Abstract
OBJECTIVE
The intraislet insulin hypothesis proposes that glucagon secretion during hypoglycemia is triggered by a decrease in intraislet insulin secretion. A more recent hypothesis based on in vivo data from hypoglycemic rats is that it is the decrease in zinc cosecreted with insulin from β-cells, rather than the decrease in insulin itself, that signals glucagon secretion from α-cells during hypoglycemia. These studies were designed to determine whether closure of the α-cell ATP-sensitive K+ channel (KATP channel) is the mechanism through which the zinc switch-off signal triggers glucagon secretion during glucose deprivation.
RESEARCH DESIGN AND METHODS
All studies were performed using perifused isolated islets.
RESULTS
In control experiments, the expected glucagon response to an endogenous insulin switch-off signal during glucose deprivation was observed in wild-type mouse islets. In experiments with streptozotocin-treated wild-type islets, a glucagon response to an exogenous zinc switch-off signal was observed during glucose deprivation. However, this glucagon response to the zinc switch-off signal during glucose deprivation was not seen in the presence of nifedipine, diazoxide, or tolbutamide or if KATP channel knockout mouse islets were used. All islets had intact glucagon responses to epinephrine.
CONCLUSIONS
These data demonstrate that closure of KATP channels and consequent opening of calcium channels is the mechanism through which the zinc switch-off signal triggers glucagon secretion during glucose deprivation.
Treatment with exogenous insulin places patients with type 1 or advanced type 2 diabetes at high risk for hypoglycemia. Under normal circumstances, the physiologic response to hypoglycemia involves multiple intrinsic defense mechanisms. These include inputs from the central nervous system and release of glucagon, epinephrine, growth hormone, and cortisol. Chief among these is the increase in glucagon secretion. In diabetic patients, the counterregulatory glucagon response is severely compromised. A leading hypothesis to explain this abnormality is referred to as the “intraislet insulin hypothesis” (1–3). Banarer et al. (1) based this hypothesis on an earlier observation by Samols et al. (4), who identified a unidirectional blood flow within the pancreatic islet whereby arterial blood from the systemic circulation first reaches β-cells in the islet core, then α-cells in the islet periphery, and finally leaves the islet to enter the hepatic portal venous circulation. In this manner, products released from upstream β-cells influence the function of downstream α-cells. Many in vitro and in vivo studies support this hypothesis that proposes that insulin tonically suppresses glucagon secretion in the presence of high or normal glucose levels and that a sudden cessation of insulin secretion caused by hypoglycemia triggers glucagon secretion.
However, it has been pointed out that, together with insulin, β-cells release other mediators that suppress glucagon secretion and thus could provide switch-off signals to the α-cell to enhance glucagon secretion. Among them, attention has been focused on γ-aminobutyric acid (5–7) and zinc (8,9). We concentrated on the role zinc might play in the intraislet insulin hypothesis. In this scenario, zinc coreleased with insulin from upstream β-cells in the presence of high glucose inhibits glucagon secretion; when blood glucose levels fall, zinc secretion from β-cells decreases and glucagon secretion is triggered. The potential role of zinc as a switch-off signal has been demonstrated by us in an in vivo study in which switching off either an insulin or a zinc infusion into the pancreatic artery of streptozotocin (STZ)-treated rats during hypoglycemia stimulated glucagon secretion. In contrast, switching-off zinc-free insulin failed to elicit glucagon secretion (9).
The current studies were designed to identify the mechanism of action whereby zinc regulates α-cell function during hypoglycemia. We hypothesized that KATP channels in α-cells play a central role. We experimented by pharmacologically manipulating the KATP channel activity of wild-type mouse islets as well as with a mouse model derived from the wild-type control but in which the SUR1 subunit of the KATP channel (SUR1KO) had been knocked out (10). We raised two independent questions. First, can a role for cessation of secretion of insulin (and by implication zinc coreleased with insulin) as a switch-off signal during hypoglycemia be demonstrated in vitro using perifused mouse islets? This initial question was essential to answer because of a report by Ravier and Rutter (11) that zinc does not regulate glucagon secretion in mice. Second, does the mechanism of action for the switch-off signal from zinc specifically rely on functional KATP channels? An endogenous insulin switch-off signal was created by stimulating endogenous insulin secretion with glucose perifusion then abruptly decreasing insulin secretion by discontinuing the glucose perifusion. An exogenous zinc switch-off signal during glucose deprivation in the absence of insulin secretion was created in STZ-treated wild-type mouse islets by perifusing and then discontinuing the perifusion of zinc chloride and glucose. This zinc switch-off signal during glucose deprivation was further investigated in the presence of diazoxide (to maintain KATP channels open), nifedipine (to close calcium channels), or tolbutamide (to close potassium channels). Finally, the zinc switch-off signal was studied using mouse islets with knocked out KATP channels.
