Abstract
OBJECTIVE
We have hypothesized that variation in imprinted growth-promoting fetal genes may affect maternal glucose concentrations in pregnancy. To test this hypothesis we evaluated the effects of fetal disruption of murine H19Δ13 on maternal glucose concentrations in pregnancy.
RESEARCH DESIGN AND METHODS
Experimental mice were pregnant females that had inherited the disrupted H19Δ13 from their fathers and were therefore phenotypically wild type due to imprinting; approximately half of their litters were null for H19Δ13 through maternal inheritance of the disrupted gene. In control mice approximately half the litter paternally inherited the disrupted H19Δ13, so the pups were either genetically wild type or phenotypically wild type due to imprinting. Blood glucose concentrations were assessed by intraperitoneal glucose tolerance tests on days 1, 16, and 18 of pregnancy.
RESULTS
There were no differences in the glucose concentrations of control and experimental pregnant mice at day 1. However, at day 16 mothers carrying H19Δ13-null pups had a significantly higher area under the glucose tolerance test curves than controls (1,845 ± 378 vs. 1,386 ± 107 mmol · min · l−1 [P = 0.01]) in association with increasing pregnancy-related insulin resistance. Although this difference lessened toward term, overall, mothers of maternally inherited H19Δ13 mutants had significantly higher glucose concentrations during the last trimester (1,602 ± 321 [n = 17] vs. 1,359 ± 147 [n = 18] mmol · min · l−1 [P = 0.009]).
CONCLUSIONS
This study provides evidence that maternal glucose concentrations in pregnant mice can be affected by targeted disruption of fetal H19Δ13. This implies that variable fetal IGF2 expression could affect risk for gestational diabetes.
Gestational diabetes mellitus (GDM) has traditionally been considered a condition originating through combined effects of maternal genetics (1,2) and her environment, including effects associated with maternal obesity. The current increasing incidence of GDM (3) is thought to result from the increasing prevalence of obesity, with effects of the associated insulin resistance combining with those of the physiological insulin resistance of pregnancy to impair glucose tolerance.
Following Haig's suggestion (4) that maternal metabolism during pregnancy could be influenced by variation in the fetal genome, we recently proposed that risk of GDM could also be modified by variation in the fetal genome, particularly in fetal growth genes (5). This might occur as part of the genetic conflict of pregnancy where maternal genes limit fetal growth and paternally inherited fetal genes enhance it by increasing the amount of metabolic fuel supplied by the mother to the fetus (6). Candidates include imprinted genes, where expression of transmitted alleles depends on their parent of origin (7,8). Preliminary evidence for this phenomenon comes from our studies of contemporary birth cohorts that have shown that both third-trimester maternal glucose tolerance and offspring birth weights are associated with common polymorphic variation in the H19 gene (9), which is reciprocally imprinted with respect to IGF2 and regulates its imprinting and expression.
The following study was therefore performed to test the hypothesis that changes in maternal glucose concentrations during pregnancy could be mediated by genetic variation in the murine fetal H19 region (5), which is usually expressed only from the maternally inherited fetal allele, using mice with targeted disruption of H19Δ13 that are born 30% heavier than controls (10,11).
RESEARCH DESIGN AND METHODS
Animals.
All experiments were performed under the Animals (Scientific Procedures) Act 1986 after Cambridge University Animal Ethics Committee approval. The mice were kept under controlled conditions with a 12-h light/dark cycle and had free access to food and water throughout (except during the glucose tolerance test [GTT] starvation period when only water was available).
H19Δ13−/+ mice (in which insertion of a neomycin resistance cassette originally replaced the entire 3-kb coding region of the gene and the 10-kb 5′ flanking region including the Igf2 control region [10,12]) were bred on C57BL/6 backgrounds and their offspring genotyped by PCRs of ear biopsy DNA (extracted using DNeasy kits; Qiagen, Crawley, U.K.). The 20-μl reaction mix contained 2 units of BIO-X-ACT polymerase (Bioline Ltd., London, U.K.), 10 μl Buffer A (Bioline), 8 pmol of forward (5′-TGCCACAGAGGAAGAAACCAG-3′) and reverse (5′-AGTCATAGCCGAATAGCC-3′) oligonucleotide primers, and 25 ng DNA. The reaction mix was incubated at 94°C for 7 min and then 20 cycles of 94°C (30 s), 49°C (30 s) dropping 0.5°C each cycle, and 72°C (1 min). After this, the mix underwent 30 cycles of 94°C (30 s), 39°C (30 s), and 72°C (1 min) and a final incubation at 72°C (10 min). Knockout mice produced a 895-bp band when separated electrophoretically on a 1% (wt/vol) agarose gel. Presence of the wild-type H19 gene was tested using similar reaction conditions but a different reverse primer (5′-TTCAGTCACTTCCCTCAGCCTC-3′) and increasing the annealing temperatures by 10°C throughout to produce a 494-bp band.
