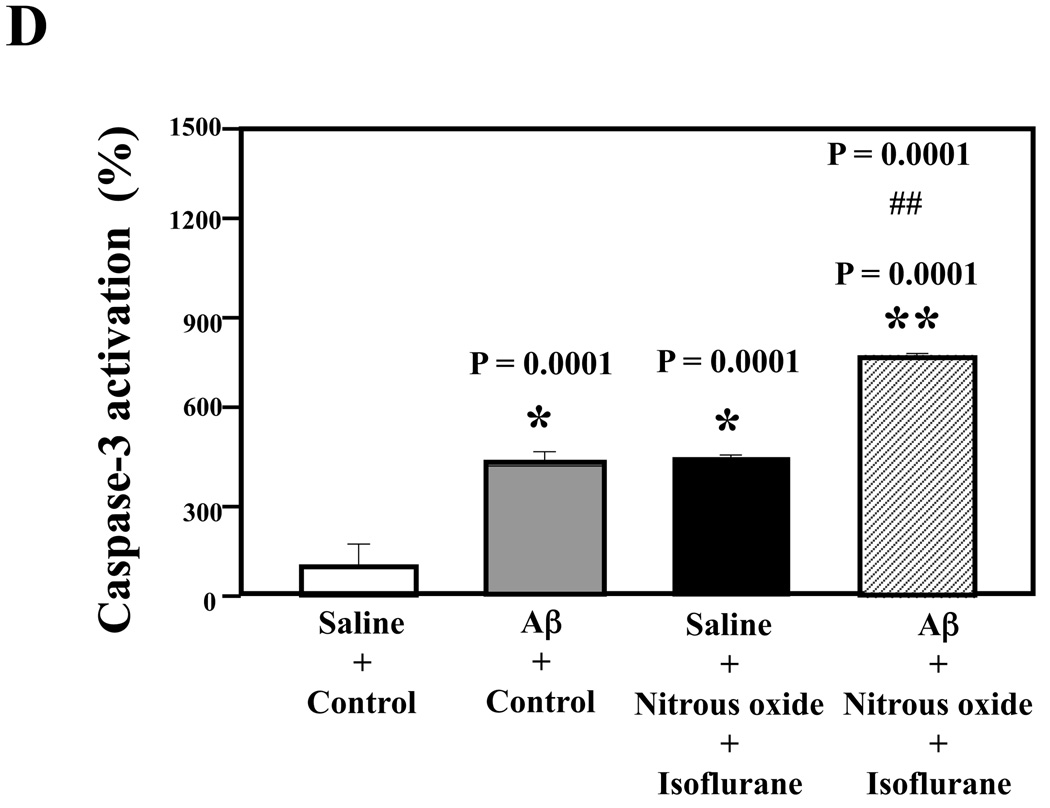
Nitrous oxide plus isoflurane-induced caspase-3 activation was attenuated by the γ-secretase inhibitor L-685,458 but was potentiated by Aβ
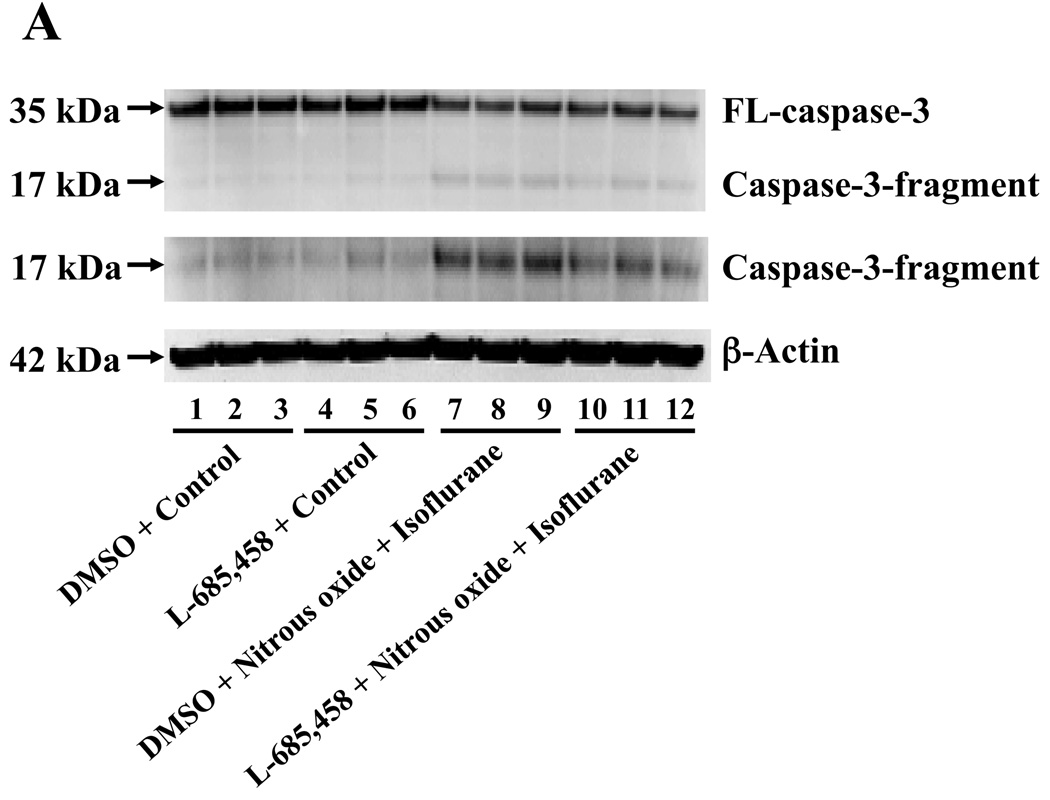
Next, we asked whether pharmacologically-based reductions in Aβ levels can attenuate nitrous oxide plus isoflurane-induced caspase-3 activation. For this purpose, we assessed the effects of the γ-secretase inhibitor L-685,458 on nitrous oxide plus isoflurane-induced caspase-3 activation in H4-APP cells. L-685,458 reduced the nitrous oxide plus isoflurane-induced caspase-3 activation: 287% versus 232%, P = 0.0001 (Figure 7A and 7B). Next, we asked whether a pro-apoptotic stimulus, 7.5 uM Aβ42, could potentiate nitrous oxide plus isoflurane-induced caspase-3 activation. We were able to show the Aβ treatment enhanced the nitrous oxide plus isoflurane-induced caspase-3 activation: 447% versus 772%, P = 0.0004 (Figure 7C and 7D). Collectively, these results suggest that Aβ can potentiate the nitrous oxide plus isoflurane-induced caspase-3 activation.
Figure 7. γ-Secretase inhibitor L-685,458 attenuates, but Aβ potentiates, the nitrous oxide plus isoflurane-induced caspase-3 activation.
A. Effects of L-685,458 on the nitrous oxide plus isoflurane-induced caspase-3 activation. Each band in the Western blot represents an independent experiment. B. Quantification of the Western blot shows that L-685,458 treatment (net bar) attenuates the nitrous oxide plus isoflurane-induced caspase-3 activation, normalized to β-actin levels. We have averaged results from three independent experiments. C. Effects of Aβ on the nitrous oxide plus isoflurane-induced caspase-3 activation. Each band in the Western blot represents an independent experiment. D. Quantification of the Western blot shows that Aβ treatment potentiates the nitrous oxide plus isoflurane-induced caspase-3 activation. We have averaged results from three independent experiments. Aβ, β-amyloid protein. DMSO, dimethyl sulfoxide; FL, full length. * P < 0.05; ** P <0.01; # P < 0.05.
