Abstract
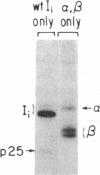
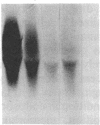
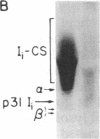
The invariant chain (Ii), a nonpolymorphic glycoprotein that associates with the immunoregulatory Ia proteins encoded by the major histocompatibility complex, has a proteoglycan form (Ii-CS) that bears a chondroitin sulfate glycosaminoglycan. In this proteoglycan form, Ii may remain associated with Ia at the cell surface. Inhibitors that prevent the addition of glycosaminoglycan to Ii have been found to depress antigen-presenting function. Ii does not have multiple candidate glycosaminoglycan-attachment sites, and we used site-directed mutagenesis to replace a candidate serine glycosaminoglycan-acceptor site with alanine at position 201 in the murine Ii protein. Transfection of the normal or altered gene into Ii-negative COS-7 cells showed that equivalent amounts of core Ii protein and its acidic, terminally glycosylated forms were synthesized, but the Ala-201 mutant Ii did not give rise to Ii-CS. The mutant protein had apparently normal transport through the Golgi compartment and associated stably with Ia molecules. Thus, this mutation directly identifies the site of glycosaminoglycan addition and shows that it can be eliminated without adversely affecting the overall biosynthesis of Ii.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Bourdon M. A., Krusius T., Campbell S., Schwartz N. B., Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci U S A. 1987 May;84(10):3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M. A., Oldberg A., Pierschbacher M., Ruoslahti E. Molecular cloning and sequence analysis of a chondroitin sulfate proteoglycan cDNA. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1321–1325. doi: 10.1073/pnas.82.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Colon S., Smith C., Freed J. H., Miles C., Grey H. M. Interaction between a "processed" ovalbumin peptide and Ia molecules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3968–3971. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Campbell P., Jacobsson I., Benzing-Purdie L., Rodén L., Fessler J. H. Silk--a new substrate for UDP-d-xylose:proteoglycan core protein beta-D-xylosyltransferase. Anal Biochem. 1984 Mar;137(2):505–516. doi: 10.1016/0003-2697(84)90119-2. [DOI] [PubMed] [Google Scholar]
- Chopra R. K., Pearson C. H., Pringle G. A., Fackre D. S., Scott P. G. Dermatan sulphate is located on serine-4 of bovine skin proteodermatan sulphate. Demonstration that most molecules possess only one glycosaminoglycan chain and comparison of amino acid sequences around glycosylation sites in different proteoglycans. Biochem J. 1985 Nov 15;232(1):277–279. doi: 10.1042/bj2320277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L. A., Carlstedt I., Cöster L., Malmström A. Binding of transferrin to the core protein of fibroblast proteoheparan sulfate. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5657–5661. doi: 10.1073/pnas.81.18.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L. A., Silverberg I., Carlstedt I. Structure of the heparan sulfate-protein linkage region. Demonstration of the sequence galactosyl-galactosyl-xylose-2-phosphate. J Biol Chem. 1985 Nov 25;260(27):14722–14726. [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet J. G., Lai M. Z., Briner T. J., Smith J. A., Gefter M. L. Interaction of peptide antigens and class II major histocompatibility complex antigens. Nature. 1986 Nov 20;324(6094):260–262. doi: 10.1038/324260a0. [DOI] [PubMed] [Google Scholar]
- Hanau D., Fabre M., Schmitt D. A., Garaud J. C., Pauly G., Tongio M. M., Mayer S., Cazenave J. P. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis "nonclassical" major histocompatibility complex class I molecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987 May;84(9):2901–2905. doi: 10.1073/pnas.84.9.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt G. D., Swiedler S. J., Freed J. H., Hart G. W. Murine Ia-associated invariant chain's processing to complex oligosaccharide forms and its dissociation from the I-Ak complex. J Immunol. 1985 Jul;135(1):399–407. [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N., Koch S., Hämmerling G. J. Ia invariant chain detected on lymphocyte surfaces by monoclonal antibody. Nature. 1982 Oct 14;299(5884):644–645. doi: 10.1038/299644a0. [DOI] [PubMed] [Google Scholar]
- Koch N., Lauer W., Habicht J., Dobberstein B. Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii). An alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO J. 1987 Jun;6(6):1677–1683. doi: 10.1002/j.1460-2075.1987.tb02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal recognition particle-dependent membrane insertion of mouse invariant chain: a membrane-spanning protein with a cytoplasmically exposed amino terminus. J Cell Biol. 1986 Jun;102(6):2169–2175. doi: 10.1083/jcb.102.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O. In search of a function for the invariant chain associated with Ia antigens. Surv Immunol Res. 1985;4(1):27–34. doi: 10.1007/BF02918583. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Cresswell P. Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens. J Immunol. 1982 Dec;129(6):2564–2569. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Germain R. N. Efficient cell surface expression of class II MHC molecules in the absence of associated invariant chain. J Exp Med. 1986 Nov 1;164(5):1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Malek T. R., Leonard W. J., Greene W. C., Shevach E. M., Germain R. N. Nucleotide sequence and expression of a mouse interleukin 2 receptor cDNA. J Immunol. 1985 Jun;134(6):4212–4217. [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. E., Pravtcheva D. D., Day C., Ruddle F. H., Jones P. P. Murine invariant chain gene: chromosomal assignment and segregation in recombinant inbred strains. Immunogenetics. 1985;22(2):193–199. doi: 10.1007/BF00563518. [DOI] [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- Rodén L., Koerner T., Olson C., Schwartz N. B. Mechanisms of chain initiation in the biosynthesis of connective tissue polysaccharides. Fed Proc. 1985 Feb;44(2):373–380. [PubMed] [Google Scholar]
- Rosamond S., Brown L., Gomez C., Braciale T. J., Schwartz B. D. Xyloside inhibits synthesis of the class II-associated chondroitin sulfate proteoglycan and antigen presentation events. J Immunol. 1987 Sep 15;139(6):1946–1951. [PubMed] [Google Scholar]
- Ross A. H., Cossu G., Herlyn M., Bell J. R., Steplewski Z., Koprowski H. Isolation and chemical characterization of a melanoma-associated proteoglycan antigen. Arch Biochem Biophys. 1983 Aug;225(1):370–383. doi: 10.1016/0003-9861(83)90042-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant A. J., Cullen S. E., Giacoletto K. S., Schwartz B. D. Invariant chain is the core protein of the Ia-associated chondroitin sulfate proteoglycan. J Exp Med. 1985 Dec 1;162(6):1916–1934. doi: 10.1084/jem.162.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant A. J., Cullen S. E., Schwartz B. D. Biosynthetic relationships of the chondroitin sulfate proteoglycan with Ia and invariant chain glycoproteins. J Immunol. 1985 Jul;135(1):416–422. [PubMed] [Google Scholar]
- Sant A. J., Cullen S. E., Schwartz B. D. Identification of a sulfate-bearing molecule associated with HLA class II antigens. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1534–1538. doi: 10.1073/pnas.81.5.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant A. J., Schwartz B. D., Cullen S. E. Identification of a new component in the murine Ia molecular complex. J Exp Med. 1983 Dec 1;158(6):1979–1992. doi: 10.1084/jem.158.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N. B. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J Biol Chem. 1977 Sep 25;252(18):6316–6321. [PubMed] [Google Scholar]
- Sekaly R. P., Tonnelle C., Strubin M., Mach B., Long E. O. Cell surface expression of class II histocompatibility antigens occurs in the absence of the invariant chain. J Exp Med. 1986 Nov 1;164(5):1490–1504. doi: 10.1084/jem.164.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P. A., Lauer W., Dembić Z., Mayer W. E., Lipp J., Koch N., Hämmerling G., Klein J., Dobberstein B. Structure of the murine Ia-associated invariant (Ii) chain as deduced from a cDNA clone. EMBO J. 1984 Apr;3(4):873–877. doi: 10.1002/j.1460-2075.1984.tb01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivak L. E., Harris M. R., Kindle C., Cullen S. E. Inhibition of proteoglycan synthesis eliminates the proteoglycan form of Ia-associated invariant chain and depresses antigen presentation. J Immunol. 1987 Mar 1;138(5):1319–1321. [PubMed] [Google Scholar]
- Stein B. S., Bensch K. G., Sussman H. H. Complete inhibition of transferrin recycling by monensin in K562 cells. J Biol Chem. 1984 Dec 10;259(23):14762–14772. [PubMed] [Google Scholar]
- Strubin M., Berte C., Mach B. Alternative splicing and alternative initiation of translation explain the four forms of the Ia antigen-associated invariant chain. EMBO J. 1986 Dec 20;5(13):3483–3488. doi: 10.1002/j.1460-2075.1986.tb04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubin M., Long E. O., Mach B. Two forms of the Ia antigen-associated invariant chain result from alternative initiations at two in-phase AUGs. Cell. 1986 Nov 21;47(4):619–625. doi: 10.1016/0092-8674(86)90626-4. [DOI] [PubMed] [Google Scholar]
- Strubin M., Mach B., Long E. O. The complete sequence of the mRNA for the HLA-DR-associated invariant chain reveals a polypeptide with an unusual transmembrane polarity. EMBO J. 1984 Apr;3(4):869–872. doi: 10.1002/j.1460-2075.1984.tb01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiedler S. J., Hart G. W., Freed J. H. Characterization of the oligosaccharides from the invariant chain associated with murine Ia antigens. J Immunol. 1983 Jul;131(1):352–358. [PubMed] [Google Scholar]
- Tantravahi R. V., Stevens R. L., Austen K. F., Weis J. H. A single gene in mast cells encodes the core peptides of heparin and chondroitin sulfate proteoglycans. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9207–9210. doi: 10.1073/pnas.83.23.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Floyd-Smith G., Francke U., Koch N., Lauer W., Dobberstein B., Schäfer R., Hämmerling G. J. The gene encoding the Ia-associated invariant chain is located on chromosome 18 in the mouse. Immunogenetics. 1985;21(1):83–90. doi: 10.1007/BF00372244. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]