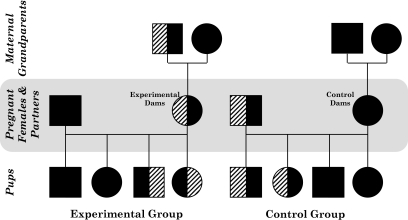
The aim of our breeding program was to produce experimental dams, in which approximately half of their litter was H19Δ13 null due to inheriting the disrupted gene from their mothers, and control dams, in which approximately half of their genetically matched litter inherited the disrupted H19Δ13 from their fathers and were therefore phenotypically wild type due to imprinting. To achieve this, experimental females were genotypically heterozygous knockout mice (H19Δ13+/−) that were phenotypically wild type due to paternal inheritance of the disrupted gene and were mated to wild-type C57BL/6 male mice (Charles River Ltd., Margate, U.K.) (Fig. 1). Controls were wild-type C57BL/6 females mated to genotypically heterozygous H19Δ13+/− males. Due to imprinting, the genotype, but not the gene expression distribution among the pups, was therefore the same for both groups. Pregnancy was assumed at the expulsion of a vaginal plug (day 0), although for the studies on days 16 and 18 of pregnancy only those animals who gained weight suggestive of pregnancy were assessed.
FIG. 1.
Schematic of the matings in the H19Δ13 knockout study. Male mice are represented by squares and female mice by circles. Wild-type mice are represented by solid black shapes and heterozygote knockout mice by half-solid black shapes and half-striped shapes (the stripes are on the left-hand side if the disrupted allele was inherited from the father and on the right-hand side if it was inherited from the mother). Penetrance of the disrupted allele is estimated to be 50% in each case, and half of the offspring are assumed to be males. All the experimental mothers, although they were heterozygous H19Δ13 knockouts, were phenotypically wild type due to having inherited the disrupted allele from their fathers and imprinting.
Glucose tolerance tests.
For each day, 8–10 different experimental and control mice had their glucose concentrations assessed on day 1, 16, or 18 of pregnancy (day 1 was used as baseline; the other days were used because in previous experiments using an alternative mouse model in which placental Igf2 expression was manipulated, there were differences in effects on placental nutrient transport among these days [13]). The animals were starved for 15 h and were then tail bled to produce samples for the measurement of blood glucose and other analytes. The mice were then injected intraperitoneally with 1 g/kg body wt glucose (administered as a 10% [wt/vol] solution). Further blood glucose measurements were then taken 15, 30, 60, 120, and 180 min later.
Blood glucose, serum insulin, and Igf-ii concentrations.
Blood glucose was measured using a 201+ glucose meter (Hemocue Ltd., Sheffield, U.K.). Fasting serum Igf-ii (following standard acid-ethanol extraction and neutralization; RayBiotech Mouse Igf-ii ELISA; Innovate Biotechnology, London, U.K.) and insulin (Linco Rat & Mouse Insulin ELISA; Millipore, London, U.K.) concentrations were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions. Fasting insulin sensitivity was assessed indirectly by homeostasis model assessment (HOMA) (http://www.dtu.ox.ac.uk/index.php?maindoc=/homa/index.php). Although its underlying principles are invalid in rodents (14), the values produced by this model do correlate with hyperinsulinemic-euglycemic clamp measures of insulin sensitivity in mice (15) without the need for prior surgery or general anesthesia.
Statistical analysis.
Glucose concentrations were assessed as areas under the (0–180 min) glucose curves (AUCs), calculated using the trapezoid rule. When comparing normally distributed data from two groups on a particular day of pregnancy, Student t tests were used (having performed the Levine test for equality of variances); otherwise, comparison was by Mann-Whitney U test. Where overall comparisons were made using all 3 days of pregnancy in the same model, two-way ANOVA was used with the experimental group and day of pregnancy as the fixed variable and glucose AUC as the dependent variable. All statistical analysis was performed using SPSS, version 14.0 (SPSS, Chicago). Data are presented as mean ± SD for the GTT data and median (interquartile range) for everything else. P < 0.05 was considered statistically significant.
RESULTS
On day 1 of pregnancy there was no detectable difference in glucose AUCs during the GTT between experimental and control groups: 1,478 ± 197 (n = 10) vs. 1,708 ± 318 (n = 10) mmol · min · l (P = 0.07). There were also no detectable differences in fasting serum insulin or Igf-ii concentrations or in insulin sensitivity (Table 1).