Discussion
The commonly used inhalation anesthetic isoflurane has previously been shown to promote Aβ aggregation and to enhance toxicity of Aβ 39. We have shown that isoflurane 7,40–42, sevoflurane 53, and desflurane plus hypoxia 54 can induce cellular apoptosis and increase Aβ generation. Nitrous oxide plus isoflurane are common anesthetics for patients. Moreover, animal studies have shown that anesthesia with 70% nitrous oxide plus 1.2% isoflurane can cause a long-term impairment of learning and memory in aged rats 55. We therefore set out to assess effects of nitrous oxide plus isoflurane on apoptosis and Aβ generation. We were able to show that treatment with 70% nitrous oxide plus 1% isoflurane for six hours induced caspase activation and apoptosis, and increased levels of BACE and secreted Aβ in H4-APP cells. Moreover, we have found that the treatment with 70% nitrous oxide plus 1% isoflurane for six hours can induce caspase activation and apoptosis without alteration in secreted Aβ levels in H4 naïve cells and primary neurons from naïve mice. However, it is possible that the treatment of isoflurane plus nitrous oxide may lead to undetectable changes in secreted Aβ levels in the H4 naïve cells and the primary neurons because the endogenous levels of Aβ in the H4 naïve cells and the primary neurons from naïve mice are low. The cultured neurons in the experiments were postnatal day 10 neurons, therefore, the findings in the primary neurons that nitrous oxide plus isoflurane can induce caspase activation and apoptosis suggest that the clinically relevant treatment of nitrous oxide plus isoflurane may cause neurotoxicity in developing neurons. The findings in H4-APP cells that isoflurane plus nitrous oxide can induce caspase activation and apoptosis, and can increase levels of BACE and secreted Aβ suggest that the clinically relevant treatment of nitrous oxide plus isoflurane can cause neurotoxicity, including apoptosis and Aβ generation, which are features of AD neuropathogenesis.
Consistent with our previous findings 41, we were able to show that treatment with 1% isoflurane alone for six hours did not induce caspase-3 activation. Furthermore, we found the 1% isoflurane treatment did not increase Aβ levels in H4-APP cells. We found that treatment of 70% nitrous oxide alone for six hours induced neither apoptosis nor increases in Aβ levels in current experiments. It is interesting that treatment with 70% nitrous oxide alone for six hours induced casapse-3 activation in H4-APP cells. McLaughlin et al. 52 have shown that caspase-3 activation alone may not represent apoptotic cell damage; therefore, it is possible that the treatment of 70% nitrous oxide induces caspase-3 activation without causing apoptosis and increasing Aβ levels. In the future studies, we will employ cellular imaging methods to determine the location of the activated caspase-3: nuclear or cytoplasm, because activated caspase-3 in the nucleus may be strongly associated with apoptosis 52. These results would further support our conclusion that apoptosis, but not caspase activation alone, can enhance BACE levels to facilitate APP processing, leading to Aβ generation.
It has been reported that N-methyl-D-aspartic acid (NMDA) receptor antagonists and gamma-aminobutyric acid (GABA) receptor agonists can induce apoptosis in the developing rodent brain. Moreover, simultaneous use of NMDA receptor antagonists and GABA receptor agonists can induce a greater degree of apoptosis 56–58. Nitrous oxide is a NMDA receptor antagonist 59 and isoflurane is a GABA receptor agonist 60–62. Therefore, we have postulated that the NMDA receptor antagonist nitrous oxide (70%) and the GABA receptor agonist isoflurane (1%) can have a synergistic effect, leading to apoptosis, whereas the 70% nitrous oxide or 1% isoflurane alone is not potent enough to induce the apoptosis. Previous studies have suggested that a treatment with 2% isoflurane for six hours may induce apoptosis by elevating cytosolic calcium levels 24. Collectively, we have hypothesized that low concentration isoflurane (e.g., 1%) may act as GABA receptor agonist, together with NMDA receptor antagonist (e.g., 70% nitrous oxide), to induce apoptosis, whereas high concentration isoflurane (e.g., 2%) alone will be able to induce apoptosis via enhancing cytosolic calcium levels. Future studies are needed to test this hypothesis by systemically assessing effects of nitrous oxide and/or isoflurane, as well as other NMDA receptor antagonists and GABA agonists, alone or in combination, on caspase activation and apoptosis.
In order to further explore the mechanisms by which nitrous oxide plus isoflurane induces apoptosis, affects APP processing and increases Aβ levels, we assessed the effects of nitrous oxide plus isoflurane on levels of BACE, the enzyme that cleaves APP to generate APP-C99, leading to Aβ generation. We have found that treatment of nitrous oxide plus isoflurane can increase levels of BACE. Early studies have shown that caspase activation and apoptosis, induced by isoflurane in vitro 42 and in vivo 7, sevoflurane 53, desflurane plus hypoxia 54 and ischemia 9, can increase BACE levels, leading to Aβ generation. Nitrous oxide plus isoflurane has been shown to induce caspase activation and apoptosis, to increase levels of BACE and Aβ in the present experiments. Thus, it is likely that treatment with nitrous oxide plus isoflurane can cause apoptosis, which then increases levels and activity of BACE to facilitate APP processing, finally leading to an increase in Aβ levels.