RESEARCH DESIGN AND METHODS
Male and female wild-type C57BL6/J mice (8–15 weeks old) were purchased from Jackson Labs (JAX no. 000664). SUR1 knockout mice (SUR1KO) were bred at PNDRI (10). Animals were maintained with a 12-h light-dark cycle and constant temperature and were given free access to food and water. All experiments were approved by the PNDRI Institutional Animal Care and Use Committee.
Islet isolation.
On the day of pancreas removal, animals were anesthetized with ketamine-xylazine and then killed by removing blood from the heart. Pancreata were cannulated for infusion of collagenase and then removed from the animal for processing. Mice pancreata were processed as described by Tanaka (12), using 0.75 mg/ml collagenase in wild-type mice and 1.5 mg/ml collagenase in SUR1KO mice. A higher concentration was used for SUR1KO mouse islets since they tended to adhere to ducts when the lower concentration was used. Once processed, islets were hand picked and cultured overnight in RPMI 1640 media with 11.1 mmol/l glucose, 10% FBS, and antibiotic/antimycotic (100 units/ml penicillin G and 0.1 mg/ml streptomycin sulfate and 0.25 μg/ml amphoteracin B). The pool of islets for each experiment was derived from at least eight animals. In some experiments STZ was used to render pancreatic islets devoid of β-cells. In these experiments, isolated islets were cultured for 2 h in RPMI media with 11.1 mmol/l glucose, 10% FBS, and antibiotic/antimycotic then for 30 min in RPMI media with STZ (5 mmol/l), 11.1 mmol/l glucose, 0.8% FBS, and antibiotic/antimycotic. Then, STZ was washed out of the islets and they were cultured overnight in RPMI media with 11.1 mmol/l glucose, 10% FBS, and antibiotic/antimycotic.
Perifusion.
The day after isolation, islets were split into groups of 200 islets. Each group was placed into one of the individual perifusion chambers for simultaneous study. The islets were placed in the chambers on top of mesh filters (60 μm) small enough to prevent islets from escaping into the effluent. Each experiment was preceded by a 60-min stabilization period using a perifusion of Kreb's Ringer buffer (KRB) containing 0.1% BSA, 0.238% HEPES, 0.1 mmol/l isobutylmethylxanthine (IBMx), and 2.8 mmol/l glucose. Thereafter, experimental KRB buffers contained 0.1% BSA, 0.238% HEPES, 0.1 mmol/l IBMx, an amino acid mixture (3), and either 16.7 or 0 mmol/l glucose. When STZ-treated islets were used, 30 μmol/l zinc chloride was also included in the buffer. Samples for measurement of insulin and glucagon were collected at −10, −5, −2, −1, and 0 min before and 2, 5, 10, 15, 20, 25, and 30 min after endogenous insulin (Fig. 1) and exogenous zinc (Figs. 2, 3 and 4) switch-off signals were provided at time 0.
FIG. 1.
A. Endogenous insulin switch-off signal to α-cells in WT islets. Isolated islets were exposed to 16.7 mmol/l glucose (16.7G) for 30 min (from −30 to 0 min). At min 0 the perifusion was either changed to 0 glucose or not changed for the ensuing 30 min. The closed circles at times −10 to 0 min represent the average of experimental and control values when islets were exposed to 16.7 mmol/l glucose. Insulin secretion declined when glucose infusion was changed to 0 mmol/l at time 0 min, generating an endogenous insulin switch-off signal from β-cells to neighboring α-cells (closed boxes). Insulin levels did not decrease significantly when the glucose perifusion was not changed (closed diamonds). Results are expressed as mean ± SE of eight replicate perifusions in experimental studies (closed boxes) and seven replicate perifusions in control studies (closed diamonds). See text for statistical information. A and B: Glucagon responses to an endogenous insulin switch-off signal during perifusion of wild-type mouse islets. Glucagon levels increased significantly only when endogenous insulin secretion (A) was switched-off in response to glucose deprivation at time 0 min (■, n = 8). No glucagon response was observed if the endogenous insulin switch-off signal was absent (♦, n = 7). ● at times −10 to 0 min represent the average of experimental and control values. See text for statistical information.