TABLE 1.
Fasting plasma insulin and Igf-ii concentrations, with fasting insulin sensitivities on days 1, 16, and 18 of pregnancy in mice carrying litters containing H19Δ13−/+knockout pups
| Experimental mice | Controls | P | |
|---|---|---|---|
| Day 1 | (n = 10) | (n = 10) | |
| Insulin (pmol/l) | 46 (35–74) | 35 (35–61) | 0.4 |
| Igf-ii (ng/ml) | All <6 | All <6 | 1.0 |
| Insulin sensitivity (HOMA %S) | 113.9 (64.3–147.8) | 140.0 (78.6–145.1) | 0.8 |
| Day 16 | (n = 8) | (n = 9) | |
| Insulin (pmol/l) | 137 (92–237) | 112 (52–154) | 0.3 |
| Igf-ii (ng/ml) | All <6 | All <6 | 1.0 |
| Insulin sensitivity (HOMA %S) | 40 (23–61) | 50 (40–119) | 0.2 |
| Day 18 | (n = 9) | (n = 9) | |
| Insulin (pmol/l) | 111 (63–279) | 141 (83–1,472) | 0.7 |
| Igf-ii (ng/ml) | All <6 | All <6 | 1.0 |
| Insulin sensitivity (HOMA %S) | 52 (21–102) | 40 (6–65) | 0.8 |
Data are median (interquartile range).
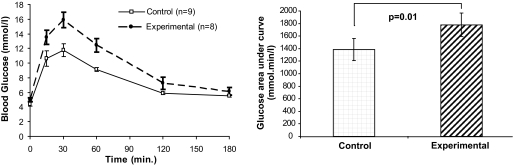
On day 16 of pregnancy, when H19Δ13−/+ pups weighed on average 118% that of wild-type pups (supplementary Table 1 in the online appendix, which is available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0757/DC1), all mice were more insulin resistant than on day 1 (insulin sensitivity dropping as the day of pregnancy increased [P = 0.002]). Experimental dams had significantly higher glucose AUCs than controls (P = 0.01, Fig. 2) despite having no detectable differences in fasting insulin or Igf-ii concentrations or insulin sensitivity (Table 1).
FIG. 2.
Intraperitoneal GTT curves of pregnant mice carrying litters containing H19Δ13−/+ knockout pups on day 16 of pregnancy and total AUC bar charts. Each time point in the GTT is presented as a mean ± SEM, with the total AUCs as mean (95% CI).
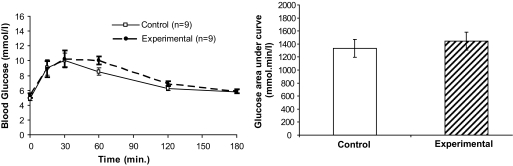
At day 18, when H19Δ13−/+ pups weighed on average 123% that of wild-type pups (supplementary Table 1), mice carrying litters containing H19Δ13−/+ mice still had higher mean glucose AUCs, but the difference was not statistically significant (P = 0.2, Fig. 3). There was no difference in either day 18 fasting insulin or Igf-ii concentrations or in insulin sensitivity (Table 1). Combining data from across the third trimester, the experimental mice still had significantly higher glucose AUCs than the controls: 1,602 ± 321 (n = 17) vs. 1,359 ± 147 (n = 18) mmol · min · l−1 (P = 0.009).
FIG. 3.
Intraperitoneal GTT curves of pregnant mice carrying litters containing H19Δ13−/+ knockout pups on day 18 of pregnancy and total AUC bar charts. Each time point in the GTT is presented as a mean ± SEM, with the total AUCs as mean (95% CI).
There were no differences in the number of pups carried by experimental and control mice (data not shown). Nor was the number of pups carried correlated with any measure of glucose concentrations in either of the groups. No difference was made to the results of analyzing the area under the GTT curves when adjusting for the numbers of pups being carried (data not shown). When comparing glucose AUCs on all the different days in the same statistical model, there were significant effects of both the day of pregnancy (P = 0.02) and the interaction between the day of pregnancy and the group (P = 0.001). Also, a lowering of glucose AUCs between days 16 and 18 of pregnancy was observed that was independent of group (P = 0.014; supplementary Table 2).