The mechanism by which nitrous oxide plus isoflurane increases BACE levels is still unknown. Recent studies have suggested that isoflurane 7 and ischemia 9 can induce caspase activation and apoptosis, which subsequently cause reductions in protein levels of Golgi-localized γ-ear-containing adenonisine disphosphate-ribosylation factor binding protein-3, a protein that can facilitate trafficking and metabolism of BACE, ultimately leading to increases in levels of BACE and activity of β-secretase. The future studies should include assessing whether the treatment with nitrous oxide plus isoflurane might likewise reduce levels of Golgi-localized γ-ear-containing adenonisine disphosphate-ribosylation factor binding protein-3 to enhance BACE levels and β-secretase activity subsequent to caspase activation and apoptosis in vitro and in vivo.
Z-VAD, a broad caspase activation inhibitor, has been shown to attenuate not only the nitrous oxide plus isoflurane-induced caspase-3 activation, but also the nitrous oxide plus isoflurane-induced APP processing and Aβ generation. Moreover, the same treatment of nitrous oxide plus isoflurane can induce caspase-3 activation and apoptosis without detectable changes in APP processing and Aβ levels in H4 naïve cells and primary neurons from naïve mice. Collectively, these results suggest that the nitrous oxide plus isoflurane-induced alterations in APP processing and Aβ generation are largely dependent on the ability of nitrous oxide plus isoflurane to induce caspase activation and apoptosis.
Finally, we assessed whether increases and reductions in Aβ levels can potentiate and attenuate, respectively, nitrous oxide plus isoflurane-induced caspase-3 activation. We have found that L-685,458, a γ-secretase inhibitor which reduces Aβ generation, can inhibit caspase-3 activation. Conversely, treatment with Aβ can potentiate nitrous oxide plus isoflurane-induced caspase-3 activation. Taken together, these results further suggest that nitrous oxide plus isoflurane can induce apoptosis, which then facilitates APP processing, leading to increases in Aβ generation; the increased Aβ levels can potentiate nitrous oxide plus isoflurane-induced apoptosis to form another round of apoptosis and Aβ generation.
Even though the findings in the current studies suggest that nitrous oxide plus isoflurane may cause neurotoxic effects including AD neuropathogenesis, these experiments were performed only in cultured cells. The determination of the in vivo relevance of nitrous oxide plus isoflurane on AD neuropathogenesis in animals and humans will be necessary before we can conclude that the inhalation anesthetic nitrous oxide plus isoflurane facilitates or exacerbates neurotoxicity including AD neuropathogenesis.
