Abstract
To date there are potential chronology-based but not conclusive reasons to believe that at least some of the gadolinium complexes play a causative role in the pathophysiology of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). Still, the exact pathogenesis and the risk for patients is unclear beside the obvious connection to moderate to severe renal insufficiency. So far, MR imaging with Gd-enhancement was regarded as the safest imaging modality in these patients—the recent development creates tremendous uncertainty in the MR-community. Nevertheless, one should remember that, despite the over 200 cases of NSF and about 100 with proven involvement of Gd3+, the vast majority of over 200 million patients exposed to gadolinium since the 1980s have tolerated these agents well. Importantly, NSF is a rare disease and does not appear to occur in patients without renal impairment. Many patients and researchers have undergone MR investigations with Gd exposure in the past. For those, it is essential to know about the safety of the agents at normal renal function. We can hope that pharmacoepidemiological and preclinical studies will allow us to better understand the pathophysiology and role of the various MR contrast agents in the near future.
Keywords: Gadolinium chelates, MRI, Renal insufficiency, Nephrogenic systemic fibrosis, Nephrogenic fibrosing dermopathy
Introduction
In the recent years Gd-enhanced MRI has been the accepted way to go for contrast-enhanced (CE) imaging in patients with renal insufficiency to avoid the use of iodinated contrast media (CM)as for CT. For the same patients group, CE-MRA replaced diagnostic DSA mainly due to its favourable renal profile and reduced nephrotoxicity. On a molar level there are little differences between iodinated and gadolinium-containing agents for what concerns nephrotoxicity, but it is evident that the dosing is very much in favour of CE-MRA [1–3].
Hypothesis on pathogenesis
Meanwhile, after reviewing the issue of nephrogenic systemic fibrosis (NSF) and on the basis of current evidence, it seems that some gadolinium-containing contrast agents may be implicated in its pathogenesis or may even trigger NSF [4–10]. The suspicion has further been enforced by the recent detection of gadolinium in tissue biopsy specimens in patients suffering from NSF [11, 12]. Thus, the main hypothesis is that NSF is a rare, chronic disorder following systemic gadolinium de-chelation causing intoxication with renal dysfunction at the core of this condition. Released gadolinium ions once outside the complex forms hydroxides and phosphates, which are insoluble at a pH > 6.2 and probably engulfed by various cells of the mononuclear phagocytic system [13]. This might depress the reticuloendothelial system [14, 15], inhibit the activity of certain enzymes with cutaneous lesions as consequences [16, 17], and cause activation of foreign body—and of fibrous reactions (in case of NSF with involvement of dendritic cells, synthesis of TFGβ precipitating the fibrotic process, attraction of fibrocytes, skin induration, and mucin production) [10, 18].
Since the recent revision and publication of new guidelines from various regulatory authorities, suddenly radiologists and researchers are faced with a radical shift of paradigm—with drastic measures concerning patients with moderate to severe renal impairment or a presumed increased nephrotoxic risk (GFR < 30 ml/min per 1.73 m2) [19–22]. The goal of this editorial review is to sensitise healthcare professionals about NSF and the new security guidelines, which also imply differences between the clinically used gadolinium agents and to discuss shortly the following related questions:
How do we deal with the new regulations for clinical routine MRI and which alternatives exist for patients with renal impairment?
How could it come, that such a distinct and severe disorder was only recognised after more than 200 cases worldwide and after such a long time delay?
How can we impede similar safety risks in the future?
What are the consequences besides clinical routine for research and ongoing clinical studies?
Gd-compounds and transmetallisation
As most of the cases were only discovered in 2006 following the first warnings of experts such as Grobner or Thomsen [4, 6, 23], there are still differences between the single national or international guidelines (FDA/EMEA). So far, over 90% of all NSF cases occurred after gadodiamide (Omniscan®) explaining the more severe contraindications and reluctance for this agent. Single cases have been reported in the USA and Europe after gadopentetate dimeglumine (Magnevist®) and gadoversetamide (Optimark®), which both form linear complexes. Macrocyclic agents such as gadoteridol (Prohance®), gadobutrol (Gadovist®) or gadoterate meglumine (Dotarem®) are slightly more stable than linear complexes because the Gd3+ is caged in a cavity. Linear compounds exhibit more toxic effects after repeated administrations than cyclic compounds, particularly on the reproductive functions and the skin [17, 24, 25] (Fig. 1).