DISCUSSION
This study provides direct evidence that changes in murine maternal glucose concentrations during pregnancy can be mediated by alteration in a fetal growth gene, in this case H19Δ13. Previously, there has been only indirect evidence from humans to support this proposal (5), partially due to the difficulty in differentiating effects of fetal genes on maternal metabolism from effects of maternal genes. Evidence implicating variation in fetal IGF2 expression comes from the observed trend for an increased risk of developing GDM in mothers carrying offspring with Beckwith-Wiedemann syndrome (16). Additional indirect evidence for the fetal genome modifying the risk for maternal GDM comes from studies showing an increased risk in women carrying males rather than smaller females (17), and in multiple pregnancies (18).
Our studies provide further support for this hypothesis. On day 1 of pregnancy there was no change in glucose concentrations in mice carrying H19Δ13−/+ pups. However by day 16 significant increases in maternal glucose concentrations were observed in dams carrying H19Δ13−/+ pups despite overall similar decreases in HOMA-derived insulin sensitivity in both groups. These raised glucose concentrations could have resulted from reduced first-phase insulin secretion and/or insulin-stimulated glucose uptakes, neither of which would have influenced HOMA measurements.
Our mice also displayed a generalized lowering of glucose concentrations between days 16 and 18, as has recently been observed in another mouse model (19). This may have limited our power to detect continuing differences between groups, as there was no effect of fetal H19Δ13−/+ on maternal glucose concentrations evident on day 18 of pregnancy. Whereas in humans it is known that glucose tolerance generally deteriorates as pregnancy progresses (20), data relating to the very last weeks of pregnancy corresponding to day 18 in the mouse are less certain. Women with preexisting type 1 diabetes have a fall in insulin requirements in the last 2 weeks of pregnancy (21), suggesting a possible improvement in insulin sensitivity. If effects on maternal glucose metabolism in our mice were mediated by placental hormones (5), the generalized fall in maternal glucose concentrations at the end of pregnancy may therefore have resulted from a prepartum decrease in placental metabolic activity similar to that which has been observed in other model systems (22).
H19Δ13 disruption affects Igf2 imprinting and expression, and fetal growth in mice (10,11). We hypothesize that our results therefore relate to increased placental and fetal Igf2 expression. However we cannot rule out a role for the disruption of H19 per se or changes in 91H (antisense H19) RNA expression (23). We propose that the increased placental Igf2 expression, rather than causing changes in maternal Igf-ii concentrations that were not detectably raised in our mice, affects the expression and release of metabolically active placental hormones into the maternal circulation that worsens their glucose tolerance (5). Indirect support for this comes from the enhanced lowering of glucose concentrations we observed in H19Δ13−/+ mice between days 16 and 18 of pregnancy, as at equivalent stages in human pregnancies at least, placental protein concentrations in the maternal circulation fall (24). One candidate hormone for this process is mouse placental lactogen II because its placental expression has been linked to that of Igf2 (25), it regulates pancreatic β-cell expansion in pregnancy (26), and pregnant mice without functional prolactin receptors, for which it is a ligand, become glucose intolerant (19). These animal experimental data may be important in the understanding of the pathogenesis of human GDM. Previously, we found that in humans a common H19 polymorphism is associated with variation in birth weight, maternal glucose concentrations, and cord blood IGF-II concentrations (9). In humans, there is a linear relationship between maternal glucose tolerance during pregnancy and birth weights, even in the absence of GDM (27). Our study would suggest that this may not be explained solely by maternal genetic and environmental factors determining glucose concentrations crossing the placenta and stimulating fetal insulin secretion with ensuing fetal weight gain. These relationships could be affected by the fetal genome influencing maternal glucose concentrations by factors secreted by the placenta into the maternal circulation. In conclusion, this study provides the first direct evidence that a variation in a fetal gene affecting growth rates (10,11) may also alter maternal glucose concentrations during pregnancy, in association with increasing pregnancy-related insulin resistance, raising the possibility that this process could contribute to the etiology of human GDM.
Supplementary Material
Acknowledgments
This study was supported by Medical Research Council funding (grant reference G0500733) and the Medical Research Council Centre for Obesity and Related Metabolic Disorders.
No potential conflicts of interest relevant to this article were reported.