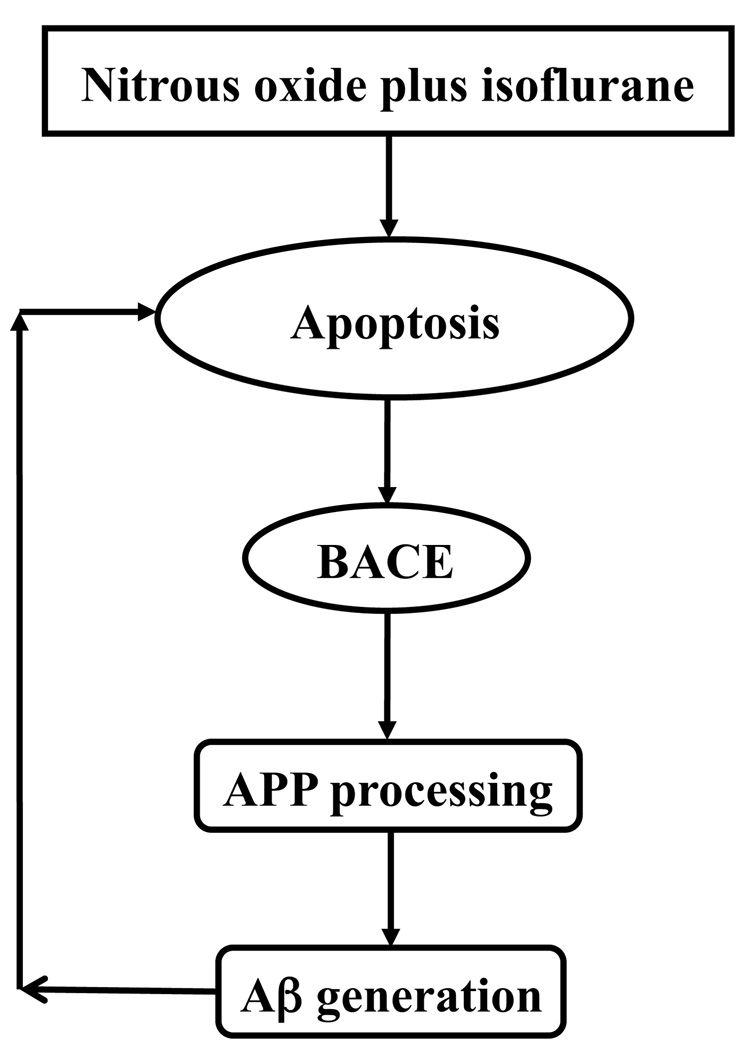
In conclusion, we have found that treatment with 70% nitrous oxide plus 1% isoflurane, but neither alone, can induce apoptosis, facilitate APP processing and increase Aβ generation. Figure 8 shows a scheme in which the treatment of nitrous oxide plus isoflurane induces apoptosis, which leads to increases in BACE and Aβ levels. Increased Aβ levels would then induce further apoptosis, resulting in another round of apoptosis and Aβ generation. Further investigation, especially in vivo studies, will be necessary to assess the potential role of nitrous oxide and/or isoflurane in triggering or driving neurotoxicity including AD neuropathogenesis. These efforts should promote more attempts to assess the effects of anesthetics on AD neuropathogenesis in vitro and in vivo. The findings from all of these studies will ultimately lead to safer and better anesthesia care to patients, especially elderly patients and AD patients.
Figure 8. Hypothetical pathway by which nitrous oxide plus isoflurane induces apoptosis and Aβ generation.
Nitrous oxide plus isoflurane induces apoptosis. Apoptosis, in turn, increases BACE levels, which serves to facilitate APP processing and to increase Aβ generation. Elevated Aβ generation then further induces apoptosis leading to another round of nitrous oxide plus isoflurane-induced apoptosis and Aβ generation. BACE, β-site amyloid precursor protein-cleaving enzyme; APP, amyloid precursor protein; Aβ, β-amyloid protein.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants (Bethesda, Maryland) (K08 NS048140, R21 AG029856 and R01 GM088801); American Geriatrics Society Jahnigen Award (New York, NY); William F. Milton Fund of Harvard University (Cambridge, MA); Investigator-Initiated Research Grant from Alzheimer’s Association (Chicago, IL) to Dr. Zhongcong Xie. The cost of anesthetic isoflurane and partial support of Yuanlin Dong were provided by the Department of Anesthesia and Critical Care, Massachusetts General Hospital and Harvard Medical School.
Footnotes
Conflict of interest statement: Dr. Zhongcong Xie is a consultant of Baxter Healthcare Corporation, New Providence, NJ, the company that produces isoflurane. The company neither supported nor had any other connections with the current study.
Part of the work was presented in the annual meeting of American Society of Anesthesiologists on Oct. 18, 2008 in Orlando, FL.
Summary Statement: 70% nitrous oxide plus 1% isoflurane induces apoptosis and increases β-amyloid protein levels in vitro. Pending on in vivo confirmation, these results suggest that nitrous oxide plus isoflurane may cause neurotoxicity.