Fig. 1.
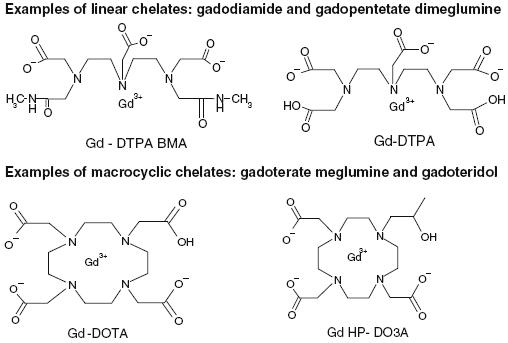
Structural formulas of gadolinium (III) complexes. Examples of linear chelates: gadodiamide and gadopentetate dimeglumine. Examples of macrocyclic chelates: gadoterate meglumine and gadoteridol
Further on, a high number of ionic bonds between the ligand and gadolinium increase the stability of the chelates. Therefore, the dynamic process of gadolinium release differs from one chelate to another (dissociation kinetic rate e.g., at low pH; Table 1). Actually, there is always a balance between the free ligand and gadolinium and the complex-bound form, with in general a pronounced over-balance to the right [26\2-28]:
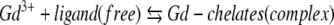 |
1 |
Table 1.
| Contrast agent | Trade name | Chemical structure | log Ktherm | log K′ | T1/2 in 0.1N HCl [34, 42] |
|---|---|---|---|---|---|
| Gd-DTPA-BMA | Omniscan® | Linear, non-ionic | 16.9 | 14.9 | 35s |
| Gd-DTPA-BMEA | OptiMARK® | Linear, non-ionic | 16.6 | 15.0 | |
| Gd-BOPTA | MultiHance® | Linear, di-ionic | 22.6 | 16.9 | |
| Gd-DTPA | Magnevist® | Linear, di-ionic | 22.1 | 17.7 | 10 min |
| Gd-EOB-DTPA | Primovist® | Linear, di-ionic | 23.5 | N/A | |
| Gd-BT-DO3A | Gadovist® | Macrocyclic, non-ionic | 21.8 | N/A | |
| Gd-HP-DO3A | ProHance® | Macrocyclic, nonionic | 23.8 | 17.1 | 3 h |
| Gd-DOTA | Dotarem® | Macrocyclic, ionic | 25.8 | 18.8 | >1 month |
NA Not available; log Ktherm thermodynamic stability constant; log K′ conditional sability constant at a pH of 7.4
To minimize the risk of release of free gadolinium or in order to shift more towards the right, manufacturers have often added additional, excess or free chelate in their pharmaceutical preparations [10].
Importantly, the elimination half-life of these agents is prolonged 20-fold and more (34.2 h for gadodiamide) in case of terminal renal insufficiency up from a normal 1.5 h in healthy subjects [29–33]. The delayed excretion of the contrast agent in patients with renal impairment dramatically increases the contact time with the body and thus enhances the risk of biological reactions. The different conditional stabilities and dynamics affect their behaviour in the body and might lead to a release of free (toxic) gadolinium. Moreover, transmetallisation which occurs more likely in case of unstable complexes or in case of prolonged interaction with endogenous ions might be explained by contestation of the binding sites of gadolinium [34–41, 45]. Probably, in case of dialysis and accompanied treatment an increased number of free electrolytes together with the delayed elimination of the gadolinium contrast agent promote such liberation. Ultimately, this may favour the occurrence of toxic manifestations for compounds with poorer stability—but this has not yet been proven clinically for NSF:
Equation for transmetallisation:
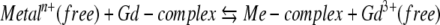 |
(2) |
Guidelines
In Europe the CHMP (Committee for Medicinal Products for Human Use) has issued on 7 Feb 2007 the following warning which should be implemented rapidly in each country [20–22]:
-
For gadodiamide (Omniscan®) :
Gadodiamide is contraindicated in patients with severe renal impairment (GFR < 30 ml/min per 1.73 m2), and those who have had or are undergoing liver transplantation
Special warnings and precautions for use:
Severe renal impairment and liver transplant patients:
There have been reports of nephrogenic systemic fibrosis (NSF) associated with use of gadodiamide and some other gadolinium-containing contrast agents in patients with severe renal impairment (GFR < 30 ml/min per 1.73 m2) and those who have had or are undergoing liver transplantation. Therefore Omniscan® should not be used in these populations.