FIG. 2.
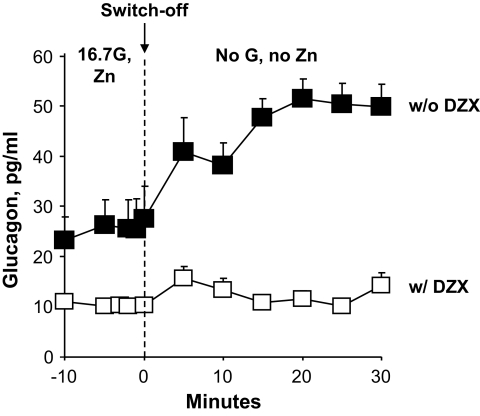
Glucagon response in the presence and absence of an exogenous zinc switch-off signal during perifusion of STZ-induced diabetic wild-type mouse islets. Islets were pretreated with STZ to kill β-cells, thereby preventing endogenous insulin and zinc secretion. After an initial 30-min perifusion with 16.7 mmol/l glucose and zinc (Zn), at time 0 min the perifusate was changed. Glucagon levels increased significantly only when a perifusate containing no glucose and no zinc, which generated an exogenous zinc switch-off signal to α-cells during glucose deprivation, was begun at 0 min (■, n = 5). Glucagon secretion did not increase if at time 0 min only glucose, but not zinc, was switched off (○, n = 4) or if only zinc but not glucose was switched off (●, n = 4). If at time 0 min nifedipine (NIF) was added to the perifusate, glucagon secretion not only failed to rise but was suppressed despite the presence of the zinc switch-off signal during glucose deprivation (□, n = 3). ▴ at times −10 to 0 min represent the average of experimental values and control values.
FIG. 3.
Glucagon responses from STZ-induced diabetic wild-type mouse islets to an exogenous zinc switch-off signal during glucose deprivation in the presence of diazoxide. After an initial 30-min perifusion with 16.7 mmol/l glucose, diazoxide (DZX), and zinc, both glucose and zinc were discontinued at time 0 min and only diazoxide was continued for the last 30 min. Diazoxide prevented any significant increase in glucagon secretion despite the presence of a zinc switch-off signal during glucose deprivation (□, n = 3). For the purpose of comparison, ■ are the same data reported in Fig. 2 and represent the glucagon response to a zinc switch-off signal during glucose deprivation in the absence of diazoxide infusion. See text for statistical information.
FIG. 4.
Glucagon responses from STZ-induced diabetic wild-type mouse islets to an exogenous zinc switch-off signal during glucose deprivation in the presence of tolbutamide (50 μmol/l). After an initial 30-min perifusion with 16.7 mmol/l glucose, tolbutamide (TLB), and zinc, both glucose and zinc were discontinued at time 0 min and only tolbutamide was continued for the last 30 min. Tolbutamide prevented any significant increase in glucagon secretion despite the presence of a zinc switch-off signal during glucose deprivation (□, n = 3). For the purpose of comparison, ■ are the same data reported in Fig. 2 and represent the glucagon response to a zinc switch-off signal during glucose deprivation in the absence of tolbutamide infusion. The glucagon response is expressed as percentage of baseline because of the higher glucagon baseline in the islets perifused with tolbutamide due to tolbutamide-mediated closure of the KATP channels. See text for statistical information.
The rationale for choosing the concentration of 30 μmol/l for zinc chloride is provided in a previous publication (9). During exposure to physiologic or elevated glucose levels, the zinc-insulin complex is released from the β-cell into the periportal circulation that travels to α-cells. This design assumes that zinc dissociates from insulin when exposed to the higher pH of blood (13) and acts to open α-cell KATP channels, which causes tonic suppression of glucagon secretion. It also assumes that zinc is normally bound to protein in blood, as it dissociates from insulin in the periportal circulation and circulates to the α-cell, and that zinc concentration during glucose stimulation of insulin release is fourfold greater (9) than the 10 mmol/l concentration found in systemically circulating blood. This fourfold higher concentration of zinc is assumed to drop to the same levels found in systemically circulating blood during glucose deprivation and cessation of insulin secretion.
Drugs: nifedipine, diazoxide, tolbutamide, epinephrine.