References
- 1.Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: Sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: A genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer's disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 4.Gu Y, Misonou H, Sato T, Dohmae N, Takio K, Ihara Y. Distinct intramembrane cleavage of the beta-amyloid precursor protein family resembling gamma-secretase-like cleavage of Notch. J Biol Chem. 2001;276:35235–35238. doi: 10.1074/jbc.C100357200. [DOI] [PubMed] [Google Scholar]
- 5.Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron MM, Teplow DB, Haass C. Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep. 2001;2:835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu C, Kim SH, Ikeuchi T, Xu H, Gasparini L, Wang R, Sisodia SS. Characterization of a presenilin-mediated amyloid precursor protein carboxyl-terminal fragment gamma. Evidence for distinct mechanisms involved in gamma -secretase processing of the APP and Notch1 transmembrane domains. J Biol Chem. 2001;276:43756–43760. doi: 10.1074/jbc.C100410200. [DOI] [PubMed] [Google Scholar]
- 7.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesco G, Koh YH, Tanzi RE. Caspase activation increases beta-amyloid generation independently of caspase cleavage of the beta-amyloid precursor protein (APP) J Biol Chem. 2003;278:46074–46080. doi: 10.1074/jbc.M307809200. [DOI] [PubMed] [Google Scholar]
- 9.Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masliah E, Mallory M, Alford M, Tanaka S, Hansen LA. Caspase dependent DNA fragmentation might be associated with excitotoxicity in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:1041–1052. doi: 10.1097/00005072-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Yang F, Sun X, Beech W, Teter B, Wu S, Sigel J, Vinters HV, Frautschy SA, Cole GM. Antibody to caspase-cleaved actin detects apoptosis in differentiated neuroblastoma and plaque-associated neurons and microglia in Alzheimer's disease. Am J Pathol. 1998;152:379–389. [PMC free article] [PubMed] [Google Scholar]
- 12.Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- 13.Shimohama S, Tanino H, Fujimoto S. Changes in caspase expression in Alzheimer's disease: Comparison with development and aging. Biochem Biophys Res Commun. 1999;256:381–384. doi: 10.1006/bbrc.1999.0344. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc A. Increased production of 4 kDa amyloid beta peptide in serum deprived human primary neuron cultures: Possible involvement of apoptosis. J Neurosci. 1995;15:7837–7846. doi: 10.1523/JNEUROSCI.15-12-07837.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeBlanc A, Liu H, Goodyer C, Bergeron C, Hammond J. Caspase-6 role in apoptosis of human neurons, amyloidogenesis, and Alzheimer's disease. J Biol Chem. 1999;274:23426–23436. doi: 10.1074/jbc.274.33.23426. [DOI] [PubMed] [Google Scholar]
- 16.Galli C, Piccini A, Ciotti MT, Castellani L, Calissano P, Zaccheo D, Tabaton M. Increased amyloidogenic secretion in cerebellar granule cells undergoing apoptosis. Proc Natl Acad Sci U S A. 1998;95:1247–1252. doi: 10.1073/pnas.95.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sodhi CP, Rampalli S, Perez RG, Koo EH, Quinn B, Gottardi-Littell NR. The endocytotic pathway is required for increased A beta 42 secretion during apoptosis. Brain Res Mol Brain Res. 2004;128:201–211. doi: 10.1016/j.molbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP. Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Florent S, Malaplate-Armand C, Youssef I, Kriem B, Koziel V, Escanye MC, Fifre A, Sponne I, Leininger-Muller B, Olivier JL, Pillot T, Oster T. Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-beta oligomers. J Neurochem. 2006;96:385–395. doi: 10.1111/j.1471-4159.2005.03541.x. [DOI] [PubMed] [Google Scholar]
- 20.Kriem B, Sponne I, Fifre A, Malaplate-Armand C, Lozac'h-Pillot K, Koziel V, Yen-Potin FT, Bihain B, Oster T, Olivier JL, Pillot T. Cytosolic phospholipase A2 mediates neuronal apoptosis induced by soluble oligomers of the amyloid-beta peptide. Faseb J. 2005;19:85–87. doi: 10.1096/fj.04-1807fje. [DOI] [PubMed] [Google Scholar]