Neonates and infants:
Due to immature kidney function in neonates and infants up to 1 year of age, Omniscan® should only be used in these patients after careful consideration.
Undesirable effects:
Cases of NSF have been reported with Omniscan®.
All other gadolinium — containing contrast agent s should add strong warnings about potential NSF with the following proposed SPC wording :
-
Special warnings and precautions for use:
There have been reports of NSF associated with use of some gadolinium-containing contrast agents in patients with severe renal impairment (GFR < 30 ml/min per 1.73 m2). As there is a possibility that NSF may occur with XXX, it should only be used in these patients after careful consideration.
The most recent knowledge must be taken into account, forcing us to be aware of the revised national and international guidelines concerning renal insufficiency, which might vary from one country to another! Among others, most national and international Radiological and MR—Societies provide the respective LINKs on their homepage [46–49].
How do we deal with the new guidelines in clinical routine MRI and what are the alternatives?
Especially, the population with an eGFR (estimated GFR) < 30 ml/min per 1.73 m2) that shall be protected by the new guidelines will also potentially suffer the most from this regulation. It is important to underline the fact, that most gadolinium complexes may still be used in case of terminal renal insufficiency, but the risk/benefit ratios must directly be compared to the application of CT with iodinated CM. More stable Gd complexes should be preferred. The standard Gd CM dose of 15–20 ml for an adult corresponding to 0.1 mmol/kg body-weight contains only about 7.5–10 mmol (=0.0075–0.01 mol), while 100 ml of a iodinated contrast agent correspond to almost 0.1 mol of active substance (for monomeric non-ionic CM). Nephrotoxicity is directly related to the applied dose in mole [2, 3]. Despite this evident advantage for CE-MRI vs CE-CT, indication and renal function must be assessed. If necessary, renal protection must be carefully ensured. The following practical recommendations might help [7, 10, 22]:
- Definition of serum creatinine (SCr) (not older than 2 weeks) and calculation of eGFR for
- patients bearing an increased nephrotoxic risk (see below)
- all patients with a history of chemotherapy, blood or connective tissue disorder or a “pro-inflammatory” disease (major surgery, vasculitis, thrombosis, systemic infection, liver cirrhosis, acidosis) within 6 months prior to the examination.
Assessment of further renal risk factors such as advanced age (>70 years), suspected or known renal diseases, diabetes mellitus, arteriosclerosis or peripheral vascular disease, congestive cardiac failure, nephrotoxic medication (contrast load in the previous 72 h, diuretics, NSAID, aminoglycosides, amphotericin, cyclosporine A), hyperuricemia, history of dialysis.
In case of eGFR < 30 ml/min per 1.73 m2 one should be reluctant to use gadolinium, prefer more stable gadolinium complexes and limit the contrast application to single dose (0.1 mMol/kg bw). Repeated dosing within a time frame of 1 week should be avoided. Hemodialysis does not necessarily protect from the appearance of NSF, and therefore is not generally recommended after the administration of gadolinium agents [10, 43].
Gd should be avoided if ever possible in case of the following:
Known allergies to any gadolinium contrast agent.
Known or diagnosed NSF
During and after the examination, a standardized scheme of hydration might help to prevent nephrotoxicity and eventual accumulation of Gd (no evidence yet).
Finally, it cannot be recommended as alternative to Gd-enhanced MRI, to perform CE-CT in patients with severe renal insufficiency (if not under dialysis). Non-enhanced MRI or other non-contrast imaging modalities are the methods of choice.
(b) How could it come that such a distinct and severe disorder was only recognised after more than 200 cases worldwide and after such a long time delay?