Nifedipine: a 1 μmol/l perifusion solution was prepared from a 1 mmol/l stock solution in 100% ethanol. Diazoxide: a 50 μmol/l perifusion solution was prepared from a 50 mmol/l stock solution in 0.1 mol/l NaOH. Tolbutamide: a 50 μmol/l perifusion solution was prepared from a 50 mmol/l stock solution in 100% ethanol. Epinephrine: a 1 μmol/l perifusion solution was prepared from a 1 mmol/l stock solution in 1 mol/l HCl. Epinephrine was infused in the absence of glucose and the amino acid mixture for 5–7 min at the end of each experiment as alternative stimulus for glucagon secretion to ensure that α-cells were still functional after STZ treatment. Nifedipine, diazoxide, tolbutamide, and epinephrine were purchased from Sigma.
Assays.
Perifusion samples were collected into tubes containing 500 units of aprotinin to prevent degradation of glucagon. Insulin was measured by radioimmunoassay according to the method of Morgan and Lazarow (14). Glucagon was measured using a glucagon radioimmunoassay kit (Millipore, St. Charles, MO).
Statistical analysis.
Results for each perifusion experiment were calculated as means ± SE. Measurements of at least three lanes were considered as n = 1. Comparisons were made by paired Student's t test, unpaired Student's t test with or without Welch correction, Wilcoxon matched-pairs signed-ranks test, and ANOVA, where appropriate. P values <0.05 were considered significant.
RESULTS
Endogenous insulin switch-off signal in wild-type mouse islets.
Wild-type mouse islets were perifused with 16.7 mmol/l glucose for 30 min to stimulate endogenous insulin secretion. At time 0, the perifusate was switched to a 0 mmol/l glucose buffer for 30 min so that endogenous insulin secretion would decrease. In control experiments, at time 0, 16.7 mmol/l glucose was continued rather than discontinued. Insulin levels in the effluent decreased when the 16.7 mmol/l glucose perifusion was discontinued (0 min = 108 ± 7 μU/ml, 30 min = 5 ± 1 μU/ml, n = 8; P < 0.001) (Fig. 1A). When the 16.7 mmol/l glucose perifusion was not discontinued, insulin levels did not change significantly (0 min = 107 ± 10 μU/ml, 30 min = 90 ± 8 μU/ml, n = 7; P = NS) (Fig. 1A). Glucagon levels increased significantly only when 16.7 mmol/l glucose buffer was discontinued (0 min = 61 ± 8 pg/ml vs. 30 min = 133 ± 26 pg/ml, n = 8; P < 0.01) (Fig. 1B) and not when the glucose perifusate was continued for the duration of the experiment (0 min = 54 ± 9 pg/ml vs. 30 min = 71 ± 14 pg/ml, n = 7; P = NS) (Fig. 1B).
Exogenous zinc switch-off signal in STZ-induced diabetic wild-type mouse islets.
STZ-induced diabetic wild-type islets were perifused with 16.7 mmol/l glucose and zinc (30 μmol/l) for 30 min. Then, at time 0, both glucose and zinc perifusions were discontinued. In controls, either glucose or zinc alone was discontinued. In other experiments, at time 0 nifedipine was added to the perifusate when zinc was switched off during glucose deprivation (Fig. 2). Glucagon levels increased only when zinc perifusion was switched off during glucose deprivation (0 min = 28 ± 6 pg/ml vs. 30 min = 50 ± 4 pg/ml, n = 5; P < 0.02) (Fig. 2). If the zinc perifusion was continued during glucose deprivation, glucagon levels did not rise significantly (0 min = 15 ± 2 pg/ml vs. 30 min = 23 ± 3 pg/ml, n = 4; P = NS) (Fig. 2). Similarly, if perifusion containing glucose was continued after the zinc perifusion was switched off, the glucagon levels did not rise (0 min = 18 ± 4 pg/ml vs. 30 min = 25 ± 7 pg/ml, n = 4; P = NS) (Fig. 2). In the presence of nifedipine, glucagon levels after the zinc switch-off signal during glucose deprivation did not increase but significantly decreased (0 min = 36 ± 5 pg/ml vs. 30 min = 11 ± 1 pg/ml, n = 3; P < 0.05) (Fig. 2). Insulin levels were undetectable throughout.
Exogenous zinc switch-off signal during diazoxide infusion in STZ-induced diabetic wild-type mouse islets.