- 21.Pillot T, Drouet B, Queille S, Labeur C, Vandekerchkhove J, Rosseneu M, Pincon-Raymond M, Chambaz J. The nonfibrillar amyloid beta-peptide induces apoptotic neuronal cell death: Involvement of its C-terminal fusogenic domain. J Neurochem. 1999;73:1626–1634. doi: 10.1046/j.1471-4159.1999.0731626.x. [DOI] [PubMed] [Google Scholar]
- 22.Sponne I, Fifre A, Drouet B, Klein C, Koziel V, Pincon-Raymond M, Olivier JL, Chambaz J, Pillot T. Apoptotic neuronal cell death induced by the non-fibrillar amyloid-beta peptide proceeds through an early reactive oxygen species-dependent cytoskeleton perturbation. J Biol Chem. 2003;278:3437–3445. doi: 10.1074/jbc.M206745200. [DOI] [PubMed] [Google Scholar]
- 23.Albrecht S, Bourdeau M, Bennett D, Mufson EJ, Bhattacharjee M, LeBlanc AC. Activation of caspase-6 in aging and mild cognitive impairment. Am J Pathol. 2007;170:1200–1209. doi: 10.2353/ajpath.2007.060974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang G, Dong Y, Zhang B, Ichinose F, Wu X, Culley DJ, Crosby G, Tanzi RE, Xie Z. Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci. 2008;28:4551–4560. doi: 10.1523/JNEUROSCI.5694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeBlanc AC. The role of apoptotic pathways in Alzheimer's disease neurodegeneration and cell death. Curr Alzheimer Res. 2005;2:389–402. doi: 10.2174/156720505774330573. [DOI] [PubMed] [Google Scholar]
- 26.Raina AK, Hochman A, Ickes H, Zhu X, Ogawa O, Cash AD, Shimohama S, Perry G, Smith MA. Apoptotic promoters and inhibitors in Alzheimer's disease: Who wins out? Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:251–254. doi: 10.1016/S0278-5846(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 27.Bohnen NI, Warner MA, Kokmen E, Beard CM, Kurland LT. Alzheimer's disease and cumulative exposure to anesthesia: A case-control study. J Am Geriatr Soc. 1994;42:198–201. doi: 10.1111/j.1532-5415.1994.tb04952.x. [DOI] [PubMed] [Google Scholar]
- 28.Bohnen N, Warner MA, Kokmen E, Kurland LT. Early and midlife exposure to anesthesia and age of onset of Alzheimer's disease. Int J Neurosci. 1994;77:181–185. doi: 10.3109/00207459408986029. [DOI] [PubMed] [Google Scholar]
- 29.Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer's disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005;7:319–324. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- 30.Knopman DS, Petersen RC, Cha RH, Edland SD, Rocca WA. Coronary artery bypass grafting is not a risk factor for dementia or Alzheimer disease. Neurology. 2005;65:986–990. doi: 10.1212/01.wnl.0000171954.92119.c7. [DOI] [PubMed] [Google Scholar]
- 31.Gasparini M, Vanacore N, Schiaffini C, Brusa L, Panella M, Talarico G, Bruno G, Meco G, Lenzi GL. A case-control study on Alzheimer's disease and exposure to anesthesia. Neurol Sci. 2002;23:11–14. doi: 10.1007/s100720200017. [DOI] [PubMed] [Google Scholar]
- 32.Harris RA, Eger EI., 2nd Alzheimer's disease and anesthesia: out of body, out of mind…or not? Ann Neurol. 2008;64:595–597. doi: 10.1002/ana.21575. [DOI] [PubMed] [Google Scholar]
- 33.Kokmen E, Whisnant JP, O'Fallon WM, Chu CP, Beard CM. Dementia after ischemic stroke: A population-based study in Rochester, Minnesota (1960–1984) Neurology. 1996;46:154–159. doi: 10.1212/wnl.46.1.154. [DOI] [PubMed] [Google Scholar]
- 34.Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Joachim C, Litchfield S, Barnetson L, Smith AD. The effects of additional pathology on the cognitive deficit in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:165–170. doi: 10.1097/00005072-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–817. [PubMed] [Google Scholar]
- 36.Jendroska K, Hoffmann OM, Patt S. Amyloid beta peptide and precursor protein (APP) in mild and severe brain ischemia. Ann N Y Acad Sci. 1997;826:401–405. doi: 10.1111/j.1749-6632.1997.tb48492.x. [DOI] [PubMed] [Google Scholar]