High doses of Gd agents are mostly applied for indications such as MRA—and the majority of NSF cases occurred especially after high dose MRA. It is interesting to note that the onset of first reported NSF in 1997 [8] coincides closely with the success of CE-MRA. Actually, in the middle of the 1990s, suddenly CE-MRA allowed not only to diagnose and overlook much more rapidly the vascular situation, but additionally promised to reduce nephrotoxicity—even with triple dose Gd applied [44]. The literature shows only very few clinical studies addressing more in depth the security question in case of terminal renal insufficiency [3, 30–33]. Mostly they limit the patient follow-up to 72 h, mainly renal endpoints were assessed and only the acute phase was followed. No prolonged studies were performed as it was assumed that these diagnostic agents were injected only once—without taking into consideration the wide extension of indications, increased use also in case of follow-up studies (multiple sclerosis, oncology, CE-MRA) or the delayed elimination rate in case of renal insufficiency.
Underreporting of chronic or long-term effects is a well known reality—not only valid for gadolinium agents, but also in general for all drugs. Still nowadays few data are available about the incidence of thyroid reactions after iodinated agents or about type IV hypersensitivity reactions after gadolinium agents. Also renal insufficiency following the non-specific gadolinium complexes is rarely reported and so on.
Besides demanding for more clinical studies with clearly defined risk-groups the radiologic community should also be aware of its own responsibility regarding the reporting of adverse events. The overarching aim must be to gain more data about adverse effects observed after the patient has left the imaging department in narrow conjunction with clinicians. Clear documentation on all contrast procedures as well as on the type of contrast agent must be available for the clinicians at any time.
(c) How can we impede similar safety risks in the future?
As has been said previously it is primarily a matter of sensitisation of all involved partners, the clinicians, the radiologists, the pharmaceutical companies and also the health authorities. As in case of previous safety risks with drugs, it became evident that apparently there was uncertainty if not lacking data about the population with terminal renal insufficiency. Radiologists, despite the evident shift of clinical use, must remain prudent in such cases. More prospective clinical data must be acquired and published to justify the use of just any agent in case of pronounced risk.
Secondly, as soon as a possible risk is recognized, health authorities and responsible pharmaceutical companies must inform others around the world. In the present case one must also ask why warnings were so different from one country to another. As soon as the higher risk associated with gadodiamide was evident, which seemed to be the case already in July 2006, a general warning to healthcare professionals would have been opportune. Transparency of information is a key-issue as we all have rapid access to online information and should be used just to avoid any single unnecessary patient risk.
(d) What are the consequences besides clinical routine for research and ongoing clinical studies?
All ongoing clinical studies with gadolinium agents must be carefully checked and must comply as well with those or similar guidelines proposed by the authorities. An exception might be research studies deliberately following the NSF issue or renal tolerance. But even in these cases, ethical considerations with a thorough risk/benefit analysis must be taken into account, thus leading at least to a modification of informed consent. Ongoing studies must be adapted instantaneously after informing the ethical committees about this issue.
Clearly, as there remains much uncertainty, new studies addressing the various questions about NSF should be performed.
Conclusion
Compliance with the new extended directives by authorities such as EMEA and FDA on the application of Gd complexes in patients with known renal impairment must be assured. Radiologists must familiarise themselves with the calculation of eGFR and the risk of NFS in renal insufficiency. Nevertheless, it is important to underline, that gadolinium agents remain one of the safest drug groups and, last but not least, are less nephrotoxic than iodinated agents on a volume level. We must be aware, that this is not a problem of one single drug, but statistically it is just a matter of time to notify NSF incidents for all Gd compounds. In practice, careful anamnesis to retrieve cases of terminal renal impairment is mandatory to avoid NSF.