STZ-induced diabetic wild-type mouse islets were perifused with 16.7 mmol/l glucose and diazoxide (50 μmol/l) for 30 min (−60 to −30 min), followed by a perifusate containing 16.7 mmol/l glucose, diazoxide, and zinc (30 μmol/l) for another 30 min (−30 to 0 min). At 0 min, both glucose and zinc were discontinued and diazoxide infusion only was continued for 30 more minutes (Fig. 3). Glucagon secretion remained suppressed despite the absence of glucose and zinc in the perifusate (0 min = 10 ± 0 pg/ml vs. 30 min = 14 ± 2 pg/ml, n = 3; P = NS) (Fig. 3). Insulin levels were undetectable throughout.
Exogenous zinc switch-off signal during tolbutamide infusion in STZ-induced diabetic wild-type mouse islets.
STZ-induced diabetic wild-type mouse islets were perifused with 16.7 mmol/l glucose and tolbutamide (50 μmol/l) for 30 min (−60 to −30 min), followed by a perifusate containing 16.7 mmol/l glucose, tolbutamide, and zinc (30 μmol/l) for another 30 min (−30 to 0 min). At 0 min, both glucose and zinc were discontinued and tolbutamide infusion only was continued for 30 more minutes (Fig. 4). Glucagon secretion reached a higher baseline than in the previous STZ-induced diabetic islet experiments and it did not rise significantly when zinc was switched off during glucose deprivation (0 min = 53 ± 5 pg/ml vs. 30 min = 67 ± 3 pg/ml, n = 3; P = NS) (Fig. 4). Insulin levels were undetectable throughout.
Exogenous zinc switch-off signal in STZ-induced diabetic SUR1KO mouse islets.
An exogenous zinc switch-off signal during glucose deprivation was provided to STZ-induced diabetic SUR1KO mouse islets by infusing 16.7 mmol/l glucose and zinc (30 μmol/l) for 30 min and then switching to a perifusate without glucose and zinc at time 0 (Fig. 5). No significant glucagon increase was observed despite the provision of a zinc switch-off signal during glucose deprivation (0 min = 55 ± 17 pg/ml vs. 30 min = 61 ± 20 pg/ml; n = 4, P = NS). Insulin levels were undetectable throughout.
FIG. 5.
Glucagon responses in the presence of an exogenous zinc switch-off signal during perifusion of STZ-induced diabetic SUR1KO mouse islets. STZ-induced diabetic SUR1KO mouse islets were perifused with 16.7 mmol/l glucose and zinc for 30 min. Then, at time 0 min, both glucose and zinc were discontinued. Glucagon secretion from SUR1KO mouse islets failed to increase despite the presence of a zinc switch-off signal during glucose deprivation (□, n = 4). For the purpose of comparison, ■ are the same data reported in Fig. 2 and represent the glucagon rise in response to a zinc switch-off signal during glucose deprivation in STZ-induced diabetic wild-type mouse islets. The glucagon response is expressed as a percentage of baseline because of the higher glucagon baseline in SUR1KO mouse due to the absence of KATP channels. See text for statistical information.
Summary of glucagon responses with and without interfering with the KATP channel activity.
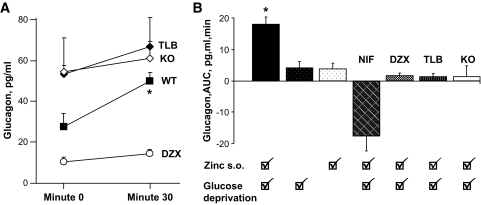
Glucagon levels before and after infusion of drugs that interfere with KATP channel activity in wild-type or SUR1KO islets are shown in Fig. 6A. Compared with wild-type values, depolarizing conditions (tolbutamide, knockout) were associated with higher glucagon levels (P < 0.05), and the polarized condition (diazoxide) was associated with lower glucagon levels (P < 0.05) at baseline (time 0). Glucagon responses to changes in the perifusate in STZ-induced diabetic mouse islets are compared as area under the curve in Fig. 6B. Glucagon response was statistically significant (P < 0.01–0.001) only when a zinc switch-off signal during glucose deprivation was provided to wild-type islets pretreated with STZ. No statistically significant glucagon responses were observed if zinc or glucose perifusions alone were discontinued or a zinc switch-off signal during glucose deprivation was provided in the presence of nifedipine, diazoxide, tolbutamide, or to SUR1KO islets.