- 37.Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 38.Xie Z, Moir RD, Romano DM, Tesco G, Kovacs DM, Tanzi RE. Hypocapnia induces caspase-3 activation and increases abeta production. Neurodegener Dis. 2004;1:29–37. doi: 10.1159/000076667. [DOI] [PubMed] [Google Scholar]
- 39.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Xie Z, Dong Y, Maeda U, Moir R, Inouye SK, Culley DJ, Crosby G, Tanzi RE. Isoflurane-induced apoptosis: A potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 42.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang B, Dong Y, Zhang G, Moir RD, Xia W, Yue Y, Tian M, Culley DJ, Crosby G, Tanzi RE, Xie Z. The inhalation anesthetic desflurane induces caspase activation and increases amyloid beta-protein levels under hypoxic conditions. J Biol Chem. 2008;283:11866–11875. doi: 10.1074/jbc.M800199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–260. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 46.Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie Z, Romano DM, Tanzi RE. Effects of RNAi-mediated silencing of PEN-2, APH-1a, and nicastrin on wild-type vs FAD mutant forms of presenilin 1. J Mol Neurosci. 2005;25:67–77. doi: 10.1385/JMN:25:1:067. [DOI] [PubMed] [Google Scholar]
- 48.Xie Z, Romano DM, Tanzi RE. RNA interference-mediated silencing of X11alpha and X11beta attenuates amyloid beta-protein levels via differential effects on beta-amyloid precursor protein processing. J Biol Chem. 2005;280:15413–15421. doi: 10.1074/jbc.M414353200. [DOI] [PubMed] [Google Scholar]
- 49.Thornberry NA. Caspases: Key mediators of apoptosis. Chem Biol. 1998;5:R97–R103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- 50.Xie Z, Romano DM, Kovacs DM, Tanzi RE. Effects of RNA interference-mediated silencing of gamma-secretase complex components on cell sensitivity to caspase-3 activation. J Biol Chem. 2004;279:34130–34137. doi: 10.1074/jbc.M401094200. [DOI] [PubMed] [Google Scholar]
- 51.Kovacs DM, Mancini R, Henderson J, Na SJ, Schmidt SD, Kim TW, Tanzi RE. Staurosporine-induced activation of caspase-3 is potentiated by presenilin 1 familial Alzheimer's disease mutations in human neuroglioma cells. J Neurochem. 1999;73:2278–2285. doi: 10.1046/j.1471-4159.1999.0732278.x. [DOI] [PubMed] [Google Scholar]
- 52.McLaughlin B, Hartnett KA, Erhardt JA, Legos JJ, White RF, Barone FC, Aizenman E. Caspase 3 activation is essential for neuroprotection in preconditioning. Proc Natl Acad Sci U S A. 2003;100:715–720. doi: 10.1073/pnas.0232966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, Xie Z. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66:620–631. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang B, Dong Y, Zhang G, Moir RD, Xia W, Yue Y, Tian M, Culley DJ, Crosby G, Tanzi RE, Xie Z. The inhalation anesthetic desflurane induces caspase activation and increases amyloid-beta protein levels under hypoxic conditions. J Biol Chem. 2008;283:11866–11875. doi: 10.1074/jbc.M800199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 56.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 57.Ikonomidou C, Stefovska V, Turski L. Neuronal death enhanced by N-methyl-D-aspartate antagonists. Proc Natl Acad Sci U S A. 2000;97:12885–12890. doi: 10.1073/pnas.220412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jevtovic-Todorovic V, Todorovic SM, Mennerick S, Powell S, Dikranian K, Benshoff N, Zorumski CF, Olney JW. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. 1998;4:460–463. doi: 10.1038/nm0498-460. [DOI] [PubMed] [Google Scholar]
- 60.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 61.Krasowski MD, Harrison NL. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br J Pharmacol. 2000;129:731–743. doi: 10.1038/sj.bjp.0703087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison NL, Kugler JL, Jones MV, Greenblatt EP, Pritchett DB. Positive modulation of human gamma-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol Pharmacol. 1993;44:628–632. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.