References
- 1.Prince MR, Arnoldus C and Frisoli JK (1996). Nephrotoxicity of high-dose gadolinium compared with iodinated contrast. J Magn Reson Imaging 6: 162–166 [DOI] [PubMed]
- 2.Elmståhl B, Nyman U, Leander P, Chai C-M, Golm K, Björk J and Almen T (2006). Gadolinium contrast media are more nephrotoxic than iodine media. The importance of osmolality in direct renal artery injections. Eur Radiol 16: 2712–2720 [DOI] [PubMed]
- 3.Thomsen HS (2004). Gadolinium-based contrast media may be nephrotoxic even at approved doses. Eur Radiol 14: 1654–1656 [DOI] [PubMed]
- 4.Grobner T (2006) Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic systemic fibrosis? Nephrol Dial Transplant 21:1104–1108 + erratum 1745 [DOI] [PubMed]
- 5.Thomsen HS, Morcos SK and Dawson P (2006). Is there a causal relation between the administration of gadolinium-based contrast media and the development of nephrogenic systemic fibrosis (NSF)?. Clin Radiol 61: 905–906 [DOI] [PubMed]
- 6.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG and Thomsen HS (2006). Nephrogenic Systemic Fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17(9): 2359–2362 [DOI] [PubMed]
- 7.Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A (2007) Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology doi: 10.1148/radiol 2431062144 [DOI] [PubMed]
- 8.Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S and Leboit PE (2000). Scleromyxoedema-like cutaneous disease in renal-dialysis patients. Lancet 356: 1000–1001 [DOI] [PubMed]
- 9.Kuo PH, Kanal E, Abu-Alfa AK and Cowper SE (2007). Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 242(3): 647–649 [DOI] [PubMed]
- 10.Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I and Kirk GA (2007). Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR 188: 586–592 [DOI] [PubMed]
- 11.High WA, Ayers RA, Cowper SE. (2007) Gadolinium is quantifiable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol [Epub on Feb 6, 2007 ahead of print] [DOI] [PubMed]
- 12.Boyd AS, Zic JA and Abraham JL (2007). Gadolinum deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 56(1): 27–30 [DOI] [PubMed]
- 13.Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J and Harpur E (1997). Gadolinium chloride toxicity in the rat. Toxicol Pathol 25(3): 245–255 [DOI] [PubMed]
- 14.Evans CH (1990). Biochemistry of the lanthanides. Plenum Press, New York
- 15.Husztik E, Lazar G and Parducz A (1980). Electron microscopic study of Kupffer-cell phagocytosis blockade induced by gadolinium chloride. Br J Exp Pathol 61: 624–630 [PMC free article] [PubMed]
- 16.Itoh N and Kawakita M. (1984). Characterization of Gd3+ and Tb3+ binding sites on Ca2+, Mg2+-adenosine triphosphatase on sarcoplasmic reticulum. J Biochem (Tokyo) 95: 661–669 [DOI] [PubMed]
- 17.Harpur ES, Worah D, Hals PA, Holtz E, Furhuhama K and Nomura H (1993). Preclinical safety assessment and phamacokinetics of gadodiamide injection, a new magnetic resonance imaging contrast agent. Invest Radiol 28(suppl 1): S28–S43 [DOI] [PubMed]
- 18.Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S and Jimenez SA (2006). Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum 35: 238–249 [DOI] [PMC free article] [PubMed]
- 19.FDA:http://www.fda.gov/cder/drug/advisory/gadolinium_agents_20061222.htm
- 20.http://www.bfarm. de/cln_043/nn_424276/DE/Pharmakovigilanz/ aktuell/gadodiamid__nierenfunkt ionseinschraenkung
- 21.http://www.mhra.gov.uk/home/idcplg?IdcService= SS_GET_ PAGE&useSecondary=true&ssDocName
- 22.http://esur.org
- 23.Thomsen HS (2006). Nephrogenic systemic fibrosis: a serious late adverse reaction to gadodiamide. Eur Radiol 16: 2619–2621 [DOI] [PMC free article] [PubMed]
- 24.Magnevist© US package insert (may 2006)
- 25.Mueller N (1992). Ionic and non-ionic gadolinium-containing magnetic resonance contrast media. Comparison of effects and their reversibility in repeated dose toxicity studies in rats.. Adv MRI contrast 1(suppl 1): 15–28