FIG. 6.
Summary of glucagon responses. A: Comparison between glucagon values baseline at time 0 min immediately before the zinc switch-off signal during glucose deprivation and at time +30 min (means ± SE). Compared with the untreated wild-type islets, baseline glucagon levels at time 0 min were higher in the depolarized state (TLB, KO) and lower in the polarized state (DZX), all P < 0.05. At time +30 min, no differences were noted among the groups. A significant difference was noted when comparing time 0 to time +30 min in the untreated wild-type islet group (P < 0.02). B: Glucagon responses expressed as area under the curve (AUC) after zinc switch-off signal. A statistically significant (P < 0.001) glucagon response occurred only when a zinc switch-off signal was provided during glucose deprivation in wild-type islets pretreated with STZ.
Responses to epinephrine.
α-Cell function was tested at the end of most experiments using a 5- to 7-min infusion of epinephrine as alternative stimulus. Glucagon responses were intact in all the conditions involving wild-type and knockout islets (Fig. 7).
FIG. 7.
Glucagon responses to epinephrine. Glucagon responses to epinephrine were intact in both wild-type and SUR1KO islets. ♦, ♢, and hatched diamonds represent data obtained, respectively, from wild-type islets, STZ-induced diabetic wild-type islets, and STZ-induced diabetic SUR1KO islets. The glucagon response correlated significantly with the prestimulated glucagon level.
DISCUSSION
These experiments were designed to identify the mechanism through which zinc provides a switch-off signal for glucagon release during glucose deprivation. We previously reported that switching off zinc and insulin, but not zinc-free insulin, triggered glucagon secretion during hypoglycemia in vivo in rats (9). The results from the first part of the current study are consistent with a role for cessation of zinc corelease with insulin as a switch-off signal to trigger glucagon secretion during glucose deprivation. Since our experiments used mouse islets, our data argue against the contention of Ravier and Rutter (11) that zinc does not regulate α-cells in mice and support the more recent findings by Gyulkhandanyan et al. (15).
The second part of our study was designed to identify the mechanism of action for zinc regulation of the α-cell. To achieve this goal, we used two approaches. The first approach was to pharmacologically manipulate the KATP channel with drugs prior to providing STZ-induced diabetic wild-type mouse islets with a zinc switch-off signal during glucose deprivation. The first drug was diazoxide that keeps the KATP channels open. The rationale was that if the zinc switch-off signal is mediated by a change in the KATP channel activity, then using diazoxide to prevent any further change in the KATP channel activity should blind the KATP channel to the zinc switch-off signal. Zinc removal from the perifusate during glucose deprivation induced no changes in glucagon secretion from diazoxide-treated islets. This suggests that by maintaining the KATP channels open, the drug masked and overrode the signal given by zinc removal. In other mechanistic studies, we interfered with the KATP channel activity using tolbutamide. This drug, which keeps KATP channels closed, led to the same result. Because of the tolbutamide-mediated prestimulation of KATP channel activity, the already closed KATP channels failed to respond to the signal provided by the switch-off of zinc during glucose deprivation. We also examined whether the post–KATP channel signal transduction pathway involved calcium channels. Nifedipine was used to prevent the calcium channels from opening. Since zinc does not act on calcium channels, no preinfusion (as with the diazoxide and tolbutamide experiments) with nifedipine was deemed necessary. In the presence of nifedipine, glucagon secretion failed to respond to the zinc switch-off signal during glucose deprivation. These results suggest that the pathway through which the zinc switch-off signal triggers glucagon secretion during glucose deprivation involves both KATP and calcium channels (Fig. 8). Our findings also demonstrate that the α-cell is responsive to the zinc switch-off signal only in the circumstance of glucose deprivation, consistent with the fact that glucose is widely recognized as a suppressant of glucagon secretion (16,17).
FIG. 8.
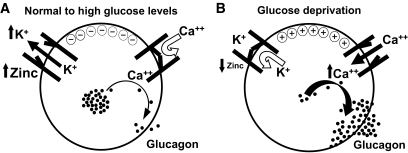
Regulation of glucagon secretion by zinc. A: α-Cell electrical and hormonal status in states of physiologic and elevated glucose conditions. β-Cells release zinc and insulin hexamers into the intraislet periportal circulation. Zinc dissociates from insulin and reaches downstream α-cells, where it binds to and opens the KATP channels. K+ ions leave the cell and hyperpolarize the α-cell, thus preventing voltage-dependent calcium channels from opening. Glucagon granules are not mobilized and remain stored inside the cell. B: When blood glucose levels decrease in response to hypoglycemic levels, β-cell insulin and zinc secretion decrease as well. KATP channels on α-cells close, K+ remains in the cell, and the α-cell depolarizes, which induces calcium channels to open and calcium enters the cell. Intracellular calcium rises, inducing glucagon exocytotic granules to migrate to the plasma membrane, where they fuse and release glucagon into the portal venous system.