We thank Alex Dittrich, Nicola Shelley, Stan Barkhuysen, Samudra Ranawaka, and Helen Steenson for excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Watanabe RM, Black MH, Xiang AH, Allayee H, Lawrence JM, Buchanan TA: Genetics of gestational diabetes mellitus and type 2 diabetes. Diabetes Care 2007(Suppl. 2);30:S134–S140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaat N, Groop L: Genetics of gestational diabetes mellitus. Curr Med Chem 2007;14:569–583 [DOI] [PubMed] [Google Scholar]
- 3.Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC: Gestational diabetes in the United States: temporal trends 1989 through 2004. Am J Obstet Gynecol 2008;198:525.e1–e5 [DOI] [PubMed] [Google Scholar]
- 4.Haig D: Placental hormones, genomic imprinting, and maternal-fetal communication. J Evol Biol 1996;9:357–380 [Google Scholar]
- 5.Petry CJ, Ong KK, Dunger DB: Does the fetal genotype affect maternal physiology during pregnancy? Trends Molec Med 2007;13:414–421 [DOI] [PubMed] [Google Scholar]
- 6.Haig D: Genetic conflicts in human pregnancy. Q Rev Biol 1993;68:495–532 [DOI] [PubMed] [Google Scholar]
- 7.Haig D, Westoby M: Parent-specific gene expression and the triploid endosperm. Am Nat 1989;134:147–155 [Google Scholar]
- 8.Reik W, Constância M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, Tycko B, Sibley C: Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol 2003;547:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petry CJ, Ong KK, Barratt BJ, Wingate D, Cordell HJ, Ring SM, Pembrey ME, Reik W, Todd JA, Dunger DB: ALSPAC Study Team: common polymorphism in H19 associated with birth weight and cord blood IGF-II levels in humans. BMC Genetics 2005;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM: Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature 1995;375:34–39 [DOI] [PubMed] [Google Scholar]
- 11.Chiao E, Fisher P, Crisponi L, Deiana M, Dragatsis I, Schlessinger D, Pilia G, Efstratiadis A: Overgrowth of a mouse model of the Simpson-Golabi-Behmel syndrome is independent of IGF signaling. Dev Biol 2002;243:185–206 [DOI] [PubMed] [Google Scholar]
- 12.Lopes S, Lewis A, Hajkova P, Dean W, Oswald J, Forné T, Murrell A, Constância M, Bartolomei M, Walter J, Reik W: Epigenetic modifications in an imprinting cluster are controlled by a hierarchy of DMRs suggesting long-range chromatin interactions. Hum Mol Genet 2003;12:295–305 [DOI] [PubMed] [Google Scholar]
- 13.Constância M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A: Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A 2005;102:19219–19224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace TM, Levy JC, Matthews DR: Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, Kim JK, Quon MJ: Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab 2008;294:E261–E270 [DOI] [PubMed] [Google Scholar]
- 16.Wangler MF, Chang AS, Moley KH, Feinberg AP, Debaun MR: Factors associated with preterm delivery in mothers of children with Beckwith-Wiedemann syndrome: a case cohort study from the BWS registry. Am J Med Genet A 2005;134:187–191 [DOI] [PubMed] [Google Scholar]
- 17.Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M: Gender does matter in perinatal medicine. Fetal Diagn Ther 2004;19:366–369 [DOI] [PubMed] [Google Scholar]
- 18.Norwitz ER, Edusa V, Park JS: Maternal physiology and complications of multiple pregnancy. Semin Perinatol 2005;29:338–348 [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Snider F, Cross JC: Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 2009;150:1618–1626 [DOI] [PubMed] [Google Scholar]
- 20.Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, Sims EA: Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol 1993;264:E60–E67 [DOI] [PubMed] [Google Scholar]
- 21.Jovanovic L, Nakai Y: Successful pregnancy in women with type 1 diabetes: from preconception through postpartum care. Endocrinol Metab Clin North Am 2006;35:79–97, vi [DOI] [PubMed] [Google Scholar]
- 22.Basch CV, Talamantes F: In vitro kinetics of synthesis and release of mouse placental lactogen. Endocrinology 1986;119:1939–1947 [DOI] [PubMed] [Google Scholar]
- 23.Berteaux N, Aptel N, Cathala G, Genton C, Coll J, Daccache A, Spruyt N, Hondermarck H, Dugimont T, Curgy JJ, Forné T, Adriaenssens E: A novel H19 antisense RNA overexpressed in breast cancer contributes to paternal IGF2 expression. Mol Cell Biol 2008;28:6731–6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuhashi N, Tachibana Y, Kono H, Shinkawa O, Takahashi T, Suzuki M: Changes in placental lactogen, beta 1-chorionic gonadotropin, and unconjugated and total estriol levels in the late course of normal human pregnancy. Tohoku J Exp Med 1984;143:47–51 [DOI] [PubMed] [Google Scholar]
- 25.Ishida M, Ohashi S, Kizaki Y, Naito J, Horiguchi K, Harigaya T: Expression profiling of mouse placental lactogen II and its correlative genes using a cDNA microarray analysis in the developmental mouse placenta. J Reprod Dev 2007;53:69–76 [DOI] [PubMed] [Google Scholar]
- 26.Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL: Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 1993;132:879–887 [DOI] [PubMed] [Google Scholar]
- 27.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA: Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.