- 26.Cacheris WP, Quay SC and Roocklage SM (1990). The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging 8: 467–481 [DOI] [PubMed]
- 27.Tweedle M (1992). Physico-chemical properties of gadoteridol and other magnetic resonance contrast agents. Invest Radiol 27(Suppl 1): 2–6 [PubMed]
- 28.Uggeri F, Aime S and Anelli PL (1995). Novel contrast agents for magnetic resonance imaging. Synthesis and characterisation of the ligand BOPTA and its Ln(III) complexes. Inorg Chem 34: 633– 642 [DOI]
- 29.Joffe P, Thomsen HS and Meusel M. (1998). Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol 5: 491–502 [DOI] [PubMed]
- 30.Bellin MF, Deray G, Assoqba U, Auberton E, Ghany F, Dion-Voirin E, Jacobs C and Grellet J (1992). Gd-DOTA: evaluation of its renal tolerance in patients with chronic renal failure. Magn Reson Imaging 10: 115–118 [DOI] [PubMed]
- 31.Tombach B, Bremer C, Reimer P, Schaefer RM, Ebert W, Geens V and Heindel W (2000). Pharmacokinetics of 1M gadobutrol in patients with chronic renal failure. Invest Radiol 35(1): 35–40 [DOI] [PubMed]
- 32.Swan SK, Lambrecht LJ, Townsend R, Davies BE, McCloud S, Parker JR, Bensel K and LaFrance ND (1999). Safety and pharmacokinetic profile of gadobenate dimeglumine in subjects with renal impairment. Invest Radiol 34(7): 443–448 [DOI] [PubMed]
- 33.Schuhmann-Giampieri G and Krestin G (1991). Pharmacokinetics of Gd-DTPA in patients with chronic renal failure. Invest Radiol 26(11): 975–979 [DOI] [PubMed]
- 34.Wedeking P, Kumar K and Tweedle MF (1992). Dissociation of gadolinium chelates in mice: relationship to chemical characteristics. Magn Reson Imaging 10: 641–648 [DOI] [PubMed]
- 35.Tweedle MF, Wedeking P and Kumar K (1995). Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate and gadodiamide in mice and rats. Invest Radiol 30: 372–380 [DOI] [PubMed]
- 36.Gibby WA, Gibby KA and Gibby WA (2004). Comparison of Gd-DTPA-BMA (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol 39: 138–142 [DOI] [PubMed]
- 37.White GW, Gibby WA and Tweedle MF (2006). Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled mass spectroscopy. Invest Radiol 41: 272–278 [DOI] [PubMed]
- 38.Corot C, Idée JM, Hentsch AM, Santus R, Mallet C, Goulas V, Bonnemain B and Meyer D (1998). Structure-relationship of macrocyclic and linear gadolinium chelates: investigation of transmetallation effect on the zinc-dependent metallopeptidase angiotensine-converting enzyme. J Magn Reson Imaging 8: 695–702 [DOI] [PubMed]
- 39.Kimura J, Ishiguchi T, Matsuda J, Ohno R, Nakumura A, Kamei S, Ohno K, Kawamura T and Murata K (2005). Human comparative study of zinc and copper excretion via urine after administration of magnetic resonance imaging contrast agents. Radiat Med 23(5): 322–326 [PubMed]
- 40.Idée JM, Berthommier C, Goulas V, Corot C, Santus R, Hermine C, Schaefer M and Bonnemain B (1998). Haemodynamic effects of macrocyclic and linear gadolinium chelates in rats: role of calcium and transmetallation. Biometals 11: 113–123 [DOI] [PubMed]
- 41.Laurent S, Vander Elst L and Muller RN (2006). Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol Imaging 1: 128–137 [DOI] [PubMed]
- 42.Kumar K (1997). Macrocyclic polyamino-carboxylate complexes of Gd(III) as magnetic resonance imaging contrast agents. J Alloys Comp 249: 163–172 [DOI]
- 43.Okada S, Katagiri K, Kumazaki T and Yokoyama H (2000). Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol 42: 339–341 [DOI] [PubMed]
- 44.Johnson DB, Lerner CA, Prince MR, Kazanjian SN, Narasimham DL, Leichtman AB and Cho KJ (1997). Gadolinium-enhanced magnetic resonance angiography of renal transplants. Magn Reson Imaging 15(1): 13–20 [DOI] [PubMed]
- 45.Khurana A, Runge VM, Narayanan M, Greene JF Jr, Nickel AE (2007) Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan). Radiology [Epub ahead of print at 31 Jan 2007] [DOI] [PubMed]
- 46.FDA: http://www.fda.gov/cder/drug/advisory/gadolinium_agents_20061222.htm
- 47.ESMRMB: http://www.esmrmb.org
- 48.ISMRM: http://www.ismrm.org
- 49.NSF Registry: http://www.icnfdr.org