The second approach used a model derived from the mouse line C57BL6/J, whose SUR1 subunit of the KATP channel is knocked out (10). Islet KATP channels consist of two subunits: the K+ inward rectifier, Kir6.2, and the high-affinity sulfonylurea receptor, SUR1, combined in 4:4 stoichiometry to form the hetero-octomeric channel. The Kir6.2 subunit forms the conducting pore for K+ ions, while the SUR1 subunit acts as a regulatory subunit conferring sensitivity to openers and blockers (18–20). A tight interaction between the two subunits is required for appropriate channel function. In our experiments, although SUR1KO mouse islets were exposed to an exogenous zinc switch-off signal during glucose deprivation, glucagon secretion failed to respond. Importantly, SUR1KO mouse islets, as well as wild-type islets, responded to the alternative stimulus epinephrine, a known stimulator of glucagon secretion (21). Glucagon responses to this stimulus indicate that α-cell glucagon synthesis and exocytotic mechanisms are intact.
Our data reinforce the hypothesis that an intercellular dialogue is critical within the pancreatic islet for optimal glycemic control (4) and that zinc participates to this dialogue and therefore to the regulation of glucagon secretion by acting through the SUR1 subunit of the KATP channels as reported by other groups (8,22–25). In this scenario, during exposure to physiologic or elevated glucose levels, the zinc-insulin complex is released from the β-cell into the periportal circulation that travels to α-cells. Zinc dissociates from insulin when exposed to the higher pH of blood (13) and acts to open α-cell KATP channels, which causes tonic suppression of glucagon secretion. However, during states of glucose deprivation, such as hypoglycemia, β-cells virtually cease releasing zinc-insulin. This results in cessation of zinc effects on α-cell KATP channels and closure of the channels, which in turn causes depolarization, voltage-dependent calcium channel opening, calcium entry, and glucagon exocytosis (Fig. 8).
In support of this mechanism is the observation that the glucagon values reached during the α-cell response to the zinc switch-off signal during glucose deprivation in wild-type mouse islets were very similar to the values of the glucagon baselines in wild-type mouse islets perifused with tolbutamide and in knockout mouse islets (Fig. 6A). Thus, switching off zinc, closing the KATP channels by means of tolbutamide, and absent KATP channels (as occurs in the KO mice) depolarize the α-cell, causing Ca2+ channels to open and increase glucagon secretion. On the other hand, adding diazoxide to the perifusate keeps KATP channels open, supporting K+ efflux, which keeps the cell polarized and thus prevents Ca2+ channels from opening, resulting in less glucagon secretion. Different from previous studies by others, our observations were obtained from intact and STZ-induced diabetic mouse islets, using a dynamic system that specifically studied glucagon secretion during glucose deprivation. This technique more closely approximates the important clinical setting in which regulation of glucagon secretion is most critical (i.e., hypoglycemia). Moreover, our results support the hypothesis that the association of high basal glucagon in type 1 diabetes could at least in part be attributed to lack of insulin-bound zinc tonically suppressing α-cell function.
In conclusion, our results strongly implicate the α-cell KATP channel and calcium channel as obligate parts of the mechanism whereby zinc suppresses glucagon secretion from the α-cell and whereby a sudden absence of zinc secretion from the β-cell during glucose deprivation triggers glucagon secretion.
Acknowledgments
Supported by National Institutes of Health Grant NIDDK RO1 39994 (to R.P.R.) and RO1DK044311(to J.B.) and an American Diabetes Association Mentor-Based Grant (to R.P.R.).
No potential conflicts of interest relevant to this article were reported.
Parts of this article have been presented in abstract form at the American Diabetes Association 69th Scientific Sessions, New Orleans, Louisiana, 5–9 June 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Banarer S, McGregor VP, Cryer PE: Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 2002;51:958–965 [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP: Regulation of alpha-cell function by the beta-cell during hypoglycemia in wistar rats: the “switch-off” hypothesis. Diabetes 2004;53:1482–1487 [DOI] [PubMed] [Google Scholar]
- 3.Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP: Regulation of α-cell function by the β-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes 2004;53:1488–1495 [DOI] [PubMed] [Google Scholar]
- 4.Samols E, Stagner JI, Ewart RB, Marks V: The order of islet microvascular cellular perfusion is β-α-δ in the perfused rat pancreas. J Clin Invest 1988;82:350–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rorsman P, Berggren PO, Bokvist K, Ericson H, Mohler H, Ostenson CG, Smith PA: Glucose-inhibition of glucagon secretion involves activation of gabaa-receptor chloride channels. Nature 1989;341:233–236 [DOI] [PubMed] [Google Scholar]
- 6.Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M: Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring β-cells. Diabetes 2004;53:1038–1045 [DOI] [PubMed] [Google Scholar]
- 7.Franklin IK, Wollheim CB: GABA in the endocrine pancreas: Its putative role as an islet cell paracrine-signalling molecule. J Gen Physiol 2004;123:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB: Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 2003;5:330–335 [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP: Zinc, not insulin, regulates the rat α-cell response to hypoglycemia in vivo. Diabetes 2007;56:1107–1112 [DOI] [PubMed] [Google Scholar]
- 10.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J: Sur1 knockout mice. A model for k(atp) channel-independent regulation of insulin secretion. J Biol Chem 2000;275:9270–9277 [DOI] [PubMed] [Google Scholar]
- 11.Ravier MA, Rutter GA: Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 2005;54:1789–1797 [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, Tran PO, Harmon J, Robertson RP: A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A 2002;99:12363–12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodson G, Steiner D: The role of assembly in insulin's biosynthesis. Curr Opin Struct Biol 1998;8:189–194 [DOI] [PubMed] [Google Scholar]
- 14.Morgan CR LA: Immunoassay of insulin: two antibody system: plasma insulin levels of normal, subdiabetic, and diabetic rats. Diabetes 1963;12:115–126 [Google Scholar]
- 15.Gyulkhandanyan AV, Lu H, Lee SC, Bhattacharjee A, Wijesekara N, Fox JE, MacDonald PE, Chimienti F, Dai FF, Wheeler MB: Investigation of transport mechanisms and regulation of intracellular zn2+ in pancreatic alpha-cells. J Biol Chem 2008;283:10184–10197 [DOI] [PubMed] [Google Scholar]
- 16.Vieira E, Salehi A, Gylfe E: Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 2007;50:370–379 [DOI] [PubMed] [Google Scholar]
- 17.MacDonald PE, De Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PRV, Cox R, Eliasson L, Rosrsman P: A KATP channel-dependent pathway within α cells regulates glucagon release from both rodent and human islets of Langerhans PLOS Biol 2007;5:1236–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguilar-Bryan L, Bryan J: Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 1999;20:101–135 [DOI] [PubMed] [Google Scholar]
- 19.Bryan J, Vila-Carriles WH, Zhao G, Babenko AP, Aguilar-Bryan L: Toward linking structure with function in ATP-sensitive K+ channels. Diabetes 53 (Suppl. 1) 2004;3:S104–S112 [DOI] [PubMed] [Google Scholar]
- 20.Seino S, Miki T: Physiological and pathophysiological roles of ATP-sensitive k+ channels. Prog Biophys Mol Biol 2003;81:133–176 [DOI] [PubMed] [Google Scholar]
- 21.Gromada J, Bokvist K, Ding W-G, Barg S, Buschar K, Renstrom E, Rorsman P: Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J Gen Physiol 1997;110:217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB: β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 2005;54:1808–1815 [DOI] [PubMed] [Google Scholar]
- 23.Bloc A, Cens T, Cruz H, Dunant Y: Zinc-induced changes in ionic currents of clonal rat pancreatic-cells: Activation of ATP-sensitive k+ channels. J Physiol 2000;3:723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prost AL, Bloc A, Hussy N, Derand R, Vivaudou M: Zinc is both an intracellular and extracellular regulator of katp channel function. J Physiol 2004;559:157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bancila V, Cens T, Monnier D, Chanson F, Faure C, Dunant Y, Bloc A: Two sur1-specific histidine residues mandatory for zinc-induced activation of the rat KATP channel. J Biol Chem 2005;280:8793–8799 [DOI] [PubMed] [Google Scholar]